Validation Criteria for PETCO2 Kinetics during the Hyperventilation Provocation Test in the Diagnosis of Idiopathic Hyperventilation Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Hyperventilation Provocation Test Procedure
2.2. Training Cohort
2.3. Validation Cohort
2.4. Statistical Analyses
2.4.1. Training Cohort
2.4.2. Validation Cohort
3. Results
3.1. Hyperventilation Provocation Test in the Training Cohort
3.2. Results of the Hyperventilation Provocation Test in the Validation Cohort and Comparison with the Training Cohort
4. Discussion
4.1. Adaptation Phase
4.2. Hyperventilation Phase
4.3. Recovery Phase
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motiejunaite, J.; Balagny, P.; Arnoult, F.; Mangin, L.; Bancal, C.; D’Ortho, M.-P.; Frija-Masson, J. Hyperventilation: A Possible Explanation for Long-Lasting Exercise Intolerance in Mild COVID-19 Survivors? Front. Physiol. 2021, 11, 614590. [Google Scholar] [CrossRef] [PubMed]
- Taverne, J.; Salvator, H.; Leboulch, C.; Barizien, N.; Ballester, M.; Imhaus, E.; Chabi-Charvillat, M.-L.; Boulin, A.; Goyard, C.; Chabrol, A.; et al. High incidence of hyperventilation syndrome after COVID-19. J. Thorac. Dis. 2021, 13, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Bouteleux, B.; Henrot, P.; Ernst, R.; Grassion, L.; Raherison-Semjen, C.; Beaufils, F.; Zysman, M.; Delorme, M. Respiratory rehabilitation for COVID-19 related persistent dyspnoea: A one-year experience. Respir. Med. 2021, 189, 106648. [Google Scholar] [CrossRef] [PubMed]
- Boulding, R.; Stacey, R.; Niven, R.; Fowler, S.J. Dysfunctional breathing: A review of the literature and proposal for classification. Eur. Respir. Rev. 2016, 25, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.A.; Howell, J.B. Definition of the hyperventilation syndrome. Bull. Eur. Physiopathol. Respir. 1986, 22, 201–205. [Google Scholar] [PubMed]
- Kerr, W.J.; Dalton, J.W.; Gliebe, P.A. Some physical phenomena associated with anxiety states and their relationship to hyperventilation. Ann. Intern. Med. 1937, 11, 961–992. [Google Scholar] [CrossRef]
- Christie, R.V. Some types of respiration in the neuroses. QJM Int. J. Med. 1935, 4, 427–428. [Google Scholar] [CrossRef]
- Collip, J.B.; Backus, P.L. The effect of prolonged hyperpnoea on the carbon dioxide combining power of the plasma, the carbon dioxide tension of alveolar air and the excretion of acid and basic phosphate and ammonia by the kidney. Am. J. Physiol. Content 1920, 51, 568–579. [Google Scholar] [CrossRef]
- O’Donnovan, D. The hyperventilation syndrome. Ir. J. Med. Sci. 1943, 213, 519–533. [Google Scholar]
- O’Donovan, D.K. The diagnosis of chronic latent tetany in adults. Ir. J. Med. Sci. 1945, 20, 146–153. [Google Scholar] [CrossRef]
- McKell, T.E.; Sullivan, A.J. The hyperventilation syndrome in gastroenterology. Gastroenterology 1947, 9, 6–16. [Google Scholar]
- Weimann, G.; Korschinsky, H. Conducting and evaluation of a study on hyperventilation. Med. Klin. 1970, 65, 56–62. [Google Scholar]
- Hardonk, H.; Beumer, H. Hyperventilation syndrome. In Handbook of Clinical Neurology; Vinken, P.J., Bruyn, G.W., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1979; Volume 38, pp. 309–360. [Google Scholar]
- Vansteenkiste, J.; Rochette, F.; Demedts, M. Evaluation of the Clinical Usefulness of Capnography Curves During a Hyperventilation Provocation Test in the Diagnosis of Hyperventilation Syndrome. Acta Clin. Belg. 1991, 46, 142–149. [Google Scholar] [CrossRef]
- Stam, J. Hyperventilatie en Hyperventilatiesyndroom. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 1974. [Google Scholar]
- Hornsveld, H.; Garssen, B.; Dop, M.F.; van Spiegel, P.; de Haes, J. Double-blind placebo-controlled study of the hyperventilation provocation test and the validity of the hyperventilation syndrome. Lancet 1996, 348, 154–158. [Google Scholar] [CrossRef]
- Van Dixhoorn, J.; Duivenvoorden, H. Efficacy of Nijmegen questionnaire in recognition of the hyperventilation syndrome. J. Psychosom. Res. 1985, 29, 199–206. [Google Scholar] [CrossRef]
- Van Dixhoorn, J.; Folgering, H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Res. 2015, 1, 00001-02015. [Google Scholar] [CrossRef] [Green Version]
- Howell, J.B. The hyperventilation syndrome: A syndrome under threat? Thorax 1997, 52, S30–S34. [Google Scholar] [CrossRef] [Green Version]
- Pauwen, N.; Alexis, G.; Vitalie, F.; Boucharessas, F.; Deboeck, G.; Colot, T.; Sergysels, R.; Ninane, V. The Loophole of Hyperventilation Curves. In: ERS Congress 2019. Available online: https://erj.ersjournals.com/content/54/suppl_63/PA604 (accessed on 15 August 2022).
- Nishioka, Y.; Hayashi, F.; Suenaga, N.; Kurosu, I.; Tachibana, H.; Sugita, M. Slow recovery of the end-tidal PCO2 after hyperventilation provocation test in hyperventilation syndrome. Nihon Kyobu Shikkan Gakkai Zasshi 1988, 26, 378–385. (In Japanese) [Google Scholar]
- Han, J.; Stegen, K.; Simkens, K.; Cauberghs, M.; Schepers, R.; Burgh, O.V.D.; Clément, J.; Van De Woestijne, K. Unsteadiness of breathing in patients with hypervenmtilation syndrome and anxiety disorders. Eur. Respir. J. 1997, 10, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Grassi, M.; Caldirola, D.; Di Chiaro, N.V.; Riva, A.; Daccò, S.; Pompili, M.; Perna, G. Are Respiratory Abnormalities Specific for Panic Disorder? A Meta-Analysis. Neuropsychobiology 2014, 70, 52–60. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Rochette, F.; Demedts, M. Diagnostic tests of hyperventilation syndrome. Eur. Respir. J. 1991, 4, 393–399. [Google Scholar] [PubMed]
- Rodenstein, D.O.; Mercenier, C.; Stănescu, D.C. Influence of the respiratory route on the resting breathing pattern in humans. Am. Rev. Respir. Dis. 1985, 131, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Han, J.N.; Stegen, K.; Cauberghs, M.; Van De Woestijne, K. Influence of awareness of the recording of breathing on respiratory pattern in healthy humans. Eur. Respir. J. 1997, 10, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornsveld, H.; Garssen, B.; van Spiegel, P. Voluntary hyperventilation: The influence of duration and depth on the development of symptoms. Biol. Psychol. 1995, 40, 299–312. [Google Scholar] [CrossRef]
- Grossman, P.; de Swart, J. Diagnosis of hyperventilation syndrome on the basis of reported complaints. J. Psychosom. Res. 1984, 28, 97–104. [Google Scholar] [CrossRef]
- Freeman, L.J.; Conway, A.; Nixon, P.G.F. Physiological Responses to Psychological Challenge under Hypnosis in Patients Considered to Have the Hyperventilation Syndrome: Implications for Diagnosis and Therapy. J. R. Soc. Med. 1986, 79, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Gardner, W.; Meah, M.; Bass, C. Controlled study of respiratory responses during prolonged measurement in patients with chronic hyperventilation. Lancet 1986, 328, 826–830. [Google Scholar] [CrossRef]
- Folgering, H. Diagnosis criteria for the hyperventilation syndrome. Symp. Respir. Psychophysiol. 1989, 29, 72. [Google Scholar]
- Folgering, H.; Durlinger, M. Time course of posthyperventilation breathing in humans depends on alveolar CO2 tension. J. Appl. Physiol. 1983, 54, 809–813. [Google Scholar] [CrossRef]
- Saltzman, H.A.; Heyman, A.; Sieker, H.O. Correlation of Clinical and Physiologic Manifestations of Sustained Hyperventilation. N. Engl. J. Med. 1963, 268, 1431–1436. [Google Scholar] [CrossRef]



| Training Cohort Baseline Characteristics | HVS+ (n = 37) | HVS- (n = 37) | p-Value |
|---|---|---|---|
| Sex F/M n (%) | 26 (35%)/11 (15%) | 26 (35%)/11 (15%) | 1.0 † |
| Age–Yrs | 43.8 ± 13.8 | 44.1 ± 13.8 | 0.913 ¥ |
| Weight–kg | 68.6 ± 13.0 | 71.8 ± 14.1 | 0.316 ¥ |
| Height–cm | 165.5 ± 7.9 | 167.4 ± 7.7 | 0.293 ¥ |
| BMI–kg/m2 | 25.0 ± 4.4 | 25.5 ± 4.6 | 0.615 ¥ |
| Nijmegen questionnaire–score | 34.9 ± 7.6 | 13.9 ± 6.8 | <0.0001 ® |
| Validation cohort Baseline characteristics | HVS+ (n = 114) | HVS- (n = 38) | p-Value |
| Sex F/M n (%) | 72 (65.5%)/13 (12%) | 17 (15.5%)/7 (%) | 0.082 † |
| Age–Yrs | 41.3 ± 16.4 | 40.9 ± 21.5 | 0.918 ® |
| Weight–kg | 67.9 ± 16.7 | 71.3 ± 18.9 | 0.421 ® |
| Height–cm | 164.0 ± 8.1 | 167.9 ± 10.1 | 0.036 ® |
| BMI–kg/m2 | 25.2 ± 5.7 | 25.1 ± 5.6 | 1.000 ® |
| Nijmegen questionnaire–score | 35.8 ± 8.4 | 15.6 ± 5.2 | <0.0001 ® |
| Hyperventilation Provocation Test Training Cohort | HVS+ (n = 37) | HVS- (n = 37) | p-Value |
|---|---|---|---|
| Adaptation phase (3 min) | |||
| VE–L/min | 13.0 ± 5.9 | 9.8 ± 2.6 | 0.013 ® |
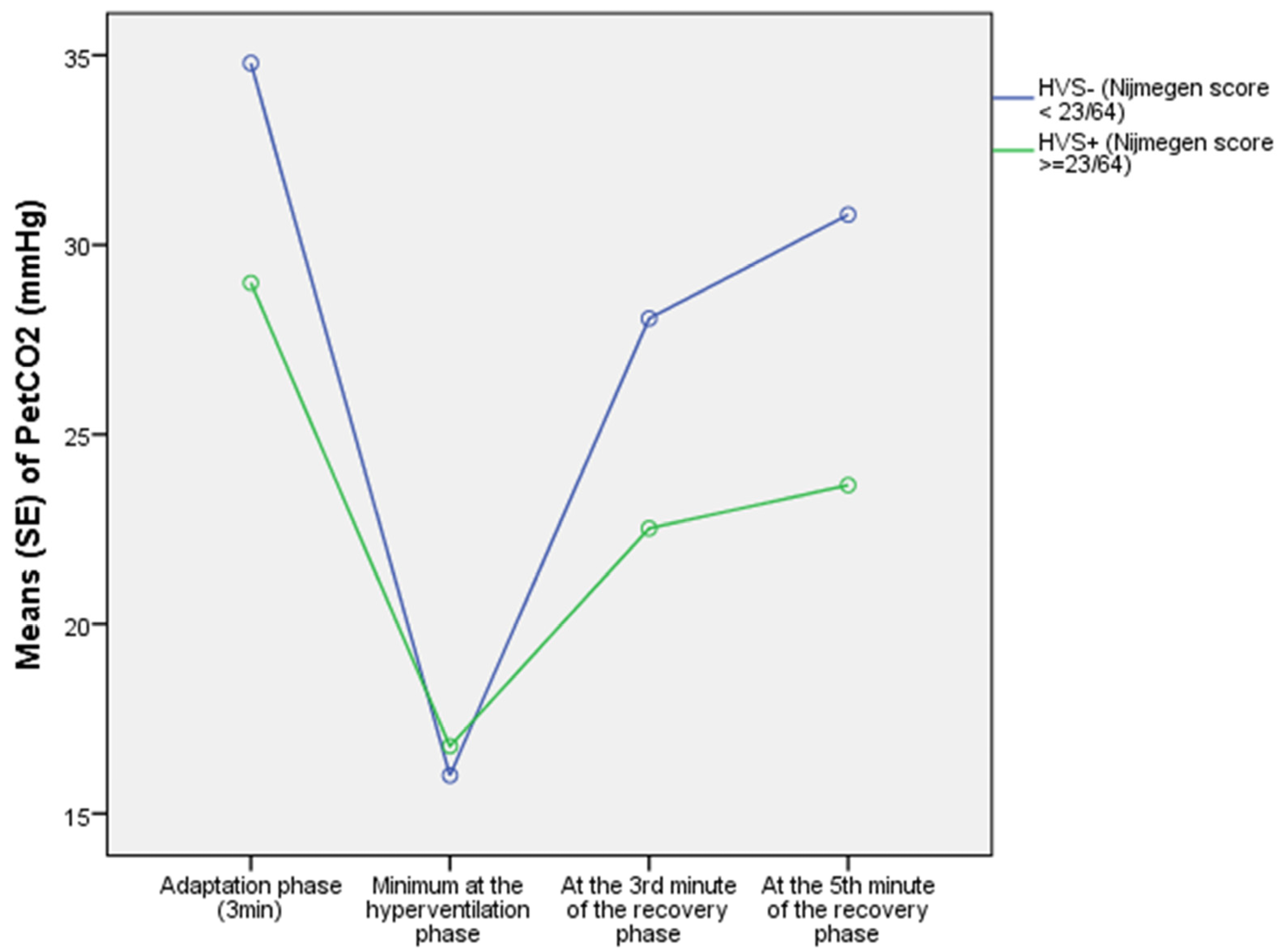
| PETCO2(adapt)–mmHg | 31.3 ± 6.0 | 36.0 ± 4.3 | 0.001 ® |
| Slope: β–mmHg/min | −0.86 ± 1.68 | +0.04 ± 0.67 | <0.0001 ® |
| PETCO2(t3)–mmHg | 29.0 ± 6.9 | 34.8 ± 4.6 | <0.0001 ® |
| Voluntary hyperventilation phase (3 min) | |||
| VE (VH)–L/min | 46.8 ± 17.7 | 61.6 ± 27.2 | 0.006 ® |
| PETCO2 (TAU A)–mmHg | 30.8 ± 6.6 | 36.3 ± 4.3 | <0.0001 ® |
| ΔPETCO2 (TAU a)–mmHg | −13.7 ± 6.3 | −20.6 ± 4.7 | <0.0001 ¥ |
| PETCO2(VH-min)–mmHg | 15.3 ± 3.4 | 14.8 ± 3.0 | 0.476 ¥ |
| AUC [95%CI] of ΔPETCO2 (TAU–3–6 min) | 0.812 [0.716; 0.907] | ||
| Sen/Sp of ΔPETCO2 (TAU–3–6 min)– (cut-off) | 0.76/0.73–(−17.6 mmHg) | ||
| Recovery phase (3 min) | |||
| VE(6–9 min)–L/min | 19.8 ± 8.6 | 16.4 ± 7.0 | 0.039® |
| PETCO2 (A’)–mmHg | 15.5 ± 4.0 | 14.6 ± 3.2 | 0.278 ¥ |
| ΔPETCO2 (TAU a’–6–9 min)–mmHg | +7.3 ± 4.2 | +14.9 ± 6.7 | <0.0001 ® |
| PETCO2(t9)–mmHg | 22.5 ± 5.1 | 28.1 ± 4.8 | <0.0001 ¥ |
| AUC [95%CI] of ΔPETCO2 (TAU a’–6–9 min) | 0.866 [0.783; 0.948] | ||
| Sen/Sp of ΔPETCO2 (TAU a’–6–9 min)–(cut-off) | 0.81/0.81–(+10.56 mmHg) | ||
| Recovery phase (5 min) | |||
| VE(6–11 min)–L/min | 18.3 ± 8.3 | 13.5 ± 5.2 | 0.002 ® |
| PETCO2 (A’)–mmHg | 15.5 ± 4.0 | 14.6 ± 3.2 | 0.278 ® |
| ΔPETCO2 (TAU a’–6–11 min)–mmHg | +8.3 ± 4.2 | +17.8 ± 6.2 | <0.0001 ¥ |
| PETCO2(t11)–mmHg | 23.7 ± 5.5 | 30.8 ± 5.1 | <0.0001 ¥ |
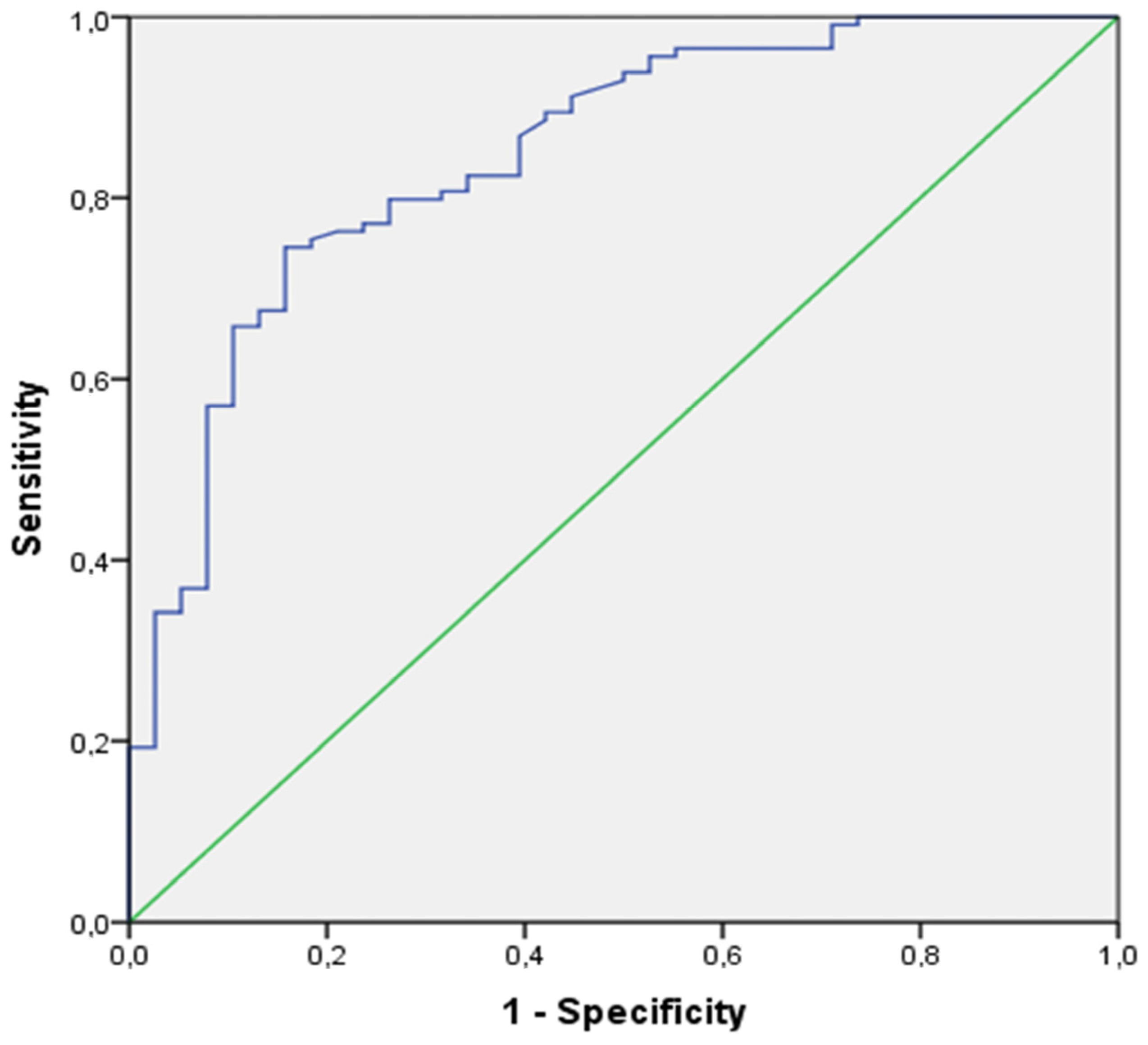
| AUC [95%CI] of ΔPETCO2 (TAU a’–6–11 min) | 0.907 [0.835; 0.979] | ||
| Sen/Sp of ΔPETCO2 (TAU a’–6–11 min)–(cut-off) | 0.92/0.84–(+12.79 mmHg) | ||
| Hyperventilation Provocation Test Validation Cohort | HVS+ (n = 114) | HVS- (n = 38) | p-Value |
|---|---|---|---|
| Adaptation phase (3 min) | |||
| PETCO2(t3)–mmHg | 30.4 ± 5.8 | 33.8 ± 4.9 | 0.002 ¥ |
| Voluntary hyperventilation phase (3 min) | |||
| PETCO2 (TAU A)–mmHg | 30.7 ± 5.7 | 34.1 ± 4.4 | 0.001 ¥ |
| ΔPETCO2 (TAU a)–mmHg | −16.1 ± 5.3 | −19.1 ± 4.5 | 0.002 ¥ |
| PETCO2(VH-min)–mmHg | 13.6 ± 2.3 | 13.9 ± 2.2 | 0.598 ® |
| AUC [95%CI] of ΔPETCO2 (TAU–3–6 min) | 0.674 [0.579; 0.769] | ||
| Sen/Sp of ΔPETCO2 (TAU–3–6 min)–(cut-off) | 0.66/0.61–(−17.7 mmHg) | ||
| Recovery phase (3 min) | |||
| PETCO2 (TAU A’)–mmHg | 14.8 ± 3.0 | 15.3 ± 3.0 | 0.175 ® |
| ΔPETCO2 (TAU a’–6–9 min)–mmHg | +7.7 ± 3.7 | +11.5 ± 5.7 | <0.0001 ® |
| PETCO2(t9)–mmHg | 22.2 ± 4.4 | 27.1 ± 5.2 | <0.0001 ¥ |
| AUC [95%CI] of ΔPETCO2 (TAU a’–6–9 min) | 0.795 [0.715; 0.876] | ||
| Sen/Sp of ΔPETCO2 (TAU a’–6–9 min)–(cut-off) | 0.80/0.63–(+10.61 mmHg) | ||
| Recovery phase (5 min) | |||
| PETCO2 (TAU A’)–mmHg | 14.8 ± 3.0 | 15.3 ± 3.0 | 0.175 ® |
| ΔPETCO2 (TAU a’–6–11 min)–mmHg | +9.2 ± 4.4 | +15.7 ± 4.5 | <0.0001 ¥ |
| PETCO2(t11)–mmHg | 23.8 ± 5.2 | 31.0 ± 5.1 | <0.0001 ¥ |
| AUC [95%CI] of ΔPETCO2 (TAU a’–6–11 min) | 0.847 [0.776; 0.918] | ||
| Sen/Sp of ΔPETCO2 (TAU a’–6–11 min)–(cut-off) | 0.80/0.74–(+12.79 mmHg) | ||
| Sensitivity and Specificity of the HP Test in the Two Cohorts | Training Cohort (n = 74) | Validation Cohort (n = 152) | p-Value |
|---|---|---|---|
| Recovery phase (3 min)–cut-off <10.56 mmHg | |||
| HVS+ (n = 37) | HVS+ (n = 114) | ||
| TP-True positives–number (%) | 30 (81%) | 91 (80%) | 1.000 ® |
| FN-False negatives–number (%) | 7 (19%) | 23 (20%) | |
| HVS- (n = 37) | HVS- (n = 38) | ||
| TN-True negatives–number (%) | 30 (81%) | 25 (66%) | 0.192 ® |
| FP-False positives–number (%) | 7 (19%) | 13 (34%) | |
| Recovery phase (5 min)–cut-off <12.79 mmHg | |||
| HVS+ (n = 37) | HVS+ (n = 114) | ||
| TP-True positives–number (%) | 34 (92%) | 89 (78%) | 0.087 ® |
| FN-False negatives–number (%) | 3 (8%) | 25 (22%) | |
| HVS- (n = 37) | HVS- (n = 38) | ||
| TN-True negatives–number (%) | 31 (84%) | 28 (74%) | 0.399 ® |
| FP-False positives–number (%) | 6 (16%) | 10 (26%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauwen, N.Y.; Faoro, V.; Boucharessas, F.; Colot, T.; Guillaume, A.; Sergysels, R.; Ninane, V. Validation Criteria for PETCO2 Kinetics during the Hyperventilation Provocation Test in the Diagnosis of Idiopathic Hyperventilation Syndrome. J. Clin. Med. 2022, 11, 6482. https://doi.org/10.3390/jcm11216482
Pauwen NY, Faoro V, Boucharessas F, Colot T, Guillaume A, Sergysels R, Ninane V. Validation Criteria for PETCO2 Kinetics during the Hyperventilation Provocation Test in the Diagnosis of Idiopathic Hyperventilation Syndrome. Journal of Clinical Medicine. 2022; 11(21):6482. https://doi.org/10.3390/jcm11216482
Chicago/Turabian StylePauwen, Nathalie Yaël, Vitalie Faoro, Françoise Boucharessas, Thierry Colot, Alexis Guillaume, Roger Sergysels, and Vincent Ninane. 2022. "Validation Criteria for PETCO2 Kinetics during the Hyperventilation Provocation Test in the Diagnosis of Idiopathic Hyperventilation Syndrome" Journal of Clinical Medicine 11, no. 21: 6482. https://doi.org/10.3390/jcm11216482
APA StylePauwen, N. Y., Faoro, V., Boucharessas, F., Colot, T., Guillaume, A., Sergysels, R., & Ninane, V. (2022). Validation Criteria for PETCO2 Kinetics during the Hyperventilation Provocation Test in the Diagnosis of Idiopathic Hyperventilation Syndrome. Journal of Clinical Medicine, 11(21), 6482. https://doi.org/10.3390/jcm11216482






