Abstract
Background. Given the rapidly evolving pandemic of COVID-19 in 2020, authorities focused on the repurposing of available drugs to develop timely and cost-effective therapeutic strategies. Evidence suggested the potential utility of remdesivir in the framework of an early access program. REMDECO-19 is a multicenter national cohort study assessing the ability of remdesivir to improve the outcome of patients hospitalized with COVID-19. Methods. We conducted a retrospective real-life study that included all patients from the early access program of remdesivir in France. The primary endpoint was the clinical course evolution of critically ill and hospitalized COVID-19 patients treated with remdesivir. Secondary endpoints were the SOFA score evolution within 29 days following the admission and mortality at 29 and 90 days. Results. Eighty-five patients were enrolled in 22 sites from January to April 2020. The median WHO and SOFA scores were respectively reduced by two and six points between days 1 and 29. Improvement in the WHO-CPS and the SOFA score were observed in 83.5% and 79.3% of patients, respectively, from day 10. However, there was no effect of remdesivir on the 90-day survival based on the control cohort for hospitalized COVID-19 patients with invasive ventilation. Conclusions. SOFA score appeared to be an attractive approach to assess remdesivir efficacy and stratify its utilization or not in critically ill patients with COVID-19. This study brings a new clinical benchmark for therapeutic decision making and supports the use of remdesivir for some hospitalized COVID-19 patients.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) inducing a significant morbidity and mortality rate [1]. In France, during the first pandemic wave of COVID-19, most patients were asymptomatic or had mild illness; however, 87,809 people (134 per 100,000) were hospitalized with COVID-19 and 15,661 people (24 per 100,000) died in hospital [2]. Risks factors associated with development of acute respiratory distress syndrome and death included older age, male sex, neutrophilia, organ dysfunction, coagulopathy, and elevated D-dimer levels [3]. Given the rapidly evolving COVID-19 pandemic during the first wave, health authorities focused on the repurposing of available drugs to develop timely and cost-effective therapeutic strategies [4]. To date, supportive care for hospitalized patients with COVID-19 including oxygen supply and the use of dexamethasone in patients who received invasive mechanical ventilation are still the cornerstone of the medical care [5]. Expanded access programs (EAP) were developed for various drugs and emergency use authorization (EUA). COVID-19 management has been greatly improved thanks to EAP that have permitted drug repositioning such as for remdesivir, baricitinib, tocilizumab, nirmatrelvir–ritonavir, and molnupiravir and early access to COVID-19 convalescent plasma, casirivimab–imdevimab, bamlanivimab–etesevimab, sotrovimab, tixagevimab-cilgavimab, and nirmatrelvir [6].
Remdesivir is a prodrug of a nucleotide analogue that is intracellularly metabolized to an analogue of adenosine triphosphate that inhibits viral RNA-dependent RNA polymerases. It has demonstrated broad-spectrum activity against members of several families, including filoviruses, paramyxoviruses, and coronaviruses [7,8,9] and has shown prophylactic and therapeutic efficacy in nonclinical models of these coronaviruses. Remdesivir was touted as a potential candidate drug for the treatment of COVID-19 [10]. EAP evaluated the safety and efficacy of remdesivir in patients with moderate or severe COVID-19 during 5 to 10 days (200 mg loading dose on day 1, followed by 100 mg daily for up to 10 days) [10,11,12,13]. ACTT-1, a randomized, double-blind clinical trial, showed that remdesivir was superior to placebo in shortening the time to recovery in adult patients with severe COVID-19 [14]. Subsequently, a randomized, open-label trial in patients with severe COVID-19 not requiring mechanical ventilation, showed no significant difference between a 5-day and 10-day course of remdesivir [15]. These results prompted the US Food and Drug Administration (FDA) to grant EUA on 1 May 2020 [16]. On the 22nd of October 2020, remdesivir became the first United States FDA-approved drug for the treatment of hospitalized COVID-19 patients [17]. DisCoVeRy, a randomized, controlled, open-label clinical trial, did not observed clinical benefit of remdesivir in hospitalized patients versus standard of care by the WHO seven-point ordinal scale [18]. Recently, the PINETREE study showed very promising results: among non-hospitalized patients at high risk for COVID-19 progression, a 3-day course of remdesivir had an acceptable safety profile and resulted in an 87% lower risk of hospitalization or death than placebo [19].
Facing mixed outcomes [20,21], we set up a real-life study assessing the efficacy of remdesivir to improve clinical features in critically ill patients hospitalized with COVID-19 and severe pneumonia.
2. Materials and Methods
2.1. Settings
This national cohort study analyzes all patients included in EAP of remdesivir in France during the first wave of COVID-19. A nominative access of remdesivir was authorized by the National Agency for the Safety of Medicines and Health Products (ANSM) and a pharmaceutical company (Gilead Sciences, Foster City, CA, USA). Approval of requests was reserved for patients meeting the following criteria: (1) adult hospitalized for COVID-19 with a positive SARS-CoV-2 RT-PCR and/or typical chest computed tomographic [CT] characterized by scan bilateral and peripheral predominant ground glass opacities not fully explained by effusions, lobar or lung collapse, or nodules, (2) an oxygen saturation of 94% or less, (3) a creatinine clearance above 30 mL per minute and serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) less than five times the upper limit of the normal range, and (4) not included in an ongoing clinical trial involving COVID-19. For each approved case, French hospitals were supplied with a stock of remdesivir needed for a 10-day treatment, consisting of a loading dose of 200 mg intravenously on day 1, plus 100 mg daily for the following 9 days. Any concomitant supportive therapy was permitted. The exclusion criteria were patients not receiving at least one dose of remdesivir and patients already enrolled in a clinical trial investigating remdesivir. The study protocol was approved by the institutional review board (IRB-00011928). Informed consent was obtained from all individual participants included in the study. Data collection has been declared to the National Commission for Data Processing and Freedoms. This trial is registered with ClinicalTrials.gov, NCT04365725.
2.2. Outcomes
The primary outcome was the clinical course evolution of patients under treatment with remdesivir using a WHO clinical progression scale (WHO-CPS) [22]. The seven-point scale consisted of the following categories: 1, not hospitalized, no limitation of activities; 2, not hospitalized, limitation of activities; 3, hospitalized, not requiring supplemental oxygen; 4, hospitalized, requiring supplemental oxygen; 5, hospitalized, requiring nasal high-flow oxygen therapy or noninvasive mechanical ventilation; 6, hospitalized, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and 7, death. Prespecified secondary outcomes were the Sequential Organ Failure Assessment (SOFA) score evolution [23], duration without mechanical ventilation within 29 days of initiation of treatment with remdesivir, and mortality at 29 and 90 days after initiation of treatment with remdesivir. Data were collected daily from the day before remdesivir introduction to 90 days after or until discharge or death. Safety outcomes were adverse events as measured by investigators using the NCI Common Terminology Criteria for Adverse Events version 3.0 and graded on a 0 to 4 scale (0, normal; 4, life-threatening).
2.3. Statistical Analysis
Summary statistics, namely median with interquartile range (IQR), and percentages, are reported unless otherwise specified. Comparison of baseline characteristics across groups used the nonparametric Wilcoxon rank sum test or the exact Fisher test. To detail the temporal dynamics of illness severity of those patients, the evolution of patient trajectories across the WHO scale over time was illustrated using multistate models based on the time-inhomogeneous Markov chain [24].
The 90-day survival was compared between patients requiring invasive mechanical ventilation with a previously described cohort [25] meeting the same four criteria mentioned above and not receiving remdesivir. To consider a confounding bias related to the non-random choice of treatment, different weightings were used to correct the sample. These weightings estimate the average effects of the treatment on different populations: ATE (average treatment effect) on the population corresponding to the sample [26], ATT (average effect in treated), or ATO (on the population balanced in prognostic terms) [27,28]. We used the weightings allowing a balance on the prognostic factors, measured on the standardized mean differences (SMD). Any value >0.10 in the pooled standard deviation (SD) was considered unbalanced.
All analyses were performed on R 3.6.2 (https://www.R-project.org, accessed on 15 June 2022). The R package mstate was used to estimate the transition and state occupation probabilities. The PSweight and survey packages were used to compare the 90-day survivals. The study was designed and conducted by the sponsor Assistance Publique—Hôpitaux de Paris (AP-HP), in accordance with the protocol. The sponsor collected the data, monitored conduct of the program, and performed the statistical analyses.
3. Results
3.1. Characteristics of Study Participants and Treatment
Eighty-five patients were enrolled in 22 sites from 24 January 2020 to 21 April 2020. The median age at inclusion was 60 years (interquartile range, IQR, 49 to 69), ranging from 25 to 85 years (Table 1). Sixty-six patients (77.6%) were men. At baseline, most patients (n = 71; 83.5%) received invasive ventilation in the intensive care unit (ICU); thirteen (15.3%) were receiving non-invasive ventilation in the intermediate care unit; and only one patient (1.2%) did not require oxygen in conventional hospitalization. Before initiating treatment with remdesivir, the median duration of symptoms was 11 days (IQR, 8 to 14) and the median duration of COVID-19 diagnosis was 5 days (IQR, 2 to 7). Those data did not differ substantially between patients receiving invasive ventilation and those receiving non-invasive ventilation. Patients had invasive mechanical ventilation for 2 days (range, 1–6) before the initiation of remdesivir. Patients who were receiving non-invasive oxygen support at baseline were younger (median age, 59 years, vs. 63 years) than those receiving invasive ventilation and were more likely to be female (22.5%, vs. 15.4%) and had higher prevalence of diabetes (14.1%, vs. 7.7%) and respiratory pathology (19.7%, vs. 15.4%). Median body mass index (BMI) and median laboratory values were similar between these groups. At baseline, patient laboratory analyses were characterized by elevated liver markers (ASAT and ALAT > 5 N, LDH), normal renal function, and normal white blood cell count despite a lymphopenia. Median WHO and SOFA scores at remdesivir initiation were six and seven, respectively.

Table 1.
Baseline Demographic and Clinical Characteristics of the Patients.
All patients received at least one dose of remdesivir from 29 January 2020 to 24 April 2020. Fifty-five patients (64.7%) received the full 10-day course of remdesivir, 22 patients (25.9%) received five to nine days of treatment, and eight patients (9.4%) received remdesivir for less than five days. Remdesivir discontinuation before 10 days was caused by limiting toxicity or contraindication (n = 12; 14.1%), clinical improvement (n = 8; 9.4%), therapeutic strategy change (n = 6; 7.1%), or death (n = 5; 5.9%). Forty-four patients (51.8%) received concomitant COVID-19 treatments: 22 (25.9%) received in addition hydroxychloroquine, 23 (27.1%) received lopinavir/ritonavir, 11 (12.9%) received corticoids, 6 (7.1%) received azithromycin, and 3 (3.6%) received other treatments. At baseline, 32 patients (37.6%) received vasopressor treatment, of whom 31 were under invasive ventilation and 1 had non-invasive oxygen support.
3.2. Control Group
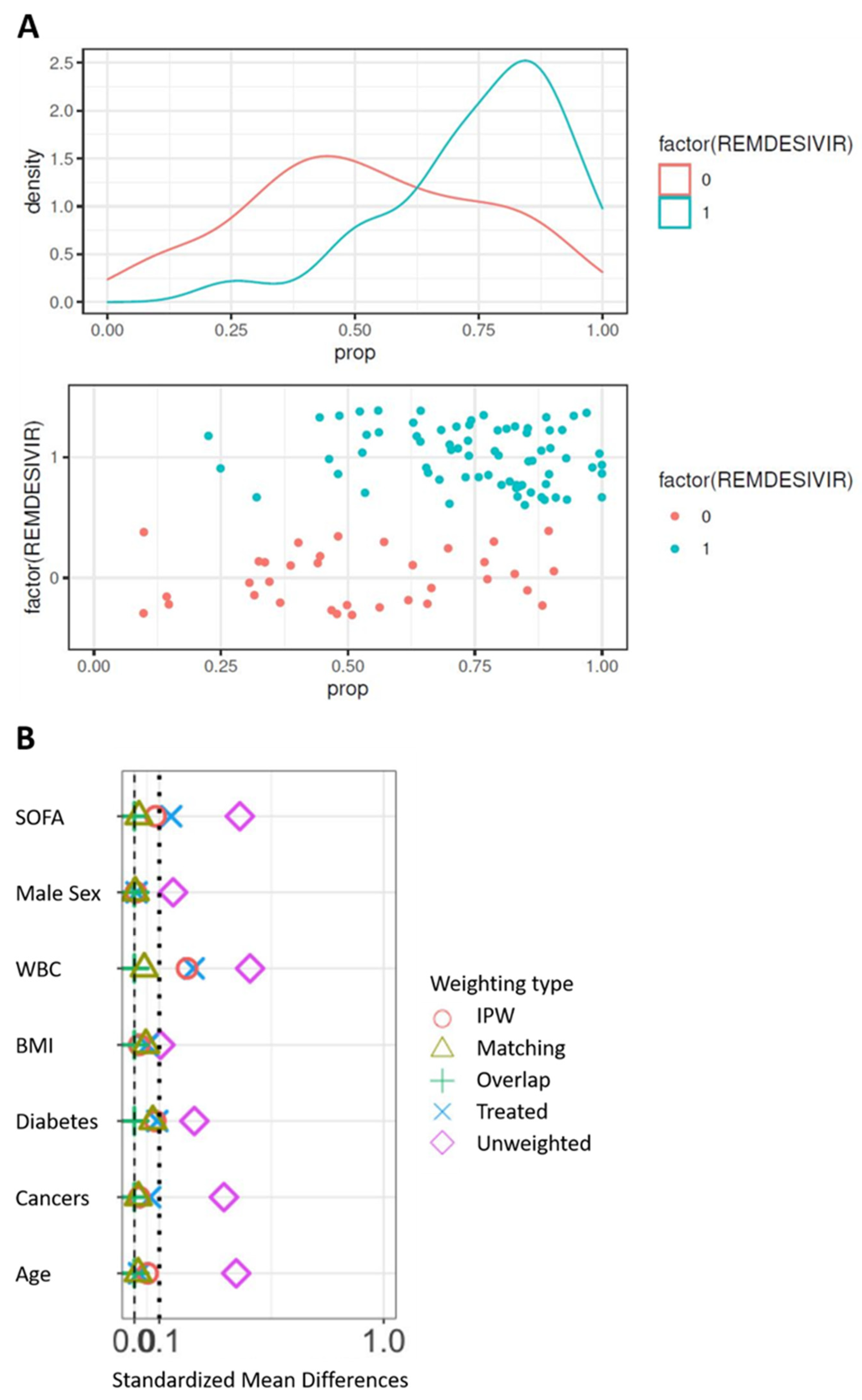
The control cohort for the comparison of 90-day survival is presented in Supplemental Table S1. The propensity score was constructed from the seven following variables: age, sex, BMI, SOFA score at admission, white blood cells at admission, and comorbidities (diabetes and cancer). At first, the model was built on complete cases, i.e., in 105 patients on invasive ventilation, including 71 patients from the remdesivir group. Significant differences are observed between groups (Table 2) with SMD > 0.10 on the seven prognostic factors. In particular, the subjects treated were younger and had a lower SOFA than the controls before the correction of the confusion bias. Difference in both treatment groups can be displayed by the distribution of the propensity score. The propensity score to have received remdesivir was estimated using a multivariate logistic model including the seven unbalanced prognostic factors. The distribution of the score in the two groups on the original basis (Figure 1A) validates the hypothesis of common support. Weightings were applied from the propensity score, resulting in pseudo-populations. The correction of the imbalances was evaluated on the bases thus weighted (Figure 1B). Only weightings with matching or overlapped weights allow all SMDs to be reduced below 0.10. These two populations were those subsequently retained.

Table 2.
Comparison of patients on invasive ventilation with controls.
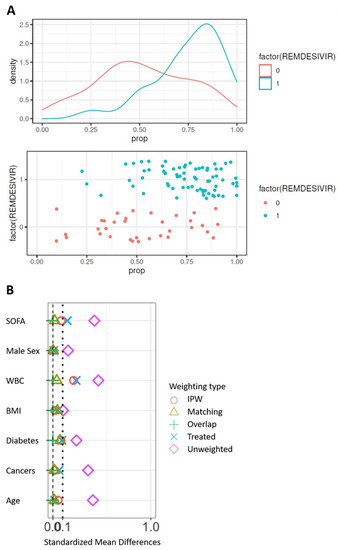
Figure 1.
Correction of confusion bias by weighting. (A) Distribution of the propensity score between groups on the original basis. (B) Correction of the imbalances according to the types of weighting. WBC, white blood cells; BMI, body mass index.
3.3. Mortality
Twelve out of 85 patients (14.1%) died before the 29 days of follow-up was completed, all with invasive ventilation at baseline. The median age of these patients was 76.5 years (IQR, 66 to 80). The median time between remdesivir initiation and death was 16 days.
Regarding the patients under invasive ventilation, 12 (16.9%) in the remdesivir group died within 90 days after the start of hospitalization, compared with 12 (35.29%) in the control arm (Table 3).

Table 3.
Comparison of 90-day survival between remdesivir and control cohorts.
3.4. WHO Score
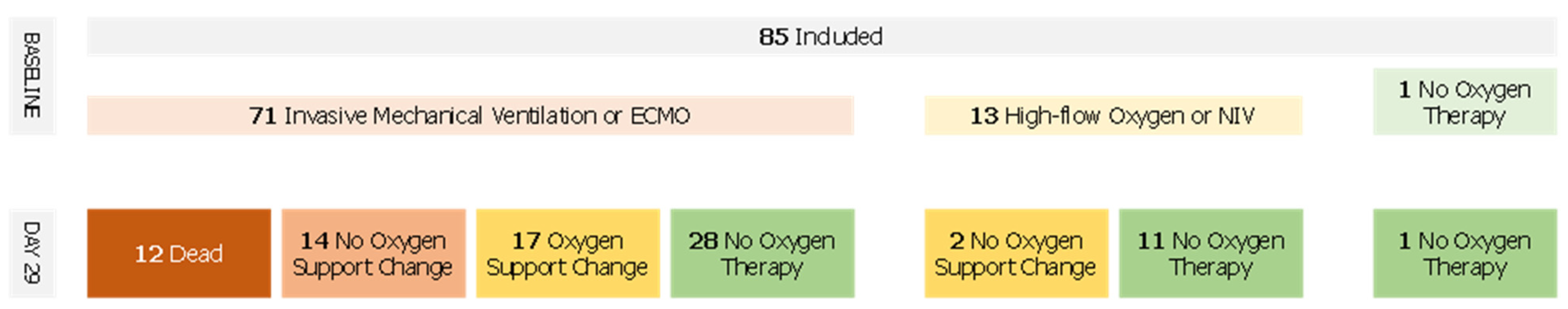
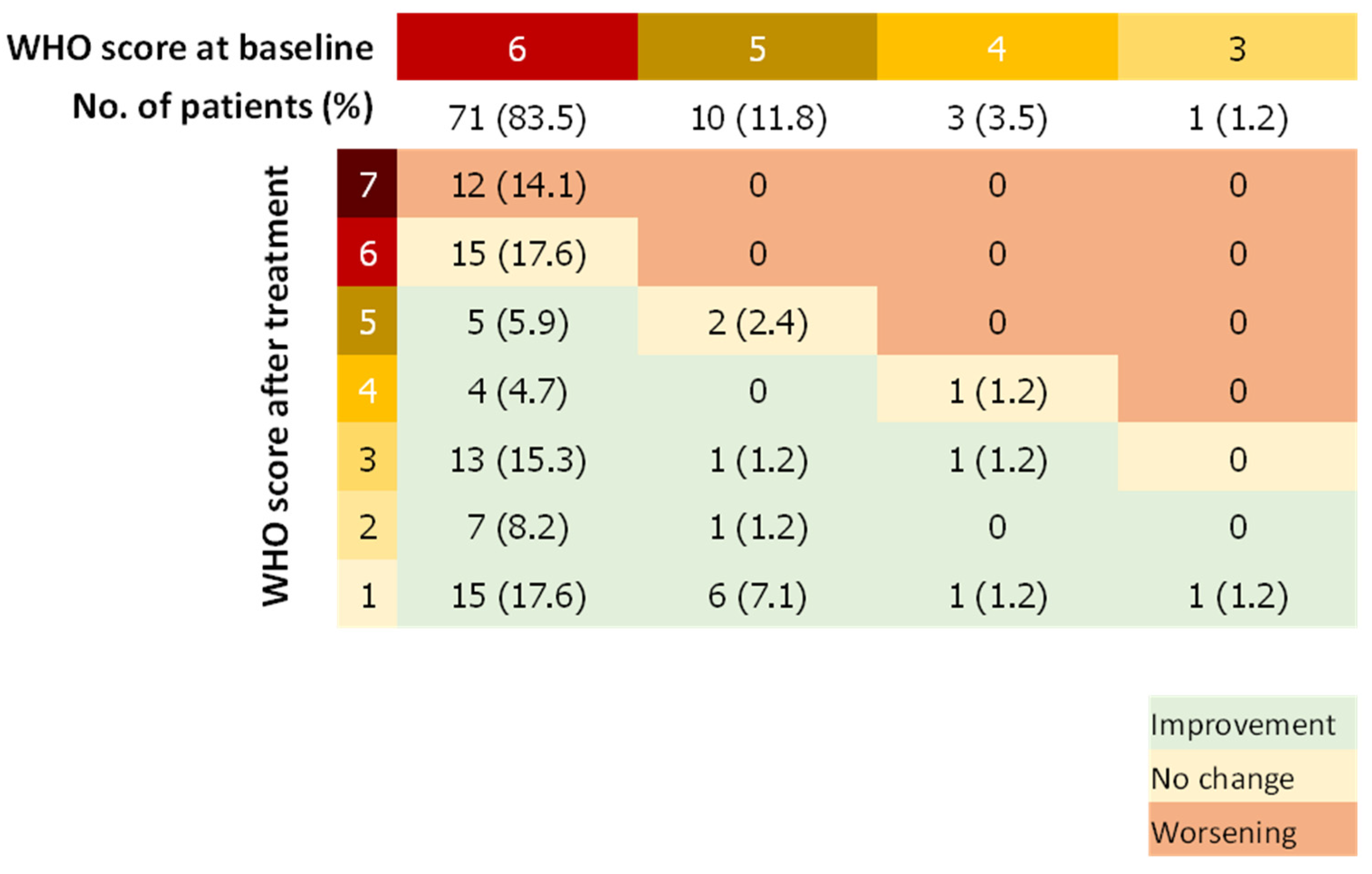
The median WHO score was reduced by two points (IQR, 0 to 4) between days 1 and 29. Forty-eight patients (56.5%) showed a WHO score reduced by at least 2 points whereas 14 patients (16.5%) had a worsening WHO score during the follow-up (Figure 2). Fifty-seven patients (67.9%) improved the category of oxygen support over a median of 9 days after the first dose of remdesivir. Median duration to change category of oxygen support was 12.5 (IQR, 8.8–17) and 14 (IQR, 8 to 18) days for patients treated with remdesivir within 11 days of symptoms onset and more than 11 days after symptoms onset, respectively. Among patients on invasive ventilation at baseline, (n = 71, 83.5%), 27 (31.7%) did not improve their WHO score after remdesivir treatment, of which 12 died (14.1%), whereas 28 patients (39.4%) were extubated or stopped receiving ECMO after the 29 days of follow-up. Among patients not on invasive ventilation (n = 13, 16.5%), 11 (84.6%) stopped receiving any oxygen and none worsened according to the WHO scale (Figure 3).
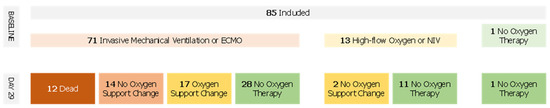
Figure 2.
Oxygen Support Flow Chart.
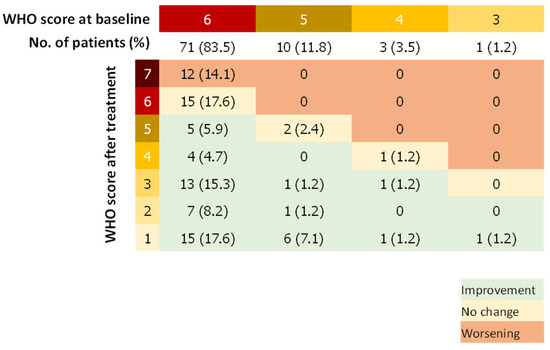
Figure 3.
WHO Score Double-Entry Board. WHO score at baseline and after remdesivir treatment; Number (%).
3.5. SOFA Score
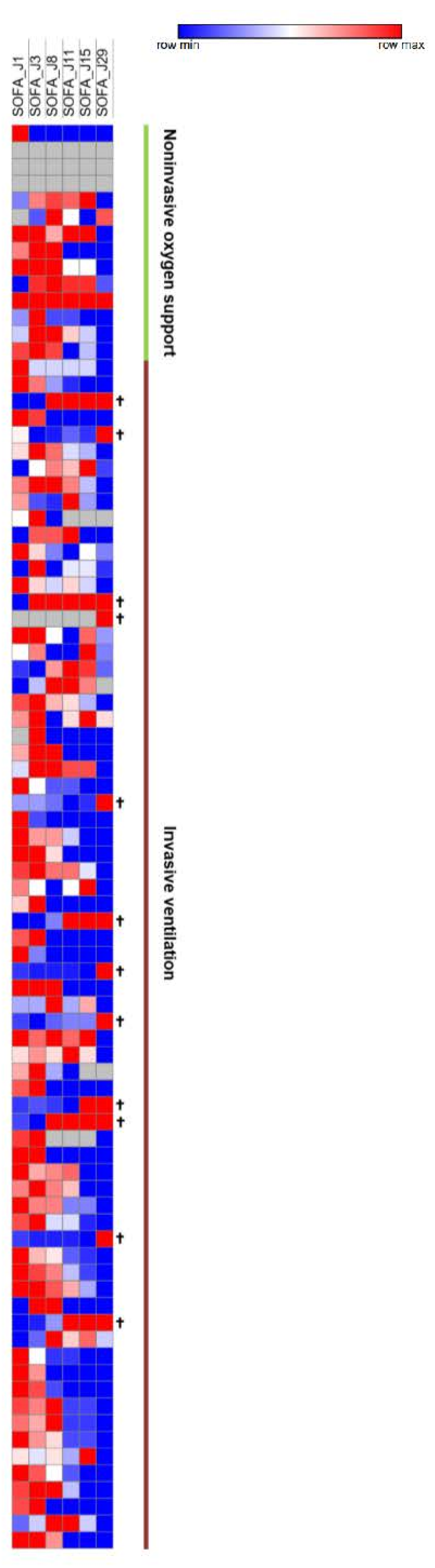
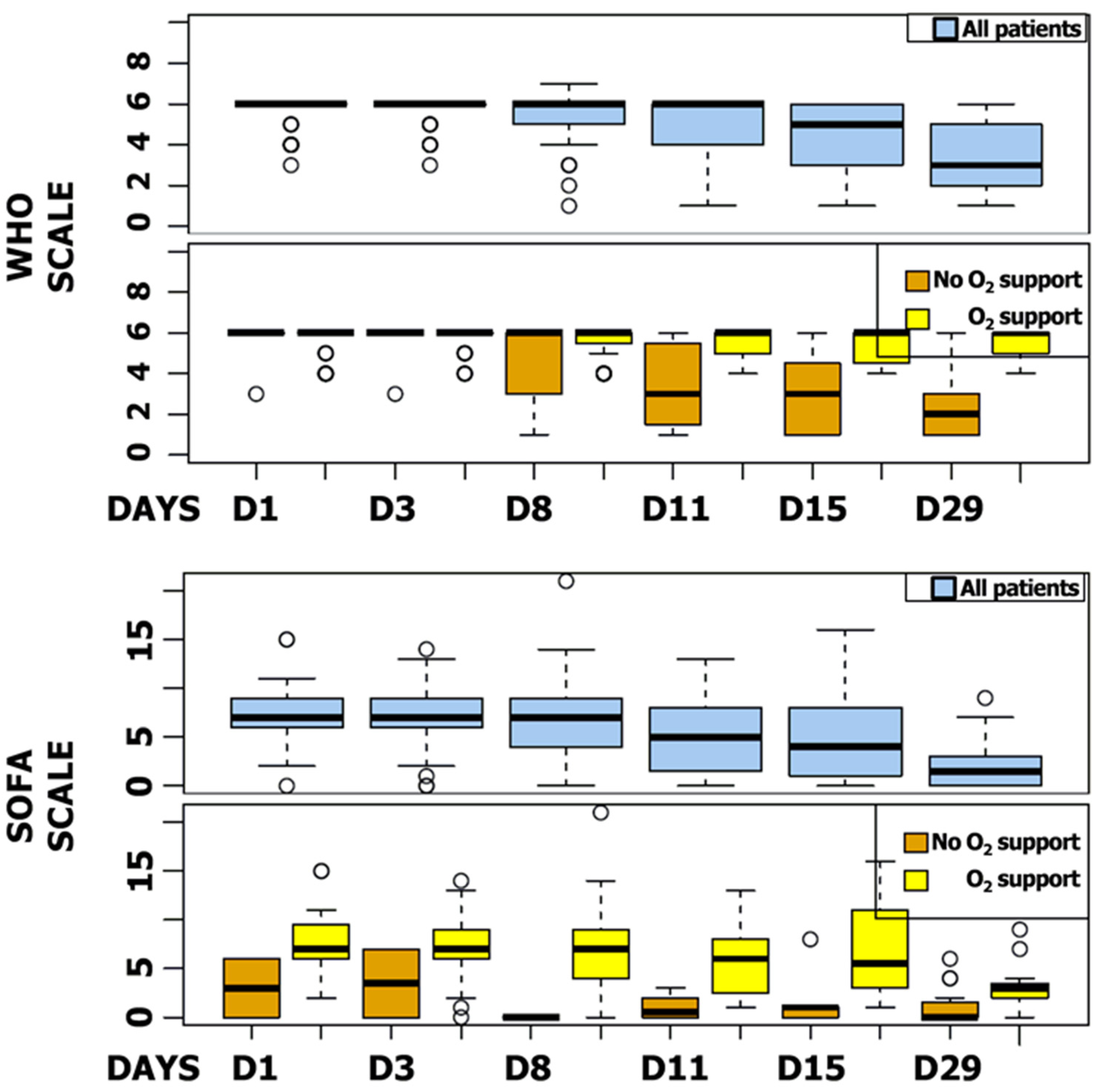
The median SOFA score was reduced by six points during follow-up (IQR, 2 to 8). Five patients (5.9%) showed a worsening SOFA score, including three patients receiving a concomitant COVID-19 treatment. The majority of patients improved their SOFA score at day 11 (n = 65, 79.3%), particularly patients receiving invasive ventilation at baseline (87.3%) (Figure 4). WHO and SOFA scores evolution depending or not on oxygen requirement are represented in Figure 5. The median duration of vasopressor treatments was 8.5 days and the median duration between the first day of remdesivir treatment and the end of the vasopressive treatment was 6 days. At the end of follow-up, 49 patients (57.6%) were still hospitalized and 22 were still oxygen-requiring including 14 still under invasive ventilation.
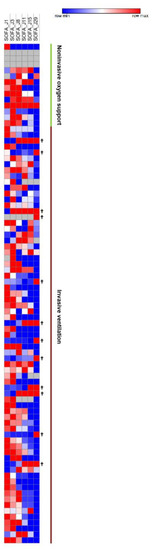
Figure 4.
SOFA Scores Heatmap. SOFA scores at day 1, 3, 8, 11, 15, and 29. Increasingly blue colors mean improved SOFA scores compared to J1; increasingly red colors mean worsened SOFA scores compared to J1. Each row corresponds to a patient.
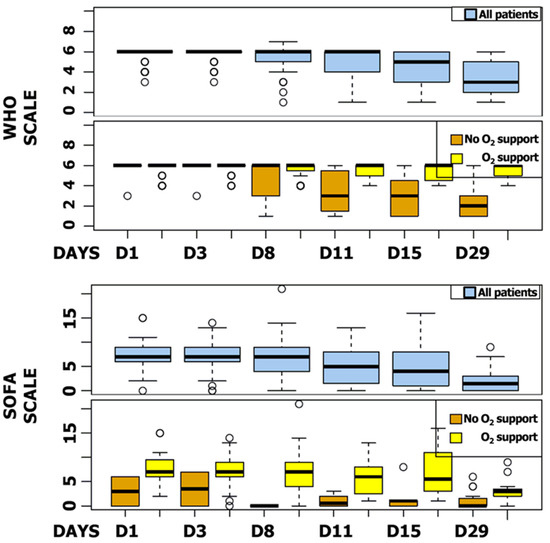
Figure 5.
SOFA and WHO Scores Box Plot. SOFA and WHO scores at day 1, 3, 8, 11, 15, and 29 for all patients and regarding oxygen support.
3.6. Safety
The most common adverse events were increased hepatic enzymes (31.8%), anemia (30.6%), renal impairment (28.2%), and hypotension (23.5%) (Table 4). Adverse events occurred both in patients with invasive and non-invasive ventilations (70.4% vs. 69.2%). Thirty-eight patients (44.7%) had serious adverse events. The most common serious adverse events were hypotension (22.3%), anemia (14.1%), renal impairment (15.3%), and deep-vein thrombosis (12.9%). Eleven patients (12.9%) discontinued remdesivir treatment prematurely because of limiting toxicity.

Table 4.
Adverse events.
4. Discussion
This retrospective, national multicenter study investigated the clinical evolution in real life of all patients with severe COVID-19 pneumonia treated in France with remdesivir outside of clinical trials during the first pandemic wave of COVID-19. Our cohort included a majority of patients in the most severe COVID-19 forms, namely requiring invasive mechanical ventilation and high-flow oxygen, and a majority of men. This patient description at admission is consistent with previous single-arm studies on remdesivir treatment for severe COVID-19 [10,16]. We showed an improvement in oxygen-support status for 67.9% of patients and an overall mortality of 14.1% over a 29-day follow-up. These clinical outcomes were consistent with the literature. Grein’s et al. described a 68% improvement in oxygen-support status [10]. Randomized, controlled trials reported an all-cause mortality rate of 11.4% [14] or 14% [29]. Improvement in the ordinal scale score was observed in 83.5% of cases and probability of change in WHO score over time was higher between 10 to 15 days following remdesivir introduction. At the same time, 79.3% of patients showed an improvement for the SOFA score from day 11 of the follow-up. At the end of the 29 days of follow-up, 36 patients (42.4%) were discharged from hospital.
In terms of safety, real-life observations are essential in addition to clinical trials—all the more so since the results of the trials are difficult to transpose to the general population, the patients most at risk of adverse effects being less often included. Mild-to-moderate elevations in ALAT, ASAT, or both were observed in this cohort, as reported [30,31]. Renal abnormalities—elevations in creatinine and declines in creatinine clearance—were also observed. However, COVID-19 itself has been found to be associated with liver and renal injuries [32,33,34]. Thus, attribution of hepatotoxicity or renal toxicity to either remdesivir or the underlying disease is challenging without control cohorts.
In addition to the efficacy results generally reported through a WHO scale dedicated to COVID-19, our study has the advantage of analyzing the SOFA, a score usually used in ICU and reflecting the clinical evolution of this category of critical patients. To our knowledge, no other study has looked at SOFA score as an efficacy feature of remdesivir. Widely established to monitor the patient’s condition in an ICU, this score was used to determine organ function or failure rate over time [23,35] so it might be an attractive approach to assess remdesivir efficacy in standard care. It included components reflective of the respiratory, coagulation, liver, cardiovascular, renal, and central nervous systems. Mine et al., had demonstrated that models based on SOFA scores at admission had performance in predicting mortality in patients in ICUs [36]. Some studies focused on this score during the COVID-19 pandemic to assess patient outcome following mechanical ventilation [37,38]. As a result, a decreased SOFA score over time in mechanically ventilated patients is associated with ICU survival. Moreover, the association between decreased SOFA score over time and survival was independent of comorbidities [38]. SOFA score could function as an effective adjunctive risk stratification tool at admission for critical COVID-19 patients [37] and allowed the identification of patients at risk of further deterioration and mortality. In our study, 65 out of the 85 patients showed an improvement of SOFA score from the 11th day of the follow-up. Although previous studies report on SOFA score in COVID-19, data are still sparse and disparate. Admission SOFA score seems not to be associated with ICU death and some individual components such as respiratory, circulatory, or renal functions of the SOFA score are more critical [38]. In our study, 19 patients had a SOFA score greater than 10 at baseline. Among them, 26% died versus 10% among those with a baseline SOFA score below 10.
The comparison of our results with a control cohort using a propensity score method to balance the cohorts, shows that treatment with remdesivir does not improve survival at D90 for patients under invasive ventilation. Based on the matched and the overlapped samples, there was no effect of remdesivir on the 90-day survival. Our real-life study confirms the literature data. No randomized trial has demonstrated an improvement for remdesivir on mortality in hospitalized COVID-19 patients. In mild to moderate forms of COVID-19, despite the results of the PINETREE trial, remdesivir no longer seems to have a place in the therapeutic arsenal in view of the recent approvals of nirmatrelvir–ritonavir and sotrovimab in this indication [37,38]. Finally, a well-known challenge in these studies is potential missing data. Despite these limitations, real-life study of remdesivir use in patients with severe COVID-19 pneumonia is a major issue to confirm the results of controlled clinical trials with data from routine care, thus reflecting current practice.
5. Conclusions
SOFA score appeared to be an attractive approach to assess remdesivir efficacy and stratify its utilization or not in critically ill patients with COVID-19. This study brings a new clinical benchmark for therapeutic decision making and supports the use of remdesivir for some hospitalized COVID-19 patients with a SOFA score below 10 but this remains to be demonstrated in a randomized controlled trial.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11216545/s1, Table S1: Control and remdesivir cohorts.
Author Contributions
Conceptualization, J.Z., S.C., F.P. and M.K.; methodology, J.Z., S.C. and M.K.; software, S.C.; validation, J.Z., M.D., J.-F.L. and M.K.; formal analysis, J.Z., S.C., M.D., J.-F.L. and M.K.; investigation, M.K., J.-F.L., C.-E.L., J.G., F.E., G.M.-B., B.S., R.C.-J., P.M., A.C., P.A., M.L., L.A., J.-F.H., P.R., L.B., M.F.-R., M.V., A.V., L.M.D., J.-D.K., C.C., F.G., F.B., S.D., Y.B., N.A., C.G. and R.B.; resources, J.Z.; data curation, J.Z. and M.K.; writing—original draft preparation, J.Z. and M.D.; writing—review and editing, J.Z., J.-F.L. and M.K.; visualization, S.C.; supervision, M.K.; project administration, J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “APHP: COVID-19 research grant from APHP center (mecenat collecte crise covid).”
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of AP-HP Center Research Ethics Committee. IRB registration number #00011928.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to all principal investigators of the REMDECO-19 study. The authors also thank the Mécénat Assistance Publique-Hôpitaux Paris. Centre-Université de Paris.
Conflicts of Interest
F.B. reports consulting and lecture fees from MSD and lecture fees from Biomérieux, outside the scope of the submitted work. J.Z. reports consulting fees from AstraZeneca, outside the scope of the submitted work. J.G. reports grants from Gilead and ViiVHealthcare and personal fees from MSD, Gilead, Astra Zeneca, Roche, and ViiVHealthcare, outside the scope of the submitted work. F.P. reports consulting fees and grants from Alexion Pharma and consulting and personal fees from Gilead outside the scope of the submitted work.
Abbreviations
| BMI | Body mass index |
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| EAP | Expanded access programs |
| EUA | Emergency use authorization |
| FDA | Food and Drug Administration |
| ANSM | National Agency for the Safety of Medicines and Health Products |
| CT | Computed tomographic |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| IRB | Institutional review board |
| WHO-CPS | World Health Organization Clinical Progression Scale |
| SOFA | Sequential Organ Failure Assessment |
| ECMO | Extracorporeal membrane oxygenation |
| IQR | Interquartile range |
| ATE | Average treatment effect |
| ATT | Average treatment effect in treated |
| ATO | Average treatment effect in the overlap |
| SMD | Standardized mean differences |
| SD | Standard deviation |
| AP-HP | Assistance Publique—Hôpitaux de Paris |
| ICU | Intensive care unit |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Semenzato, L.; Botton, J.; Drouin, J.; Cuenot, F.; Dray-Spira, R.; Weill, A.; Zureik, M. Chronic Diseases, Health Conditions and Risk of COVID-19-Related Hospitalization and in-Hospital Mortality during the First Wave of the Epidemic in France: A Cohort Study of 66 Million People. Lancet Reg. Health—Eur. 2021, 8, 100158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Rizk, J.G.; Forthal, D.N.; Kalantar-Zadeh, K.; Mehra, M.R.; Lavie, C.J.; Rizk, Y.; Pfeiffer, J.P.; Lewin, J.C. Expanded Access Programs, Compassionate Drug Use, and Emergency Use Authorizations during the COVID-19 Pandemic. Drug Discov. Today 2021, 26, 593–603. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Food and Drug Administration. Emergency Use Authorization. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs (accessed on 24 February 2021).
- Lo, M.K.; Jordan, R.; Arvey, A.; Sudhamsu, J.; Shrivastava-Ranjan, P.; Hotard, A.L.; Flint, M.; McMullan, L.K.; Siegel, D.; Clarke, M.O.; et al. GS-5734 and Its Parent Nucleoside Analog Inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017, 7, 43395. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative Therapeutic Efficacy of Remdesivir and Combination Lopinavir, Ritonavir, and Interferon Beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Burwick, R.M.; Yawetz, S.; Stephenson, K.E.; Collier, A.-R.Y.; Sen, P.; Blackburn, B.G.; Kojic, E.M.; Hirshberg, A.; Suarez, J.F.; Sobieszczyk, M.E.; et al. Compassionate Use of Remdesivir in Pregnant Women with Severe COVID-19. Clin. Infect. Dis. 2021, 73, e3996–e4004. [Google Scholar] [CrossRef]
- Méndez-Echevarría, A.; Pérez-Martínez, A.; Gonzalez Del Valle, L.; Ara, M.F.; Melendo, S.; Ruiz de Valbuena, M.; Vazquez-Martinez, J.L.; Morales-Martínez, A.; Remesal, A.; Sándor-Bajusz, K.A.; et al. Compassionate Use of Remdesivir in Children with COVID-19. Eur. J. Pediatr. 2021, 180, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 24 February 2021).
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.-P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus Standard of Care versus Standard of Care Alone for the Treatment of Patients Admitted to Hospital with COVID-19 (DisCoVeRy): A Phase 3, Randomised, Controlled, Open-Label Trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Barratt-Due, A.; Olsen, I.C.; Nezvalova-Henriksen, K.; Kåsine, T.; Lund-Johansen, F.; Hoel, H.; Holten, A.R.; Tveita, A.; Mathiessen, A.; Haugli, M.; et al. Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19: A Randomized Trial. Ann. Intern. Med. 2021, 174, 1261–1269. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; Australian and New Zealand Intensive Care Society (ANZICS); Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and QSOFA Score for In-Hospital Mortality among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Hazard, D.; Kaier, K.; von Cube, M.; Grodd, M.; Bugiera, L.; Lambert, J.; Wolkewitz, M. Joint Analysis of Duration of Ventilation, Length of Intensive Care, and Mortality of COVID-19 Patients: A Multistate Approach. BMC Med. Res. Methodol. 2020, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Llitjos, J.-F.; Bredin, S.; Lascarrou, J.-B.; Soumagne, T.; Cojocaru, M.; Leclerc, M.; Lepetit, A.; Gouhier, A.; Charpentier, J.; Piton, G.; et al. Increased Susceptibility to Intensive Care Unit-Acquired Pneumonia in Severe COVID-19 Patients: A Multicentre Retrospective Cohort Study. Ann. Intensive Care 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.M.; Hernán, M.A.; Brumback, B. Marginal Structural Models and Causal Inference in Epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Mao, H.; Li, L.; Greene, T. Propensity Score Weighting Analysis and Treatment Effect Discovery. Stat. Methods Med. Res. 2019, 28, 2439–2454. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic Efficacy of the Small Molecule GS-5734 against Ebola Virus in Rhesus Monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Mulangu, S.; Dodd, L.E.; Davey, R.T.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of Acute Kidney Injury in Patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute Kidney Injury in Critically Ill Patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver Injury in COVID-19: Management and Challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Minne, L.; Abu-Hanna, A.; de Jonge, E. Evaluation of SOFA-Based Models for Predicting Mortality in the ICU: A Systematic Review. Crit. Care 2008, 12, R161. [Google Scholar] [CrossRef]
- Liu, S.; Yao, N.; Qiu, Y.; He, C. Predictive Performance of SOFA and QSOFA for In-Hospital Mortality in Severe Novel Coronavirus Disease. Am. J. Emerg. Med. 2020, 38, 2074–2080. [Google Scholar] [CrossRef]
- Bels, J.L.M.; van Kuijk, S.M.J.; Ghossein-Doha, C.; Tijssen, F.H.; van Gassel, R.J.J.; Tas, J.; Collaborators, M.; Schnabel, R.M.; Aries, M.J.H.; van de Poll, M.C.G.; et al. Decreased Serial Scores of Severe Organ Failure Assessments Are Associated with Survival in Mechanically Ventilated Patients; the Prospective Maastricht Intensive Care COVID Cohort. J. Crit. Care 2020, 62, 38–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).