Multimodal Pain Management in Orthopedic Surgery
Abstract
:1. Introduction
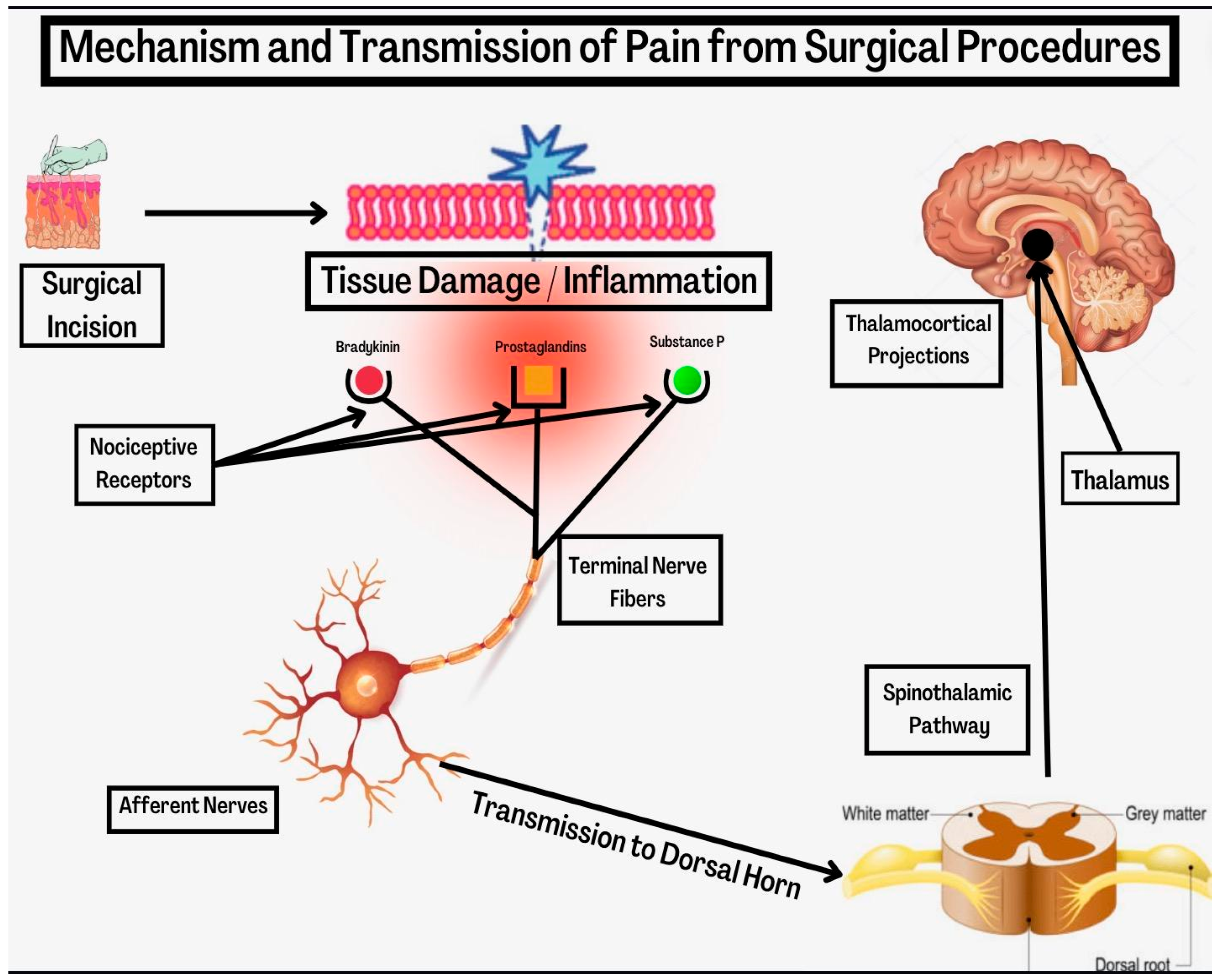
2. Mechanism of Post-Surgical Pain
3. Role of the Anesthesiologist
4. Methods of Analgesia
4.1. Pre-Emptive Analgesia
4.2. Surgical Site Infiltration
4.3. Analgesic Techniques
4.4. Central Neuraxial, Regional and Local Analgesia
4.5. Regional Anesthesia
4.6. Patient Controlled Analgesia
5. Systemic Analgesics
5.1. Opioids
5.2. Acetaminophen
5.3. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
5.4. N-Methyl D-Aspartate (NMDA) Receptor Antagonists
| Drug Class | Dose | Analgesic Duration | Metabolism | Comments |
|---|---|---|---|---|
| Para-aminophenol Derivative | ||||
| Acetaminophen | 650–1000 mg IV | 4–6 h |
|
|
| Nonselective NSAIDs | ||||
| Ketorolac | 15–30 mg IV | 4–6 h |
|
|
| Ibuprofen | 400–800 mg IV | 4–6 h |
|
|
| Selective COX-2 Inhibitor | ||||
| Parecoxib | 20–40 mg IV | 6–12 h (IV) |
|
|
| N-methyl D-asparate (NMDA) Receptor Antagonist | ||||
| Ketamine | 0.5–1 mg/kg/h IV | 30–60 min IV |
|
|
5.5. Anticonvulsants (Gamma-Aminobutyric Acid Analogues)
5.6. Beta-Blockers
5.7. Alpha-2 Agonists
| Drug Class | Dose | Duration of Hemodynamic Effect | Metabolism | Comments |
|---|---|---|---|---|
| Beta Adrenergic Receptor Blockers | ||||
| Atenolol | 25–50 mg PO | 12–24 h (PO) |
|
|
| Metoprolol | 100 mg PO 2.5–5 mg IV | 3–6 h (PO)3–4 h (IV) |
|
|
| Esmolol | 0.5–1 mg/kg (IV) | 10–30 min (IV) |
|
|
| Alpha-2 Adrenergic Receptor Agonists | ||||
| Clonidine | 0.1 mg PO | 4–6 h |
|
|
| Dexmedeto-midine | 1 mcg/kg IV over 10 min with 0.2–0.7 mcg/kg/hr continuous IV | 2–3 h (IV) |
|
|
5.8. Capsaicin (Transient Receptor Potential Vanilloid Receptor 1 (TRPV1) Agonist)
5.9. Lidocaine Infusion
5.10. Glucocorticoids
5.11. Magnesium
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, J.R.; Mir, H.; Wally, M.K.; Seymour, R.B. Clinical Practice Guidelines for Pain Management in Acute Musculoskeletal Injury. J. Orthop. Trauma 2019, 33, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, S.A.; Chelminski, P.R.; Ives, T.J.; Ranapurwala, S.I. Management of Pain in the United States—A Brief History and Implications for the Opioid Epidemic. Health Serv. Insights 2018, 11, 117863291881944. [Google Scholar] [CrossRef] [PubMed]
- Rudd, R.A.; Seth, P.; David, F.; Scholl, L. Increases in Drug and Opioid-Involved Overdose Deaths—United States, 2010–2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1445–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braden, J.B.; Fan, M.-Y.; Edlund, M.J.; Martin, B.C.; DeVries, A.; Sullivan, M.D. Trends in Use of Opioids by Noncancer Pain Type 2000–2005 among Arkansas Medicaid and HealthCore Enrollees: Results from the TROUP Study. J. Pain 2008, 9, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.C.; Fan, M.-Y.; Edlund, M.J.; DeVries, A.; Braden, J.B.; Sullivan, M.D. Long-Term Chronic Opioid Therapy Discontinuation Rates from the TROUP Study. J. Gen. Intern. Med. 2011, 26, 1450–1457. [Google Scholar] [CrossRef] [Green Version]
- Korff, M.V.; Saunders, K.; Thomas Ray, G.; Boudreau, D.; Campbell, C.; Merrill, J.; Sullivan, M.D.; Rutter, C.M.; Silverberg, M.J.; Banta-Green, C.; et al. De Facto Long-Term Opioid Therapy for Noncancer Pain. Clin. J. Pain 2008, 24, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Maheshwari, A.V.; Blum, Y.C.; Shekhar, L.; Ranawat, A.S.; Ranawat, C.S. Multimodal Pain Management after Total Hip and Knee Arthroplasty at the Ranawat Orthopaedic Center. Clin. Orthop. Relat. Res. 2009, 467, 1418–1423. [Google Scholar] [CrossRef] [Green Version]
- Phillips, W.J.; Currier, B.L. Analgesic Pharmacology: I. Neurophysiology. J. Am. Acad. Orthop. Surg. 2004, 12, 213–220. [Google Scholar] [CrossRef]
- Skinner, H.B.; Shintani, E.Y. Results of a Multimodal Analgesic Trial Involving Patients with Total Hip or Total Knee Arthroplasty. Am. J. Orthop. 2004, 33, 85–92, discussion 92. [Google Scholar]
- Dafny, N. Pain Principles (Section 2, Chapter 6) Neuroscience Online: An Electronic Textbook for the NNeurosciences|Department of Neurobiology and Anatomy-The University of Texas Medical School at Houston. Available online: https://nba.uth.tmc.edu/neuroscience/m/s2/chapter06.html (accessed on 21 October 2022).
- Levine, J.D.; Reichling, D.B. Peripheral mechanisms of inflammatory pain. In Textbook of Pain, 4th ed.; Wall, P.D., Melzack, R., Eds.; Churchill Livingstone: Edinburgh, Scotland, 1999; pp. 59–84. [Google Scholar]
- Ghori, M.K.; Zhang, Y.-F.; Sinatra, R.S. Pathophysiology of Acute Pain. Available online: https://www.cambridge.org/core/books/abs/acute-pain-management/pathophysiology-of-acute-pain/1AB5E239B3A33CE9E9F78A28E2756EA6 (accessed on 25 October 2022).
- Graefe, S.B.; Mohiuddin, S.S. Biochemistry, Substance P. Available online: https://pubmed.ncbi.nlm.nih.gov/32119470/ (accessed on 28 April 2022).
- Kehlet, H.; Dahl, J.B. The Value of Multimodal Or Balanced Analgesia In Postoperative Pain Treatment. Anesth. Analg. 1993, 77, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.M.; Zahn, P.K. From Preemptive to Preventive Analgesia. Curr. Opin. Anaesthesiol. 2006, 19, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Xiao, L. Postoperative Pain Management in Total Knee Arthroplasty. Orthop. Surg. 2019, 11, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crile, G. The Kinetic Theory of Shock and Its Prevention through Anoci-Association (Shockless Operation). Lancet 1913, 182, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J. Evidence for a Central Component of Post-Injury Pain Hypersensitivity. Nature 1983, 306, 686–688. [Google Scholar] [CrossRef]
- Joshi, G.P.; Machi, A. Surgical Site Infiltration: A Neuroanatomical Approach. Best Pr. Res. Clin. Anaesthesiol. 2019, 33, 317–324. [Google Scholar] [CrossRef]
- Moraca, R.J.; Sheldon, D.G.; Thirlby, R.C. The Role of Epidural Anesthesia and Analgesia in Surgical Practice. Ann. Surg. 2003, 238, 663–673. [Google Scholar] [CrossRef]
- Krishna Prasad, G.V.; Khanna, S.; Jaishree, S.V. Review of Adjuvants to Local Anesthetics in Peripheral Nerve Blocks: Current and Future Trends. Saudi J. Anaesth. 2020, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D. Palliative Care Clinical PharmacistMedStar Washington Hospital CenterWashington, Pearls and Pitfalls of Patient-Controlled Analgesia. Available online: https://www.uspharmacist.com/article/pearls-and-pitfalls-of-patientcontrolled-analgesia (accessed on 24 April 2022).
- Portenoy, R.K.; Mehta, Z.; Ahmed, E. Cancer Pain Management with Opioids: Optimizing Analgesia. Available online: https://www.uptodate.com/contents/cancer-pain-management-with-opioids-optimizing-analgesia?search=route+of+administration+pain+control&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 27 April 2022).
- Mariano, E.R. Management of Acute Perioperative Pain. Available online: https://www.uptodate.com/contents/management-of-acute-perioperative-pain (accessed on 25 October 2021).
- Tan, H.S.; Habib, A.S. Oliceridine: A Novel Drug for the Management of Moderate to Severe Acute Pain–A Review of Current Evidence. J. Pain Res. 2021, 14, 969–979. [Google Scholar] [CrossRef]
- Wong, I.; St John-Green, C.; Walker, S.M. Opioid-Sparing Effects of Perioperative Paracetamol and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Children. Pediatr. Anesth. 2013, 23, 475–495. [Google Scholar] [CrossRef] [Green Version]
- Cobby, T.F.; Crighton, I.M.; Kyriakides, K.; Hobbs, G.J. Rectal Paracetamol Has a Significant Morphine-Sparing Effect after Hysterectomy. Br. J. Anaesth. 1999, 83, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Zukowski, M.; Kotfis, K. Safety of Metamizole and Paracetamol for Acute Pain Treatment. Anestezjol. Intensywna Ter. 2009, 41, 170–175. [Google Scholar]
- Maund, E.; McDaid, C.; Rice, S.; Wright, K.; Jenkins, B.; Woolacott, N. Paracetamol and Selective and Non-Selective Non-Steroidal Anti-Inflammatory Drugs for the Reduction in Morphine-Related Side-Effects after Major Surgery: A Systematic Review. Br. J. Anaesth. 2011, 106, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosbøl, E.L.; Folke, F.; Jacobsen, S.; Rasmussen, J.N.; Sørensen, R.; Schramm, T.K.; Andersen, S.S.; Rasmussen, S.; Poulsen, H.E.; Køber, L.; et al. Cause-Specific Cardiovascular Risk Associated with Nonsteroidal Antiinflammatory Drugs among Healthy Individuals. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Remérand, F.; Le Tendre, C.; Baud, A.; Couvret, C.; Pourrat, X.; Favard, L.; Laffon, M.; Fusciardi, J. The Early and Delayed Analgesic Effects of Ketamine after Total Hip Arthroplasty: A Prospective, Randomized, Controlled, Double-Blind Study. Anesth. Analg. 2009, 109, 1963–1971. [Google Scholar] [CrossRef]
- Maher, D.P.; Chen, L.; Mao, J. Intravenous Ketamine Infusions for Neuropathic Pain Management. Anesth. Analg. 2017, 124, 661–674. [Google Scholar] [CrossRef]
- Zhang, J.; Ho, K.-Y.; Wang, Y. Efficacy of Pregabalin in Acute Postoperative Pain: A Meta-Analysis. Br. J. Anaesth. 2011, 106, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Palliative Drugs. Pregabalin-palliativedrugs.com. Available online: https://www.palliativedrugs.com/download/pregabalin140105aw.pdf (accessed on 10 January 2022).
- Chia, Y.Y.; Chan, M.H.; Ko, N.H.; Liu, K. Role of β-Blockade in Anaesthesia and Postoperative Pain Management after Hysterectomy. Br. J. Anaesth. 2004, 93, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Blaudszun, G.; Lysakowski, C.; Elia, N.; Tramer, M.R. Effect of Perioperative Systemic Alpha-2 Agonists on Postoperative Morphine Consumption and Pain Intensity: Systematic Review and Meta-Analysis of Randomized Controlled Trials; Centre for Reviews and Dissemination: Heslington, UK, 2012. [Google Scholar]
- Royster, R.L. Perioperative beta-blockade II: Practical clinical application. Anesthesia Patient Safety Foundation. Available online: https://www.apsf.org/article/perioperative-beta-blockade-ii-practical-clinical-application/ (accessed on 1 October 2021).
- Young, A.; Buvanendran, A. Recent Advances in Multimodal Analgesia. Anesthesiol. Clin. 2012, 30, 91–100. [Google Scholar] [CrossRef]
- Hartrick, C.T.; Pestano, C.; Carlson, N.; Hartrick, S. Capsaicin Instillation for Postoperative Pain Following Total Knee Arthroplasty. Clin. Drug Investig. 2011, 31, 877–882. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical Capsaicin for Pain Management: Therapeutic Potential and Mechanisms of Action of the New High-Concentration Capsaicin 8% Patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, C.L. Overview of peripheral nerve blocks. UpToDate. Available online: https://www.uptodate.com/contents/overview-of-peripheral-nerve-blocks?sectionName=Local+anesthetics&search=local+anesthesia+regional&topicRef=14929&anchor=H14&source=see_link#H14 (accessed on 20 October 2020).
- Pujari, V.; Siddaiah, J.; Madalu, A.; Bevinaguddaiah, Y.; Parate, L. A Comparative Study on the Effect of Addition of Intrathecal Buprenorphine to 2-Chloroprocaine Spinal Anesthesia in Short Duration Surgeries. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 533. [Google Scholar] [CrossRef] [PubMed]
- Lennard, T. Corticosteroid Use in Pain Management. Pract. Pain Manag. 2000. Available online: https://www.practicalpainmanagement.com/corticosteroid-use-pain-management (accessed on 24 October 2022).
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-Dependent Block by Mg2+ of NMDA Responses in Spinal Cord Neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, K.S.; Hägi-Pedersen, D.; Lunn, T.H.; Laursen, C.C.; Holmqvist, M.; Vinstrup, L.Ø.; Ammitzboell, M.; Jakobsen, K.; Jensen, M.S.; Pallesen, M.J.; et al. Effect of Dexamethasone as an Analgesic Adjuvant to Multimodal Pain Treatment after Total Knee Arthroplasty: Randomised Clinical Trial. BMJ 2022, 376, e067325. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Dose | Analgesic Duration | Metabolism | Comments |
|---|---|---|---|---|
| Parenteral Opioids | ||||
| Morphine | 2–4 mg IV | 3–4 h |
|
|
| Hydromorphone | 0.5–1 mg IV/SC | 2–4 h |
|
|
| Fentanyl | 1–2 mcg/kg IV | 30 min–1 h |
|
|
| Meperidine | 50–150 mg IV | 2–3 h |
|
|
| Oral immediate-release opioids | ||||
| Codeine | 15–60 mg PO | 4–6 h | Liver—CYP2D6 |
|
| Hydrocodone | 2.5–10 mg PO | 4–6 h | Liver—CYP2D6 and CYP3A4 |
|
| Oxycodone | 5–10 mg PO | 3–6 h | Liver—CYP2D6 and CYP3A4 |
|
| Tramadol | 50–100 mg PO | 3–6 h | Liver—CYP2D6 and CYP3A4 |
|
| Oxymorphone | 5–10 mg PO | 3–6 h | None known | |
| Drug Class | Dose | Analgesic Duration | Metabolism | Comments |
|---|---|---|---|---|
| Anticonvulsants | ||||
| Gabapentin | 300 mg PO | 5–7 h |
|
|
| Pregabalin | 100 mg PO Q8Hr | 12 h |
| |
| Drug Class | Dose | Analgesic Duration | Metabolism | Comments |
|---|---|---|---|---|
| Transient Receptor Potential Vanilloid Receptor (TRPV1) Agonist | ||||
| Capsaicin | 0.025% patch |
| Liver—CYP2E1 |
|
| Drug Class | Dose | Analgesia Duration | Metabolism | Comments |
|---|---|---|---|---|
| Local Anesthetics | ||||
| Lidocaine | 5 mg/kg plain 7 mg/kg with epinephrine | 30–90 min |
|
|
| Mepivacaine | 7 mg/kg plain 8 mg/kg with epinephrine | 1–2 h |
|
|
| Chloroprocaine | 14 mg/kg max 1000 mg with 1:100,000 epinephrine 11 mg/kg max 800 mg plain for peripheral nerve blocks | 40–60 min |
|
|
| 50 mg for intrathecal | Spinal 1–1.5 h | |||
| Bupivacaine | 2.5 mg/kg plain |
|
| |
| 3 mg/kg with epinephrine | 6–8 h | |||
| Spinal-1–2 mL of 0.75% bupivacaine | 2–4 h | |||
| Ropivacaine | 3 mg/kg | 1.5–8 h |
|
|
| Drug Class | Dose | Analgesia Duration | Metabolism | Comments |
|---|---|---|---|---|
| Glucocorticoids | ||||
| Methylprednisolone | 40–120 mg | ~2 weeks |
|
|
| Triamcinolone | 5–40 mg | ~2 weeks |
| |
| Dexamethasone | 8–24 mg (IV) | ~48 h |
| |
| Magnesium | ||||
| Magnesium | 30–50 mg/kg (IV) | Enhances analgesic effect of other medications |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chunduri, A.; Aggarwal, A.K. Multimodal Pain Management in Orthopedic Surgery. J. Clin. Med. 2022, 11, 6386. https://doi.org/10.3390/jcm11216386
Chunduri A, Aggarwal AK. Multimodal Pain Management in Orthopedic Surgery. Journal of Clinical Medicine. 2022; 11(21):6386. https://doi.org/10.3390/jcm11216386
Chicago/Turabian StyleChunduri, Aparna, and Amit Kumar Aggarwal. 2022. "Multimodal Pain Management in Orthopedic Surgery" Journal of Clinical Medicine 11, no. 21: 6386. https://doi.org/10.3390/jcm11216386





