Multicentric Reticulohistiocytosis Associated with an Early Form of Systemic Lupus Erythematosus: A Case Report of a Rare Disease, with Mini Review of the Literature
Abstract
:1. Introduction
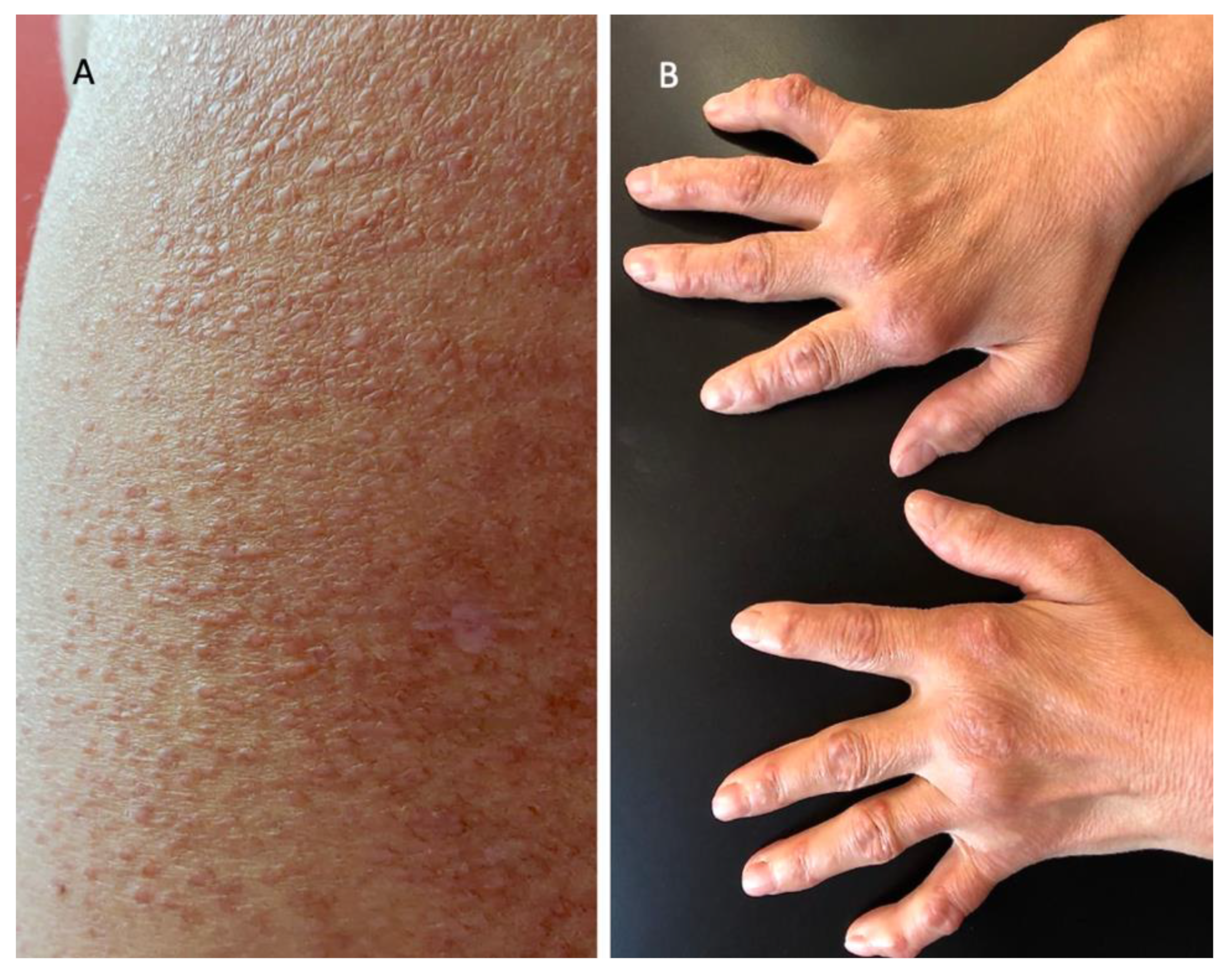
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berti, E.; Facchetti, F.; Zelger, B. Reticulohistiocytosis. In WHO Classification of Skin Tumours, 4th ed.; Elder, D.E., Massi, D., Willemze, R., Scolyer, R., Eds.; IARC Press: Lyon, French, 2018; pp. 289–290. [Google Scholar]
- Luz, F.B.; Rochael, M.C.; Ramos-E-Silva, M. Reticulohistiocytoses: A unique nosologic spectrum of non-Langerhans cell histiocytosis. Skinmed 2018, 16, 167–174. [Google Scholar] [PubMed]
- Zelger, B.W.; Sidoroff, A.; Orchard, G.; Cerio, R. Non-Langerhans cell histiocy-toses. a new unifying concept. Am. J. Dermatopathol. 1996, 18, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Zelger, B.; Cerio, R.; Soyer, H.P.; Misch, K.; Orchard, G.; Wilson-Jones, E. Reticulohistiocytoma and multicentricreticulohistiocytosis. Histopathologic and immunophenotypic distinctentities. Am. J. Dermatopathol. 1994, 16, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alvarez, C.; Sandhu, A.S.; Crowson, C.S.; Wetter, D.A.; McKenzie, G.A.; Lehman, J.S.; Makol, A. Multicentric reticulohistiocytosis: The Mayo Clinic experience (1980–2017). Rheumatology 2020, 59, 1898–1905. [Google Scholar] [CrossRef]
- Yang, H.J.; Ding, Y.Q.; Deng, Y.J. Multicentric reticulohistiocytosis with lungs and liver involved. Clin. Exp. Dermatol. 2009, 34, 183–185. [Google Scholar] [CrossRef]
- Furey, N.; Di Mauro, J.; Eng, A. Multicentric reticulohistiocytosis with salivary gland involvement and pericardial effusion. J. Am. Acad. Dermatol. 1983, 8, 679–685. [Google Scholar] [CrossRef]
- Benucci, M.; Sulla, A.; Manfredi, M. Cardiac engagement in multicentric reticulohistiocytosis: Report of a case with fatal outcome and literature review. Intern. Emerg. Med. 2008, 3, 165–168. [Google Scholar] [CrossRef]
- Tariq, S.; Hugenberg, S.T.; Hirano-Ali, S.A.; Tariq, H. Multicentric reticulohistiocytosis (MRH): Case report with review of literature between 1991 and 2014 with in depth analysis of various treatment regimens and outcomes. Springerplus 2016, 5, 180. [Google Scholar] [CrossRef] [Green Version]
- Bonometti, A.; Berti, E.; Associazione Italiana Ricerca Istiocitosi ONLUS. Reticulohistiocytoses: A revision of the full spectrum. J. Eur. Acad. Derm. Venereol. 2020, 34, 1684–1694. [Google Scholar] [CrossRef]
- Selmi, C.; Greenspan, A.; Huntley, A.; Gershwin, M.E. Multicentric reticulo-histiocytosis: A critical review. Curr. Rheumatol. Rep. 2015, 17, 511.52. [Google Scholar] [CrossRef]
- Toz, B.; Büyükbabani, N.; İnanç, M. Multicentric reticulohistiocytosis: Rheumatology perspective. Best Pract. Res. Clin. Rheumatol. 2016, 30, 250–260. [Google Scholar] [CrossRef]
- Snow, J.L.; Muller, A.S. Malignancy-associated multicentric reticulohistiocytosis: A clinical, histological and immunophenotypic study. Br. J. Dermatol. 1995, 133, 71–76. [Google Scholar] [CrossRef]
- Aldridge, R.D.; Main, R.A.; Daly, B.M. Multicentric reticulohistiocytosis and cancer. J. Am. Acad. Derm. 1984, 10, 296–298. [Google Scholar] [CrossRef]
- Nunnink, J.C.; Krusinski, P.A.; Yates, J.W. Multicentric reticulohistiocytosis and cancer: A case report and review of the literature. Med. Pediatr. Oncol. 1985, 13, 273–279. [Google Scholar] [CrossRef]
- Hinchman, K.F.; Wu, J.J.; Soden, C.E., Jr.; Waldman, J.; Dyson, S.W. Multicentric reticulohistiocytosis associated with Burkitt lymphoma and adenocarcinoma. Cutis 2008, 82, 113–114. [Google Scholar]
- Malik, M.K.; Regan, L.; Robinson-Bostom, L. Proliferating multicentric reticulohistiocytosis associated with papillary serous carcinoma of the endometrium. J. Am. Acad. Derm. 2005, 53, 1075–1079. [Google Scholar] [CrossRef]
- Ben Abdelghani, K.; Mahmoud, I.; Chatelus, E.; Sordet, C.; Gottenberg, J.E.; Sibilia, J. Multicentric reticulohis-tiocytosis: An autoimmune systemic disease? Case report of an association with erosive rheumatoid arthritis and systemic Sjogren syndrome. Jt. Bone Spine 2010, 77, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Asano, Y.; Okada, A.; Sugaya, M.; Sato, S. Ultraviolet light-induced Köbner phenomenon contributes to the development of skin eruptions in multicentric reticulohistiocytosis. Acta Derm. Venereol. 2011, 91, 160–163. [Google Scholar] [CrossRef]
- Mosca, M.; Costenbader, K.H.; Johnson, S.R.; Lorenzoni, V.; Sebastiani, G.D.; Hoyer, B.F.; Navarra, S.; Bonfa, E.; Ramsey-Goldman, R.; Medina-Rosas, J.; et al. Brief Report: How Do Patients with Newly Diagnosed Systemic Lupus Erythematosus Present? A Multicenter Cohort of Early Systemic Lupus Erythematosus to Inform the Development of New Classification Criteria. Arthritis Rheumatol. 2019, 71, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Doherty, M.; Martin, M.F.; Dieppe, P.A. Multicentric reticulohistiocytosis associated with primary biliary cirrhosis: Successful treatment with cytotoxic agents. Arthritis Rheum. 1984, 27, 344–348. [Google Scholar] [CrossRef]
- Shiokawa, S.; Shingu, M.; Nishimura, M.; Yasuda, M.; Yamamoto, M.; Tawara, T.; Wada, T.; Nobunaga, M. Multicentric reticulohistiocytosis associated with subclinical Sjögren·s syndrome. Clin. Rheumatol. 1991, 10, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-H.; Chiang, Y.-Y.; Information, P.E.K.F.C. Multicentric reticulohistiocytosis in a Taiwanese woman with Sjögren syndrome. Dermatol. Sin. 2016, 34, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Hoshina, D.; Shimizu, T.; Abe, R.; Murata, J.; Tanaka, K.; Shimizu, H. Multicentric reticulohistiocytosis associated with rheumatoid arthritis. Rheumatol. Int. 2005, 25, 553–554. [Google Scholar] [CrossRef]
- Roth, S.; Campagni, J.P.; Perrin, C.; Sanderson, F.; Castela, J.; Rosenthal, E.; Tieulié, N.; Jeandel, P.Y.; Heudier, P.; Fuzibet, J.G. Un cas de réticulohistiocytose multicentrique paranéoplasique associée à une maladie coeliaque [Paraneoplasic multicentric reticulohistiocytosis associated with a celiac disease]. Rev. Med. Interne 2006, 27, 263–265. [Google Scholar] [CrossRef]
- Codriansky, K.A.; Rünger, T.M.; Bhawan, J.; Kantarci, A.; Kissin, E.Y. Multicentric reticulohistiocytosis: A systemic osteoclastic disease? Arthritis Rheum. 2008, 59, 444–448. [Google Scholar] [CrossRef]
- Behera, A.; Devi, S.; Guru, S.; Sethy, M. A Rare Case of Multicentric Reticulohistiocytosis with Concurrent Rheumatoid Arthritis. Cureus 2019, 11, e5476. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Mikulik, Z.; Hackshaw, K.V. Multicentric reticulohistiocytosis with positive anticyclic citrullinated antibodies. J. Natl. Med. Assoc. 2007, 99, 678–680. [Google Scholar]
- Aouba, A.; Leclerc-Mercier, S.; Fraitag, S.; Martin-Silva, N.; Bienvenu, B.; Georgin-Lavialle, S. Assessment and effective targeting of Interleukin-1 in multicentric reticulohistyocytosis. Jt. Bone Spine 2015, 82, 280–283. [Google Scholar] [CrossRef]
- Bruscas Izu, C.; Hörndler Argarate, C.; García Latasa de Araníbar, F.J. Multicentric reticulohistiocytosis: A case report treated with tofacitinib. Med. Clin. 2021, 156, 310–311. [Google Scholar] [CrossRef]
- Niaki, O.Z.; Penn, E.; Scott, D.A.; Cobos, G.; Vleugels, R.A.; Weinblatt, M.E. Treatment of Severe Multicentric Reticulohistiocytosis with Upadacitinib. JAMA Dermatol. 2021, 157, 735–737. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- van de Putte, L.B.; Atkins, C.; Malaise, M.; Sany, J.; Russell, A.S.; van Riel, P.L.; Settas, L.; Bijlsma, J.W.; Todesco, S.; Dougados, M.; et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann. Rheum. Dis. 2004, 63, 508–516. [Google Scholar] [CrossRef]
- Biggioggero, M.; Crotti, C.; Becciolini, A.; Favalli, E.G. Tocilizumab in the treatment of rheumatoid arthritis: An evidence-based review and patient selection. Drug Des. Devel. Ther. 2018, 13, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Tena, C.; Reyes-Cordero, G.; Ochoa-Albíztegui, R.; Ríos-Barrera, V.; González-Chávez, S.A. Treatment of multicentric reticulohistiocytosis with tocilizumab. J. Clin. Rheumatol. 2013, 19, 272–276. [Google Scholar] [CrossRef]





| Timing of Clinical Manifestations | AIDs Associated | Serology | Timing of Diagnosis | Therapy | |
|---|---|---|---|---|---|
| Case 1. Caucasian woman, 56 y.o. | Joint manifestations preceded dermatological manifestations | Vitiligo, PBC, AI thyroiditis | Positive thyroid microsomal and thyroglobulin antibodies | MRH diagnosis following AIDs diagnosis | NSAIDs, D-penicillamine, cyclophosphamide, chlorambucil |
| Case 2 Caucasian woman, 60 y.o. | Joint manifestations preceded dermatological manifestations (7 years) | Sjogren syndrome | Positive ANA (1:80) and anti-Ro/SSA antibodies | Pre-existent Sjogren’s syndrome | Cyclophosphamide, CS, sodium aurothiomalate |
| Case 3 Taiwanese woman, 59 y.o. | Joint manifestations preceded dermatological manifestations (2 years) | Sjogren’s syndrome | Positive ANA (1:640), anti-Ro/SSA and anti-La/SSB | Pre-existent Sjogren’s syndrome | CS, hydroxychloroquine |
| Case 4 Japanese woman, 67 y.o. | Joint manifestations preceded dermatological manifestations (20 years) | Seropositive RA | Positive RF | MRH diagnosis followed RA diagnosis (22 years later) | MTX, CS |
| Case 5 Caucasian woman, 68 y.o. | - | Celiac disease | Positive anti-endomysium, anti-TG, and anti-gliadin | CS, MTX | |
| Case 6 Caucasian woman, 40 y.o. | - | Sjogren’s syndrome, RA | Positive RF and anti–CCP | Sjogren’s diagnoses preceded MRH diagnoses | CS, hydroxychloroquine, MTX, intravenous infusions of zoledronic acid |
| Case 7 Caucasian woman, 50 y.o. | Concomitant | RA, Sjogren’s syndrome | Positive RF, ACPA, and anti-Ro/SSA | MRH complicated by Sjogren’s syndrome | MTX, CS, alendronate |
| Case 8 Caucasian woman, 60 y.o. | - | Type 1 DM | Anemia, elevated VES, PCR, RF, anti- CCP titer | - | NSAIDs, MTX, CS, hydroxychloroquine, ibandronate |
| Cases of MRH Associated with AI Disease | Articular Manifestations | Dermatological Manifestation |
|---|---|---|
| Case 1 | A 4 y history of pain and stiffness affecting the knees, wrists, MCP, PIP, and DIP joints | Asymptomatic verrucous nailfold swellings on several fingers |
| Case 2 | A 7 y history of polyarthritis and stiffness involving the knees, wrists, fingers, shoulders, and elbows, with diagnosis of RA | Multiple small, reddish-brown cutaneous nodules on the fingers |
| Case 3 | A 2 y history of polyarthropathy affecting mainly the knees and the small joints of the hands | Itchy papulonodular rash affecting the dorsa of the hands, the neck and the helices of the ears |
| Case 4 | A 20 y history of pain, swelling, and tenderness in the elbows, knees, shoulders, and hip joints, with a diagnosis of RA with positive rheumatoid factor | Papules on the face, especially on the ears and the dorsum of the hands and fingers |
| Case 5 | Inflammatory arthralgias, without signs of arthritis, involving the fingers, wrists, and shoulders | Erythematosus/violaceus papulonodular lesions of the face, décolleté, hands, and knees |
| Case 6 | An 8 m history of joint symptoms | Non tender skin-colored papules and nodules over the PIP and the DIP joints, elbows, forehead, and ears; characteristic “coral beads” over the proximal nailfolds |
| Case 7 | Previous diagnosis of RA complicated by an extension of damage to the DIP joints | Rosy purple, smooth, firm, nodules, initially periungual, then spreading throughout the body and the dorsal surface of the fingers, elbows, pavilions, ears, scalp, and neck |
| Case 8 | A 30 y history of symmetrical polyarthritis, with morning stiffness | Papulonodular pruritic skin lesions over the scalp, trunk, extensor aspect of the elbow, dorsum of the hands, and pinnae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariotti, E.B.; Corrà, A.; Lemmi, E.; Laschi, L.; Aimo, C.; Quintarelli, L.; Volpi, W.; Nacci, F.; Verdelli, A.; Ruffo di Calabria, V.; et al. Multicentric Reticulohistiocytosis Associated with an Early Form of Systemic Lupus Erythematosus: A Case Report of a Rare Disease, with Mini Review of the Literature. J. Clin. Med. 2022, 11, 6529. https://doi.org/10.3390/jcm11216529
Mariotti EB, Corrà A, Lemmi E, Laschi L, Aimo C, Quintarelli L, Volpi W, Nacci F, Verdelli A, Ruffo di Calabria V, et al. Multicentric Reticulohistiocytosis Associated with an Early Form of Systemic Lupus Erythematosus: A Case Report of a Rare Disease, with Mini Review of the Literature. Journal of Clinical Medicine. 2022; 11(21):6529. https://doi.org/10.3390/jcm11216529
Chicago/Turabian StyleMariotti, Elena Biancamaria, Alberto Corrà, Elisa Lemmi, Lucrezia Laschi, Cristina Aimo, Lavinia Quintarelli, Walter Volpi, Francesca Nacci, Alice Verdelli, Valentina Ruffo di Calabria, and et al. 2022. "Multicentric Reticulohistiocytosis Associated with an Early Form of Systemic Lupus Erythematosus: A Case Report of a Rare Disease, with Mini Review of the Literature" Journal of Clinical Medicine 11, no. 21: 6529. https://doi.org/10.3390/jcm11216529





