The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review
Abstract
:1. Introduction
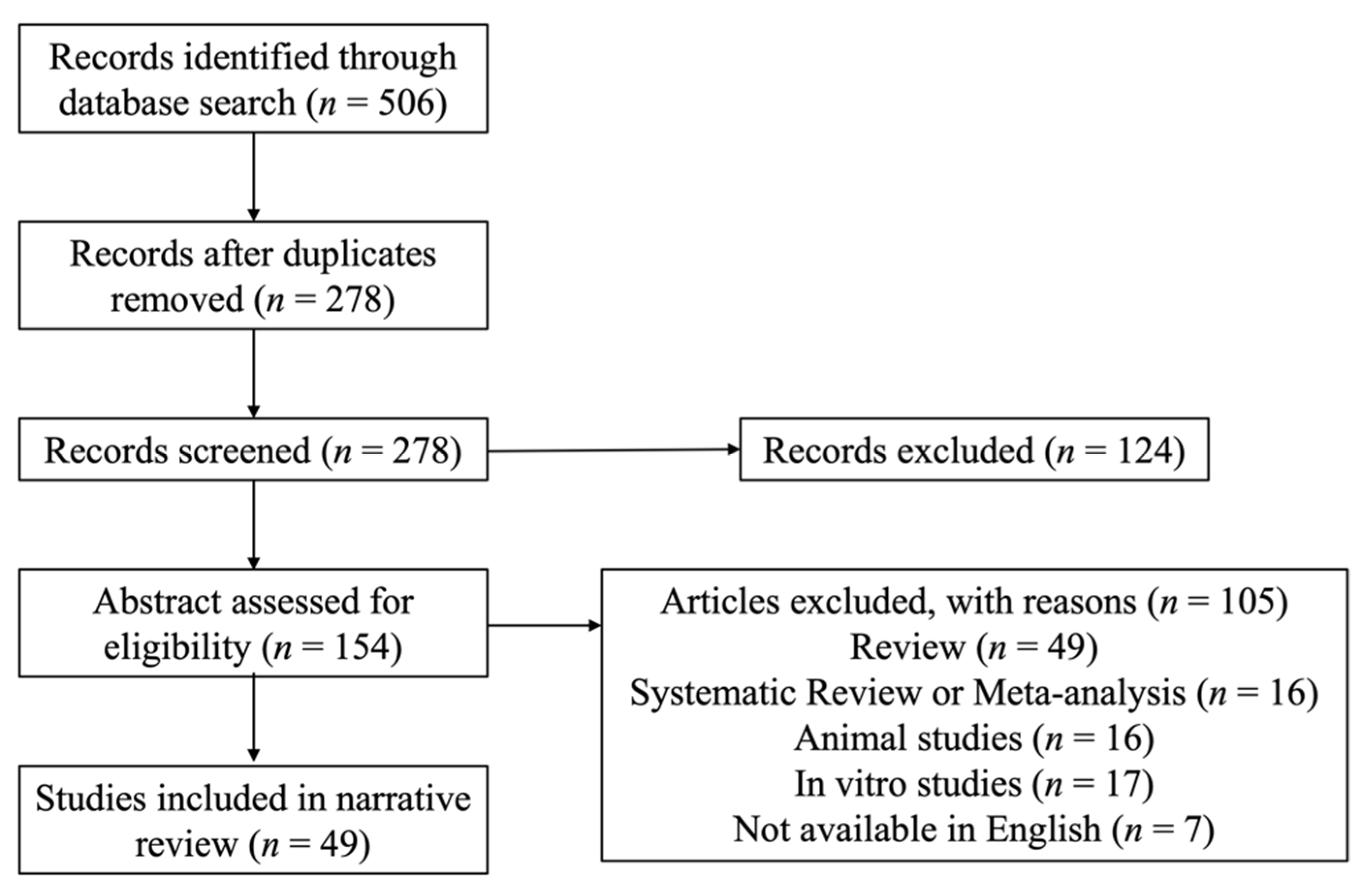
2. Methodology
3. Results
3.1. Levels of Circulating Vitamins and Diabetic Retinopathy
3.2. Dietary Vitamins and Diabetic Retinopathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Sherwin, R.; Jastreboff, A.M. Year in Diabetes 2012: The Diabetes Tsunami. J. Clin. Endocrinol. Metab. 2012, 97, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Wirostko, B.; Wong, T.Y.; Simó, R. Vascular endothelial growth factor and diabetic complications. Prog. Retin. Eye Res. 2008, 27, 608–621. [Google Scholar] [CrossRef]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. preventive services task force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef]
- van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Luca, C.T. Crosstalk between Vitamins A, B12, D, K, C, and E Status and Arterial Stiffness. Dis. Markers 2017, 2017, 8784971. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, W.; Naumnik, B.; Szczepański, M.; Myśliwiec, M. The mechanism of vascular calcification—A systematic review. Med. Sci. Monit. 2012, 18, RA1–RA11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Taddei, S.; Salvetti, A. Supplementation With Vitamins C and E Improves Arterial Stiffness and Endothelial Function in Essential Hypertensive Patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, G. The Roles of Vitamin A in the Regulation of Carbohydrate, Lipid, and Protein Metabolism. J. Clin. Med. 2014, 3, 453–479. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Villarroel, M.; Corraliza, L.; Hernández, C.; Garcia-Ramírez, M. The Retinal Pigment Epithelium: Something More than a Constituent of the Blood-Retinal Barrier—Implications for the Pathogenesis of Diabetic Retinopathy. J. Biomed. Biotechnol. 2010, 2010, 190724. [Google Scholar] [CrossRef] [Green Version]
- Rostamkhani, H.; Mellati, A.A.; Tabaei, B.S.; Alavi, M.; Mousavi, S.N. Association of Serum Zinc and Vitamin A Levels with Severity of Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Study. Biol. Trace Elem. Res. 2019, 192, 123–128. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Zhang, J.; Kuang, X.; Liu, C.; Deng, Q.; Li, D. Relationship between retinol and risk of diabetic retinopathy: A case-control study. Asia Pac. J. Clin. Nutr. 2019, 28, 607–613. [Google Scholar] [CrossRef]
- Luong, K.V.Q.; Nguyen, L.T.H. The Impact of Thiamine Treatment in the Diabetes Mellitus. J. Clin. Med. Res. 2012, 4, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Rämet, M.E.; Patel, A.R.; Pandian, N.G.; Mendelsohn, M.E.; Karas, R.H. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: Enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 2002, 144, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdman, J.W.; MacDonald, I.A.; Zeisel, S.H. Present Knowledge in Nutrition: Tenth Edition, 10th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; ISBN 9780470959176. [Google Scholar]
- Lei, X.; Zeng, G.; Zhang, Y.; Li, Q.; Zhang, J.; Bai, Z.; Yang, K. Association between homocysteine level and the risk of diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; Harper, C.A.; O’Dea, K. Homocysteine and diabetic retinopathy. Diabetes Care 2008, 31, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-vitamins and homocysteine in diabetic retinopathy: Association with vitamin-B12 deficiency and hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef] [PubMed]
- Cinici, E.; Dilekmen, N.; Senol, O.; Arpalı, E.; Cinici, O.; Tanas, S. Blood thiamine pyrophosphate concentration and its correlation with the stage of diabetic retinopathy. Int. Ophthalmol. 2020, 40, 3279–3284. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Gagliano, C.; Salomone, S.; Giordano, M.; Bucolo, C.; Pappalardo, A.; Drago, F.; Caraci, F.; Avitabile, T.; Motta, M. Folate status in type 2 diabetic patients with and without retinopathy. Clin. Ophthalmol. 2015, 9, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Tomić, M.; Vrabec, R.; Ljubić, S.; Bulum, T.; Rahelić, D. Plasma homocysteine is associated with nonproliferative retinopathy in patients with type 2 diabetes without renal disease. Diabetes Metab. Syndr. 2022, 16, 102355. [Google Scholar] [CrossRef]
- Srivastav, K.; Saxena, S.; Mahdi, A.A.; Shukla, R.K.; Meyer, C.H.; Akduman, L.; Khanna, V.K. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol. Vis. 2016, 22, 1352. [Google Scholar]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, S.; Ambati, B.K. The value of nutritional supplements in treating Age-Related Macular Degeneration: A review of the literature. Int. Ophthalmol. 2019, 39, 2975–2983. [Google Scholar] [CrossRef]
- Longo-Mbenza, B.; Muaka, M.M.; Masamba, W.; Kini, L.M.; Phemba, I.L.; Ndembe, D.K.; Mona, D.T. Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: A new phenotype in Central Africa? Int. J. Ophthalmol. 2014, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Mandal, T.; Nandi, M.; Osta, M.; Bandyopadhyay, U.; Ray, D. Oxidative stress in diabetic patients with retinopathy. Ann. Afr. Med. 2014, 13, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Pradhan, T.; Panda, T.K. Trace Minerals and Oxidative Stress in Diabetic Retinopathy. Bangladesh J. Med. Sci. 2014, 13, 175–179. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Benzie, I.F.F.; Choi, S.W.; Chan, L.Y.L.; Yeung, V.T.F.; Woo, G.C. Relationships among diabetic retinopathy, antioxidants, and glycemic control. Optom. Vis. Sci. 2011, 88, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D 3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef] [Green Version]
- Lazzara, F.; Longo, A.M.; Giurdanella, G.; Lupo, G.; Platania, C.B.M.; Rossi, S.; Drago, F.; Anfuso, C.D.; Bucolo, C. Vitamin D3 preserves blood retinal barrier integrity in an in vitro model of diabetic retinopathy. Front. Pharmacol. 2022, 13, 971164. [Google Scholar] [CrossRef]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Isaia, G.; Giorgino, R.; Adami, S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care 2001, 24, 1496. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.B.; Sivaprasad, M.; Shalini, T.; Satyanarayana, A.; Seshacharyulu, M.; Balakrishna, N.; Viswanath, K.; Sahay, M. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition 2015, 31, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537. [Google Scholar] [CrossRef] [PubMed]
- Nadri, G.; Saxena, S.; Mahdi, A.A.; Kaur, A.; Ahmad, M.K.; Garg, P.; Meyer, C.H. Serum vitamin D is a biomolecular biomarker for proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashinne, B.; Rajalakshmi, R.; Anjana, R.M.; Venkat Narayan, K.M.; Jayashri, R.; Mohan, V.; Hendrick, A.M. Association of serum vitamin D levels and diabetic retinopathy in Asian Indians with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Afarid, M.; Ghattavi, N.; Johari, M. Serum Levels of Vitamin D in Diabetic Patients with and without Retinopathy. J. Ophthalmic Vis. Res. 2020, 15, 172–177. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Shen, J.; Liu, F.; Zeng, H.; Li, L.; Yu, H.; Lu, H.; Lu, F.; Wu, Q.; Jia, W. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with Type 2 diabetes. Diabet. Med. 2014, 31, 1657–1664. [Google Scholar] [CrossRef]
- Payne, J.F.; Ray, R.; Watson, D.G.; Delille, C.; Rimler, E.; Cleveland, J.; Lynn, M.J.; Tangpricha, V.; Srivastava, S.K. Vitamin D Insufficiency in Diabetic Retinopathy. Endocr. Pract. 2012, 18, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Otí, J.M.; Galván-Manso, A.I.; Callejas-Herrero, M.R.; Vara-González, L.A.; Salas-Herrera, F.; Muñoz-Cacho, P. Vitamin d deficiency is significantly associated with retinopathy in type 2 diabetes mellitus: A case-control study. Nutrients 2022, 14, 84. [Google Scholar] [CrossRef]
- Zoppini, G.; Galletti, A.; Targher, G.; Brangani, C.; Pichiri, I.; Trombetta, M.; Negri, C.; de Santi, F.; Stoico, V.; Cacciatori, V.; et al. Lower levels of 25-hydroxyvitamin D3 are associated with a higher prevalence of microvascular complications in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2015, 3, e000058. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhou, J.B.; Zhao, W.; Zhang, R.H.; Cai, Y.H.; Shu, L.P.; Qi, L.; Yang, J.K. Could Vitamin D be Associated with Proliferative Diabetic Retinopathy? Evidence from Pooling Studies. Horm. Metab. Res. 2019, 51, 729–734. [Google Scholar] [CrossRef]
- Millen, A.E.; Sahli, M.W.; Nie, J.; LaMonte, M.J.; Lutsey, P.L.; Klein, B.E.K.; Mares, J.A.; Meyers, K.J.; Andrews, C.A.; Klein, R. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc. Diabetol. 2016, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wang, C.; Liu, D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr. Diabetes 2017, 7, e281. [Google Scholar] [CrossRef] [PubMed]
- Shimo, N.; Yasuda, T.; Kaneto, H.; Katakami, N.; Kuroda, A.; Sakamoto, F.; Takahara, M.; Irie, Y.; Horikawa, K.; Miyashita, K.; et al. Vitamin D deficiency is significantly associated with retinopathy in young Japanese type 1 diabetic patients. Diabetes Res. Clin. Pract. 2014, 106, e41–e43. [Google Scholar] [CrossRef]
- Kaur, H.; Donaghue, K.C.; Chan, A.K.; Benitez-Aguirre, P.; Hing, S.; Lloyd, M.; Cusumano, J.; Pryke, A.; Craig, M.E. Vitamin D Deficiency Is Associated with Retinopathy in Children and Adolescents with Type 1 Diabetes. Diabetes Care 2011, 34, 1400. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Ahmed, E.A.; Hassan, A.; Atkin, S.L. Relationship between total vitamin d metabolites and complications in patients with type 2 diabetes. Biomed. Rep. 2020, 14, 18. [Google Scholar] [CrossRef]
- Jee, D.; Do Han, K.; Kim, E.C. Inverse Association between High Blood 25-Hydroxyvitamin D Levels and Diabetic Retinopathy in a Representative Korean Population. PLoS ONE 2014, 9, e115199. [Google Scholar] [CrossRef] [Green Version]
- Alcubierre, N.; Valls, J.; Rubinat, E.; Cao, G.; Esquerda, A.; Traveset, A.; Granado-Casas, M.; Jurjo, C.; Mauricio, D. Vitamin D Deficiency Is Associated with the Presence and Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 374178. [Google Scholar] [CrossRef]
- Bonakdaran, S.; Shoeibi, N. Is there any correlation between vitamin D insufficiency and diabetic retinopathy? Int. J. Ophthalmol. 2015, 8, 326. [Google Scholar] [CrossRef]
- Patrick, P.A.; Visintainer, P.F.; Shi, Q.; Weiss, I.A.; Brand, D.A. Vitamin D and retinopathy in adults with diabetes mellitus. Arch. Ophthalmol. 2012, 130, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Alam, U.; Amjad, Y.; Chan, A.W.S.; Asghar, O.; Petropoulos, I.N.; Malik, R.A. Vitamin D Deficiency Is Not Associated with Diabetic Retinopathy or Maculopathy. J. Diabetes Res. 2016, 2016, 6156217. [Google Scholar] [CrossRef] [Green Version]
- Usluogullari, C.A.; Balkan, F.; Caner, S.; Ucler, R.; Kaya, C.; Ersoy, R.; Cakir, B. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr. Disord. 2015, 15, P130. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Laiginhas, R.; Madeira, C.; Neves, J.S.; Barbosa, M.; Rosas, V.; Carvalho, D.; Falcão-Reis, F.; Falcão, M. Association between Serum Vitamin D and Diabetic Retinopathy in Portuguese Patients with Type 1 Diabetes. Acta Med. Port. 2020, 33, 459–465. [Google Scholar] [CrossRef]
- Nakagawa, K.; Shibata, A.; Yamashita, S.; Tsuzuki, T.; Kariya, J.; Oikawa, S.; Miyazawa, T. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J. Nutr. 2007, 137, 1938–1943. [Google Scholar] [CrossRef] [Green Version]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Said, N.S.; Hadhoud, K.M.; Nada, W.M.; El Tarhouny, S.A. Superoxide dismutase, glutathione peroxidase and vitamin E in patients with diabetic retinopathy. Life Sci. J. 2013, 10, 1851–1856. [Google Scholar]
- Ho, J.-I.; Ng, E.Y.; Chiew, Y.; Koay, Y.Y.; Chuar, P.F.; Phang, S.C.W.; Ahmad, B.; Kadir, K.A. The effects of vitamin E on non-proliferative diabetic retinopathy in type 2 diabetes mellitus: Are they sustainable with 12 months of therapy. SAGE Open Med. 2022, 10, 205031212210953. [Google Scholar] [CrossRef]
- Smolek, M.; Notaroberto, N.F.; Jaramillo, A.G.; Pradillo, L.R. Intervention with vitamins in patients with nonproliferative diabetic retinopathy: A pilot study. Clin. Ophthalmol. 2013, 7, 1451. [Google Scholar] [CrossRef] [Green Version]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [Green Version]
- Fairulnizal, M.; Gunasegavan, R.D.N.; Khalid, N.M.; Balasubramaniam, V.; Mustar, S.; Rashed, A.A. Recent Techniques in Nutrient Analysis for Food Composition Database. Molecules 2020, 25, 4567. [Google Scholar] [CrossRef]
- Horikawa, C.; Aida, R.; Kamada, C.; Fujihara, K.; Tanaka, S.; Tanaka, S.; Araki, A.; Yoshimura, Y.; Moriya, T.; Akanuma, Y.; et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: Analysis of data from the Japan Diabetes Complications Study (JDCS). Eur. J. Nutr. 2020, 59, 1585–1594. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Tanaka, S.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akanuma, Y.; et al. Fruit Intake and Incident Diabetic Retinopathy with Type 2 Diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kawasaki, R.; Rogers, S.; Man, R.E.K.; Itakura, K.; Xie, J.; Flood, V.; Tsubota, K.; Lamoureux, E.; Wang, J.J. The Associations of Dietary Intake of Polyunsaturated Fatty Acids With Diabetic Retinopathy in Well-Controlled Diabetes. Investig. Opthalmol. Vis. Sci. 2015, 56, 7473. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Dietary Supplements|FDA. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 24 September 2022).
- Aguilera-Méndez, A.; Boone-Villa, D.; Nieto-Aguilar, R.; Villafaña-Rauda, S.; Molina, A.S.; Sobrevilla, J.V. Role of vitamins in the metabolic syndrome and cardiovascular disease. Pflügers Arch.-Eur. J. Physiol. 2022, 474, 117–140. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Vitamin D supplements and cancer incidence and mortality: A meta-analysis. Br. J. Cancer 2014, 111, 976–980. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Charrondiere, U.R.; Stadlmayr, B.; Wijesinha-Bettoni, R.; Rittenschober, D.; Nowak, V.; Burlingame, B. INFOODS Contributions to Fulfilling Needs and Meeting Challenges Concerning Food Composition Databases. Procedia Food Sci. 2013, 2, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Enko, D.; Fridrich, L.; Rezanica, E.; Stolba, R.; Ernst, J.; Wendler, I.; Fabian, D.; Hauptlorenz, S.; Halwachs-Baumann, G. 25-hydroxy-Vitamin D status: Limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin. Lab. 2014, 60, 1541–1550. [Google Scholar] [CrossRef]
- Klapkova, E.; Cepova, J.; Pechova, M.; Dunovska, K.; Kotaska, K.; Prusa, R. A Comparison of Four Methods (Immunochemistry and HPLC) for Determination of 25-(OH)-Vitamin D in Postmenopausal Women. Clin. Lab. 2017, 63, 385–388. [Google Scholar] [CrossRef]
- El Maaty, M.A.A.; Hanafi, R.S.; Aboul-Enein, H.Y.; Gad, M.Z. Design-of-experiment approach for HPLC analysis of 25-hydroxyvitamin D: A comparative assay with ELISA. J. Chromatogr. Sci. 2015, 53, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Upala, S.; Sanguankeo, A. Relationship between vitamin D deficiency and diabetic retinopathy: A meta-analysis. Can. J. Ophthalmol. 2017, 52, S39–S44. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.-A.; Gao, F.; Qin, L.-L. The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2017, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Xiong, R.; Yuan, Y.; Zhu, Z.; Wu, Y.; Ha, J.; Han, X.; Wang, W.; He, M. Micronutrients and Diabetic Retinopathy: Evidence From The National Health and Nutrition Examination Survey and a Meta-analysis. Am. J. Ophthalmol. 2022, 238, 141–156. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification. Ophthalmology 1991, 98, 786–806. [CrossRef]
- Wong, T.Y.; Sabanayagam, C. The War on Diabetic Retinopathy. Asia-Pac. J. Ophthalmol. 2019, 8, 448–456. [Google Scholar] [CrossRef]
| Reference | N (Subjects) | Measurement | Primary Outcome |
|---|---|---|---|
| Vitamin A | |||
| Zhang et al. | 126 (43 T2DM without DR, 43 T2DM with DR, 40 healthy controls) |
|
|
| Rostamkhani et al. | 60 (20 no DR, 20 NPDR, 20 PDR) |
|
|
| Vitamin B | |||
| Satyanarayana et al. | 394 (100 no DR, 194 DR, 100 healthy controls) |
|
|
| Srivastav et al. | 80 (20 no DR, 20 NPDR with DME, 20 PDR with DME, 20 healthy controls) |
|
|
| Cinici et al. | 100 (20 no DR, 20 mild-moderate NPDR, 20 severe NPDR, 20 PDR, 20 healthy controls) |
|
|
| Tomic et al. | 94 (69 no DR, 25 NPDR) |
|
|
| Malaguarnera et al. | 311 (96 no DR, 70 NPDR, 65 PDR, 80 healthy controls) |
|
|
| Vitamin C | |||
| Kumari et al. | 112 (30 no DR, 42 DR, 40 healthy controls) |
|
|
| Kundu et al. | 150 (50 DM without DR, 50 DR, 50 healthy controls) |
|
|
| Lam et al. | 420 (46 no DR, 161 background DR, 207 NPDR, 6 PDR) |
|
|
| Longo-Mbenza et al. | 200 (150 T2DM, 5 non-DM with retinopathy, 45 healthy control) |
|
|
| Vitamin D | |||
| Ahmed et al. | 750 (460 T2DM, 290 healthy controls) |
|
|
| Tomic et al. | 94 (69 no DR, 25 NPDR) |
|
|
| Afarid et al. | 60 (30 no DR, 30 DR) |
|
|
| Lopes et al. | 182 T1DM (79 no DR, 103 DR) |
|
|
| Alcubierre et al. | 283 T2DM (144 no DR, 139 DR) |
|
|
| Yuan et al. | 889 (616 no DR, 273 DR) |
|
|
| Nadri et al. | 72 (24 no DR, 24 NPDR, 24 PDR) |
|
|
| Longo-Mbenza et al. | 200 (150 T2DM, 5 non-DM with retinopathy, 45 healthy control) |
|
|
| Ashinne et al. | 3054 (1647 no DR, 1174 NPDR, 232 PDR) |
|
|
| Long et al. | 842 T2DM (541 no DR, 195 mild NPDR, 106 severe NPDR&PDR) |
|
|
| Zoppini et al. | 715 T2DM (490 no DR, 168 NPDR, 57 DR) |
|
|
| Alam et al. | 657 T2DM (257 no DR, 243 background DR, 135 pre PDR, 22 PDR) |
|
|
| Shimo et al. | 75 T1DM |
|
|
| Millen et al. | 1339 T2DM: 280 PDR (21%) |
|
|
| Usluogullari et al. | 311 (73 no DR, 238 DR) |
|
|
| He et al. | 1520 T2DM (625 no DR, 562 non-STDR, 333 STDR) |
|
|
| Jee et al. | 2113 T2DM (1738 no DR, 375 DR) |
|
|
| Reddy et al. | 263 (82 no DR, 82 DR, 99 healthy controls) |
|
|
| Bajaj et al. | 158 T2DM |
|
|
| Bonakdaran et al. | 235 (153 no DR, 64 NPDR, 18 PDR) |
|
|
| Payne et al. | 221 (47 no DM, 51 no DM with ocular disease, 41 DM without DR, 40 NPDR, 42 PDR) |
|
|
| Kaur et al. | 517 T1DM |
|
|
| Vitamin E | |||
| Kumari et al. | 112 (30 no DR, 42 DR, 40 healthy controls) |
|
|
| Said et al. | 128 (30 healthy controls, 30 DR without retinopathy, 34 NPDR, 34 PDR) |
|
|
| Longo-Mbenza et al. | 200 (150 T2DM, 5 non-DM with retinopathy, 45 healthy control) |
|
|
| Lam et al. | 420 (46 no DR, 161 background DR, 207 NPDR, 6 PDR) |
|
|
| Reference | N (Subjects) | Study Design | Measurement Methods | Primary Outcomes |
|---|---|---|---|---|
| Vitamin A | ||||
| Zhang et al. | 126 (43 no DR, 43 DR, 40 healthy controls) |
|
|
|
| Vitamin B | ||||
| Horikawa et al. | 978 T2DM no DR from Japanese Diabetic Complication Study |
|
|
|
| Smolek et al. | 10 T2DM with bilateral mild-moderate NPDR |
|
|
|
| Vitamin C | ||||
| Tanaka et al. | 978 T2DM no DR from Japanese Diabetic Complication Study |
|
|
|
| Sasaki et al. | 379 T2DM (150 no DR, 142 NPDR, 87 PDR) |
|
|
|
| Vitamin D | ||||
| Alcubierre et al. | 283 (144 no DR, 139 DR) |
|
|
|
| Millen et al. | 1339 (207 mild NPDR, 44 moderate to severe NPDR, 29 PDR, 3 DME) |
|
|
|
| Vitamin E | ||||
| Tanaka et al. | 978 T2DM no DR from Japanese Diabetic Complication Study |
|
|
|
| Sasaki et al. | 379 T2DM (150 no DR, 142 NPDR, 87 PDR) |
|
|
|
| Ho et al. | 55 NPDR |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. https://doi.org/10.3390/jcm11216490
Ruamviboonsuk V, Grzybowski A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. Journal of Clinical Medicine. 2022; 11(21):6490. https://doi.org/10.3390/jcm11216490
Chicago/Turabian StyleRuamviboonsuk, Varis, and Andrzej Grzybowski. 2022. "The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review" Journal of Clinical Medicine 11, no. 21: 6490. https://doi.org/10.3390/jcm11216490





