Quantification of Iris Atrophy by Swept-Source Optical Coherence Tomography in Posner–Schlossman Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
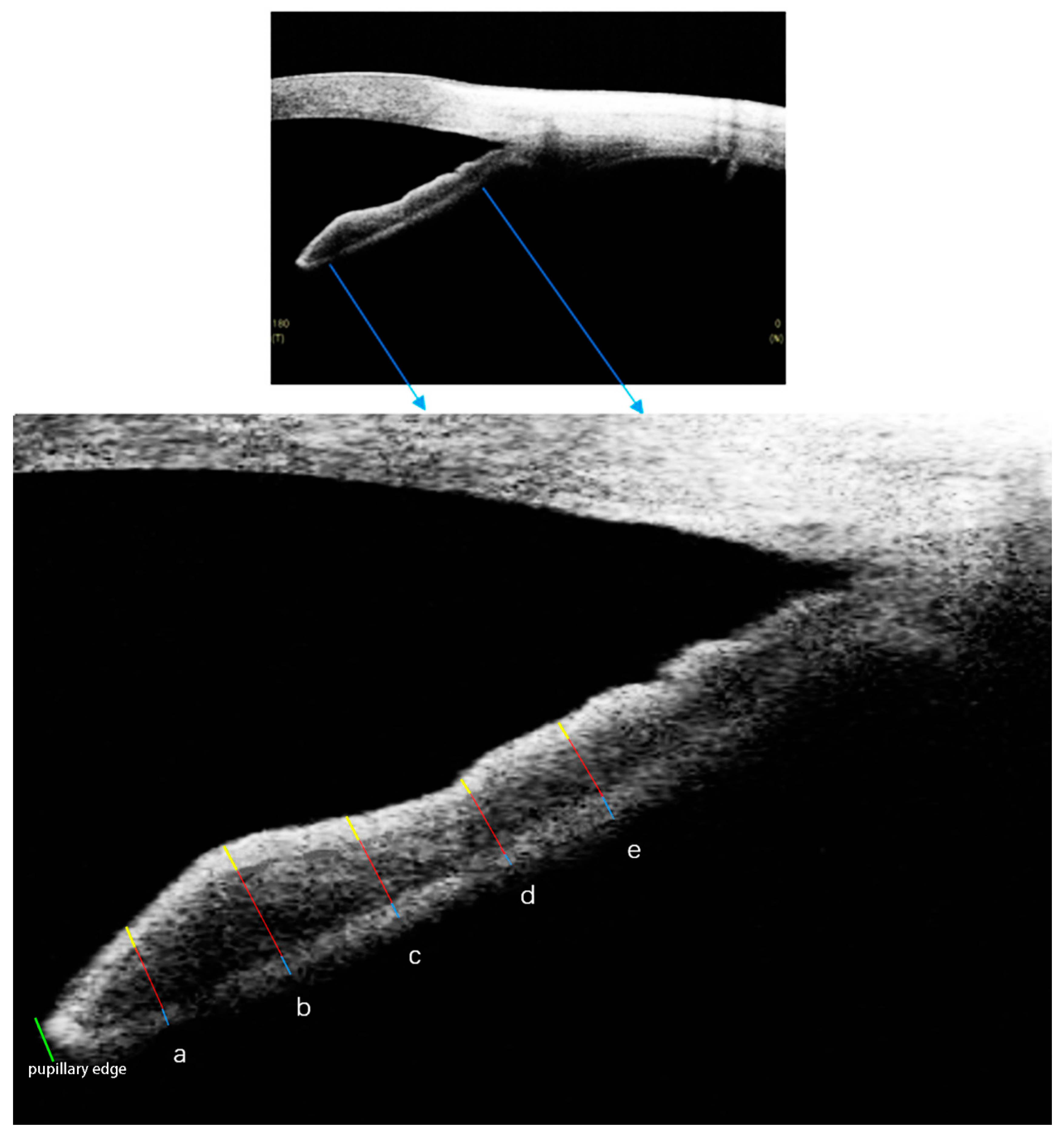
2.2. SS-OCT Imaging Acquisition and Processing
2.3. Statistical Analysis
3. Results
3.1. Univariate Linear Regression of Associations of Iris Thickness and Corneal Endothelium Density in PSS-Affected Eyes
3.2. Univariate Linear Regression of Associations of Iris Thickness and C/D Ratio in PSS-Affected Eyes
3.3. Univariate Linear Regression of Associations of Iris Thickness and RNFL Thickness in PSS-Affected Eyes
3.4. Univariate Linear Regression of Associations of Iris Thickness and IOP in PSS-Affected and Fellow Eyes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posner, A.; Schlossman, A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch. Ophthalmol. 1948, 39, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Zhang, S.D.; Dai, M.L.; Yang, J.Y.; Xie, Y.Q.; Hu, C.; Mao, G.Y.; Lu, F.; Liang, Y.B. Posner-Schlossman syndrome in Wenzhou, China: A retrospective review study. Br. J. Ophthalmol. 2017, 101, 1638–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, N.; Honjo, M.; Kaburaki, T.; Aihara, M. Effects of ROCK Inhibitors on Apoptosis of Corneal Endothelial Cells in CMV-Positive Posner–Schlossman Syndrome Patients. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5. [Google Scholar] [CrossRef]
- Pohlmann, D.; Schlickeiser, S.; Metzner, S.; Lenglinger, M.; Winterhalter, S.; Pleyer, U. Different composition of intraocular immune mediators in Posner-Schlossman-Syndrome and Fuchs’ Uveitis. PLoS ONE 2018, 13, e0199301. [Google Scholar] [CrossRef]
- Hirose, S.; Ohno, S.; Matsuda, H. HLA-Bw54 and Glaucomatocyclitic Crisis. Arch. Ophthalmol. 1985, 103, 1837–1839. [Google Scholar] [CrossRef]
- Li, J.; Ang, M.; Cheung, C.M.G.; Vania, M.; Chan, A.S.Y.; Waduthantri, S.; Yang, H.; Chee, S.P. Aqueous Cytokine Changes Associated with Posner-Schlossman Syndrome with and without Human Cytomegalovirus. PLoS ONE 2012, 7, e44453. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-C.; Hu, F.-R.; Wang, T.-H.; Huang, J.-Y.; Yeh, P.-T.; Lin, C.-P.; Wang, I.-J. Clinical Outcomes in Cytomegalovirus-Positive Posner-Schlossman Syndrome Patients Treated With Topical Ganciclovir Therapy. Am. J. Ophthalmol. 2014, 158, 1024–1031. [Google Scholar] [CrossRef]
- Bloch-Michel, E.; Dussaix, E.; Cerqueti, P.; Patarin, D. Possible role of cytomegalovirus infection in the etiology of the Posner-Schlossmann syndrome. Int. Ophthalmol. 1987, 11, 95–96. [Google Scholar] [CrossRef]
- Chee, S.-P.; Jap, A. Presumed Fuchs Heterochromic Iridocyclitis and Posner-Schlossman Syndrome: Comparison of Cytomegalovirus-Positive and Negative Eyes. Am. J. Ophthalmol. 2008, 146, 883–889.e1. [Google Scholar] [CrossRef]
- Shen, S.-C.; Ho, W.-J.; Wu, S.-C.; Yu, K.-H.; Lin, H.-C.; Lin, Y.-S.; Tsay, P.-K.; Chu, P.-H. Peripheral Vascular Endothelial Dysfunction in Glaucomatocyclitic Crisis: A Preliminary Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 272–276. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, T.; Chen, W.; Fan, B.J.; He, L.; Yang, B.; Deng, Z. Human Leukocyte Antigens-B and -C Loci Associated with Posner-Schlossman Syndrome in a Southern Chinese Population. PLoS ONE 2015, 10, e0132179. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.; Schlossman, A. Further observations on the syndrome of glaucomatocyclitic crises. Trans. Am. Acad. Ophthalmol. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1953, 57, 531–536. [Google Scholar]
- De Schryver, I.; Rozenberg, F.; Cassoux, N.; Michelson, S.; Kestelyn, P.; LeHoang, P.; Davis, J.L.; Bodaghi, B. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br. J. Ophthalmol. 2006, 90, 852–855. [Google Scholar] [CrossRef]
- Sobolewska, B.W.; Deuter, C.M.E.; Doycheva, D.G.; Zierhut, M. Long-term oral therapy with valganciclovir in patients with Posner-Schlossman syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jap, A.; Sivakumar, M.; Chee, S.-P. Is Posner Schlossman syndrome benign? Ophthalmology 2001, 108, 913–918. [Google Scholar] [CrossRef]
- Liu, P.-K.; Tseng, H.-Y.; Huang, M.-Y.; Wu, K.-Y. Glaucomatocyclitic Crises May Occur in Patients with Narrow or Closed Angles. J. Ophthalmol. 2017, 2017, 4074912. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kelly, S.R.; Montesano, G.; Bryan, S.R.; Barry, R.; Keane, P.A.; Denniston, A.K.; Crabb, D.P. Evaluating the Impact of Uveitis on Visual Field Progression Using Large-Scale Real-World Data. Am. J. Ophthalmol. 2019, 207, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Lee, J.-Y.; Choi, J.A. Long-term prognosis for glaucoma in patients with Posner–Schlossman syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3757–3767. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhai, R.; Fan, X.; Kong, X. The Analysis of Dynamic Changes and Prognosis of Posner–Schlossman Syndrome with Cytomegalovirus Infection and Antiviral Therapy. J. Ophthalmol. 2021, 2021, 6687929. [Google Scholar] [CrossRef]
- Fan, X.; Li, Z.; Zhai, R.; Sheng, Q.; Kong, X. Clinical characteristics of virus-related uveitic secondary glaucoma: Focus on cytomegalovirus and varicella zoster virus. BMC Ophthalmol. 2022, 22, 130. [Google Scholar] [CrossRef]
- Markomichelakis, N.N.; Canakis, C.; Zafirakis, P.; Marakis, T.; Mallias, I.; Theodossiadis, G. Cytomegalovirus as a cause of anterior uveitis with sectoral iris atrophy. Ophthalmology 2002, 109, 879–882. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhai, R.; Fan, X.; Kong, X. 2% Ganciclovir Eye Drops Control Posner-Schlossman Syndrome Relapses with/without Cytomegalovirus Intraocular Reactivation. Front. Med. 2022, 9, 848820. [Google Scholar] [CrossRef]
- Woo, J.H.; Lim, W.K.; Ho, S.L.; Teoh, S.C. Characteristics of Cytomegalovirus Uveitis in Immunocompetent Patients. Ocul. Immunol. Inflamm. 2015, 23, 378–383. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Yan, X.; Zhang, H. Diagnostic power of scleral spur length in primary open-angle glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1253–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, A.K.; Fischer, J.E.; Vossmerbaeumer, U. Curvature of iris profile in spectral domain optical coherence tomography and dependency to refraction, age and pupil Sizethe MIPH Eye&Health Study. Acta Ophthalmol. 2017, 95, 175–181. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, X.; Chen, W.; Wang, P.; Wang, J. Decreased iris thickness on swept-source optical coherence tomography in patients with primary open-angle glaucoma. Clin. Exp. Ophthalmol. 2021, 49, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, M.; Wang, J.; Zhang, H.; Zhou, X.; Chen, Z. Morphology of the Trabecular Meshwork and Schlemm’s Canal in Posner-Schlossman Syndrome. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1. [Google Scholar] [CrossRef]
- Li, M.; Song, Y.; Zhao, Y.; Yan, X.; Zhang, H. Influence of exercise on the structure of the anterior chamber of the eye. Acta Ophthalmol. 2018, 96, e247–e253. [Google Scholar] [CrossRef]
- Neumann, R.; Barequet, D.; Rosenblatt, A.; Amer, R.; Ben-Arie-Weintrob, Y.; Hareuveni-Blum, T.; Vishnevskia-Dai, V.; Raskin, E.; Blumenfeld, O.; Shulman, S.; et al. Herpetic Anterior Uveitis—Analysis of Presumed and PCR Proven Cases. Ocul. Immunol. Inflamm. 2019, 27, 211–218. [Google Scholar] [CrossRef]
- Bale, J.F.; O'Neil, M.E.; Lyon, B.; Perlman, S. The pathogenesis of murine cytomegalovirus ocular infection. Anterior chamber inoculation. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1575–1581. [Google Scholar]
- Bale, J.F.; O’Neil, M.E.; Folberg, R. Murine cytomegalovirus ocular infection in immunocompetent and cyclophosphamide-treated mice. Potentiation of ocular infection by cyclophosphamide. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1749–1756. [Google Scholar]
- Ang, M.; Sng, C.C.; Chee, S.-P.; Tan, D.T.; Mehta, J.S. Outcomes of Corneal Transplantation for Irreversible Corneal Decompensation Secondary to Corneal Endotheliitis in Asian Eyes. Am. J. Ophthalmol. 2013, 156, 260–266.e2. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Shiraishi, A.; Ohashi, Y.; Kandori, M.; Miyazaki, D.; Inoue, Y.; Soma, T.; Nishida, K.; et al. Clinical features and management of cytomegalovirus corneal endotheliitis: Analysis of 106 cases from the Japan corneal endotheliitis study. Br. J. Ophthalmol. 2015, 99, 54–58. [Google Scholar] [CrossRef]
- Aketa, N.; Yamaguchi, T.; Suzuki, T.; Higa, K.; Yagi-Yaguchi, Y.; Satake, Y.; Tsubota, K.; Shimazaki, J. Iris Damage Is Associated With Elevated Cytokine Levels in Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO42–BIO51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfawaz, A.M.; Holland, G.N.; Yu, F.; Margolis, M.S.; Giaconi, J.A.; Aldave, A.J. Corneal Endothelium in Patients with Anterior Uveitis. Ophthalmology 2016, 123, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Kong, X.; Sun, X. Quantification of Retinal Microvascular Density Using Optic Coherence Tomography Angiography in Primary Angle Closure Disease. Curr. Eye Res. 2021, 46, 1018–1024. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Kong, X.; Yu, X.; Sun, X. Peripapillary retinal vessel density in eyes with acute primary angle closure: An optical coherence tomography angiography study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1013–1018. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Yao, Y.; Zhu, Y. Optical Coherence Tomography Angiography in Posner-Schlossman Syndrome—A Preliminary Study. Ocul. Immunol. Inflamm. 2022, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Ko, T.; Kong, X.; Yu, X.; Min, W.; Shi, G.; Sun, X. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: An optical coherence tomography angiography study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1557–1564. [Google Scholar] [CrossRef]
- Wu, J.; Sebastian, R.T.; Chu, C.J.; McGregor, F.; Dick, A.D.; Liu, L. Reduced Macular Vessel Density and Capillary Perfusion in Glaucoma Detected Using OCT Angiography. Curr. Eye Res. 2019, 44, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Triolo, G.; Rabiolo, A.; Shemonski, N.D.; Fard, A.; Di Matteo, F.; Sacconi, R.; Bettin, P.; Magazzeni, S.; Querques, G.; Vazquez, L.E.; et al. Optical Coherence Tomography Angiography Macular and Peripapillary Vessel Perfusion Density in Healthy Subjects, Glaucoma Suspects, and Glaucoma Patients. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5713–5722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelius, A.; Pilger, D.; Riechardt, A.; Reitemeyer, E.; Rübsam, A.; Winterhalter, S.; Maier, A.-K.B. Macular, papillary and peripapillary perfusion densities measured with optical coherence tomography angiography in primary open angle glaucoma and pseudoexfoliation glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wong, T.Y.; Mitchell, P.; Friedman, D.S.; He, M.; Aung, T. Distribution of Ocular Perfusion Pressure and Its Relationship with Open-Angle Glaucoma: The Singapore Malay Eye Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, M.; Song, Y.; Guo, J.; Zhao, Y.; Chen, W.; Zhang, H. Influence of Exercise on Intraocular Pressure, Schlemm’s Canal, and the Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4733–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, H.A.; Silver, D.M.; Friedman, D.S.; He, M.; Plyler, R.J.; Eberhart, C.G.; Jampel, H.D.; Ramulu, P. Iris Cross-sectional Area Decreases With Pupil Dilation and its Dynamic Behavior is a Risk Factor in Angle Closure. J. Glaucoma 2009, 18, 173–179. [Google Scholar] [CrossRef]
- Gregersen, E. The tissue spaces in the human iris and their communication with the anterior chamber by way of the iridic crypts. Acta Ophthalmol. 1958, 36, 819–828. [Google Scholar] [CrossRef]
- Dieterich, C.E.; Witmer, R.; Franz, H.E. Iris and circulation of aqueous humor. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1971, 182, 321–340. [Google Scholar] [CrossRef]
- Yermalitski, A.; Rübsam, A.; Pohlmann, D.; Metzner, S.; Pleyer, U. Rubella Virus- and Cytomegalovirus-Associated Anterior Uveitis: Clinical Findings and How They Relate to the Current Fuchs Uveitis Syndrome Classification. Front. Ophthalmol. 2022, 2, 906598. [Google Scholar] [CrossRef]
- Szumny, D.; Szelag, A. The influence of new beta-adrenolytics nebivolol and carvedilol on intraocular pressure and iris blood flow in rabbits. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 917–923. [Google Scholar] [CrossRef]



| Affected Eye | Fellow Eye | p | |
|---|---|---|---|
| Age (years) | 36.56 ± 11.80 | 36.56 ± 11.80 | - |
| Sex (Male/Female) | 34/27 | 34/27 | - |
| Axial length (mm) | 23.88 ± 0.95 | 23.87 ± 0.91 | 0.848 |
| Corneal endothelium density (/mm2) | 2447.70 ± 423.93 | 2706.43 ± 297.06 | <0.001 * |
| C/D ratio | 0.50 ± 0.19 | 0.37 ± 0.09 | <0.001 * |
| Retinal nerve fiber layer thickness (μm) | 94.56 ± 23.34 | 101.72 ± 10.38 | 0.002 * |
| Intraocular pressure (mmHg) | 23.71 ± 11.96 | 17.43 ± 3.74 | <0.001 * |
| Pupil diameter (mm) | 4.63 ± 0.88 | 4.43 ± 0.89 | 0.032 * |
| Affected Eye | Corneal Endothelium Density (/mm2) | |||||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |||
| A500 (μm) | 0.441 | 0.740 | M500 (μm) | 0.538 | 0.047 * | P500 (μm) | −0.656 | 0.850 |
| A1000 (μm) | −0.161 | 0.938 | M1000 (μm) | 0.827 | 0.018 * | P1000 (μm) | 3.737 | 0.209 |
| A1500 (μm) | 2.140 | 0.382 | M1500 (μm) | 0.227 | 0.713 | P1500 (μm) | 3.262 | 0.268 |
| A2000 (μm) | 2.913 | 0.237 | M2000 (μm) | 0.317 | 0.639 | P2000 (μm) | 2.324 | 0.480 |
| A2500 (μm) | 1.216 | 0.412 | M2500 (μm) | 0.805 | 0.175 | P2500 (μm) | 4.922 | 0.096 |
| Affected Eye | C/D Ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |||
| A500 (μm) | 0.0001 | 0.859 | M500 (μm) | −0.0005 | 0.001 * | P500 (μm) | 0.0004 | 0.818 |
| A1000 (μm) | 0.0005 | 0.548 | M1000 (μm) | −0.0002 | 0.407 | P1000 (μm) | −0.0005 | 0.770 |
| A1500 (μm) | 0.0007 | 0.546 | M1500 (μm) | 0.0006 | 0.200 | P1500 (μm) | 0.0001 | 0.968 |
| A2000 (μm) | −0.0002 | 0.851 | M2000 (μm) | 0.0004 | 0.138 | P2000 (μm) | 0.0001 | 0.955 |
| A2500 (μm) | −0.0013 | 0.055 | M2500 (μm) | 0.0004 | 0.143 | P2500 (μm) | −0.0014 | 0.246 |
| Affected Eye | RNFL Thickness (μm) | |||||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |||
| A500 (μm) | −0.060 | 0.415 | M500 (μm) | 0.043 | 0.037 * | P500 (μm) | −0.112 | 0.541 |
| A1000 (μm) | −0.092 | 0.288 | M1000 (μm) | 0.005 | 0.824 | P1000 (μm) | 0.027 | 0.890 |
| A1500 (μm) | −0.022 | 0.873 | M1500 (μm) | −0.071 | 0.100 | P1500 (μm) | 0.009 | 0.956 |
| A2000 (μm) | −0.046 | 0.734 | M2000 (μm) | −0.023 | 0.466 | P2000 (μm) | −0.066 | 0.674 |
| A2500 (μm) | 0.201 | 0.058 | M2500 (μm) | −0.027 | 0.415 | P2500 (μm) | 0.091 | 0.601 |
| Affected Eye | IOP (mmHg) | |||||||
| β | p | β | p | β | p | |||
| A500 (μm) | −0.028 | 0.514 | M500 (μm) | 0.004 | 0.783 | P500 (μm) | −0.053 | 0.591 |
| A1000 (μm) | −0.059 | 0.208 | M1000 (μm) | −0.004 | 0.803 | P1000 (μm) | −0.114 | 0.356 |
| A1500 (μm) | 0.135 | 0.065 | M1500 (μm) | 0.006 | 0.742 | P1500 (μm) | −0.016 | 0.848 |
| A2000 (μm) | 0.035 | 0.598 | M2000 (μm) | 0.004 | 0.844 | P2000 (μm) | 0.036 | 0.787 |
| A2500 (μm) | −0.090 | 0.209 | M2500 (μm) | −0.013 | 0.513 | P2500 (μm) | 0.042 | 0.660 |
| Fellow Eye | IOP (mmHg) | |||||||
| β | p | β | p | β | p | |||
| A500 (μm) | −0.009 | 0.406 | M500 (μm) | −0.003 | 0.519 | P500 (μm) | 0.023 | 0.309 |
| A1000 (μm) | −0.041 | 0.200 | M1000 (μm) | −0.008 | 0.087 | P1000 (μm) | −0.036 | 0.120 |
| A1500 (μm) | −0.029 | 0.213 | M1500 (μm) | −0.008 | 0.078 | P1500 (μm) | −0.017 | 0.640 |
| A2000 (μm) | −0.021 | 0.132 | M2000 (μm) | −0.008 | 0.098 | P2000 (μm) | −0.022 | 0.429 |
| A2500 (μm) | −0.012 | 0.550 | M2500 (μm) | −0.008 | 0.199 | P2500 (μm) | −0.022 | 0.386 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Li, M.; Chen, W.; Chen, Z. Quantification of Iris Atrophy by Swept-Source Optical Coherence Tomography in Posner–Schlossman Syndrome. J. Clin. Med. 2022, 11, 6484. https://doi.org/10.3390/jcm11216484
Yan X, Li M, Chen W, Chen Z. Quantification of Iris Atrophy by Swept-Source Optical Coherence Tomography in Posner–Schlossman Syndrome. Journal of Clinical Medicine. 2022; 11(21):6484. https://doi.org/10.3390/jcm11216484
Chicago/Turabian StyleYan, Xiaoqin, Mu Li, Wei Chen, and Zhiqi Chen. 2022. "Quantification of Iris Atrophy by Swept-Source Optical Coherence Tomography in Posner–Schlossman Syndrome" Journal of Clinical Medicine 11, no. 21: 6484. https://doi.org/10.3390/jcm11216484





