Personal Activity Intelligence and Ischemic Heart Disease in a Healthy Population: China Kadoorie Biobank Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Measurements and Questionnaire-Based Information
2.3. Personal Activity Intelligence
2.4. End-Points and Follow-Up
2.5. Statistical Analyses
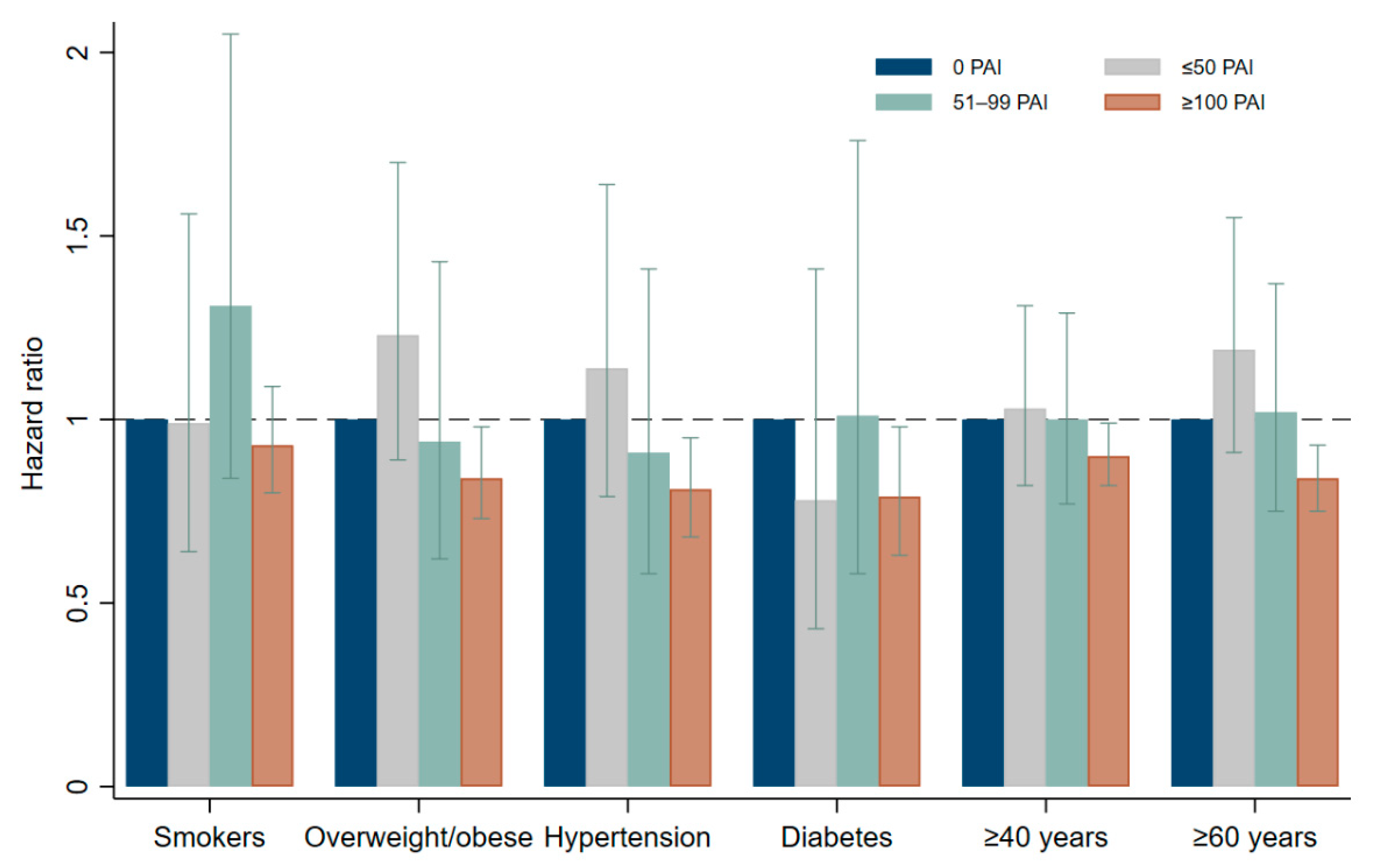
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Zeng, X.; Wang, H.; Yin, P.; Wang, L.; Liu, Y.; Liu, J.; Qi, J.; Ran, S.; et al. Burden of Cardiovascular Diseases in China, 1990–2016. JAMA Cardiol. 2019, 4, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Jiang, C.; Wang, M.; Cai, R.; Zhang, Y.; He, Z.; Wang, H.; Wu, D.; Wang, F.; Liu, X.; et al. BMI, leisure-time physical activity, and physical fitness in adults in China: Results from a series of national surveys, 2000–2014. Lancet Diabetes Endocrinol. 2016, 4, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Sallis, R.; Franklin, B.; Joy, L.; Ross, R.; Sabgir, D.; Stone, J. Strategies for Promoting Physical Activity in Clinical Practice. Prog. Cardiovasc. Dis. 2014, 57, 375–386. [Google Scholar] [CrossRef]
- Bauman, A.E.; Reis, R.S.; Sallis, J.F.; Wells, J.C.; Loos, R.J.F.; Martin, B.W.; Lancet Physical Activity Series Working Group. Correlates of physical activity: Why are some people physically active and others not? Lancet 2012, 380, 258–271. [Google Scholar] [CrossRef]
- Stalsberg, R.; Pedersen, A.V. Are Differences in Physical Activity across Socioeconomic Groups Associated with Choice of Physical Activity Variables to Report? Int. J. Environ. Res. Public Health 2018, 15, 922. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, S.; Trott, M.; Tully, M.; Shin, J.; Barnett, Y.; Butler, L.; McDermott, D.; Schuch, F.; Smith, L. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000960. [Google Scholar] [CrossRef]
- Kohl, H.W., III; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Lancet Physical Activity Series Working Group. The Pandemic of Physical Inactivity: Global Action for Public Health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Woessner, M.N.; Tacey, A.; Levinger-Limor, A.; Parker, A.G.; Levinger, P.; Levinger, I. The Evolution of Technology and Physical Inactivity: The Good, the Bad, and the Way Forward. Front. Public Health 2021, 9, 655491. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Lee, J.E. Emerging Technology in Promoting Physical Activity and Health: Challenges and Opportunities. J. Clin. Med. 2019, 8, 1830. [Google Scholar] [CrossRef] [Green Version]
- Chaddha, A.; Jackson, E.A.; Richardson, C.; Franklin, B.A. Technology to Help Promote Physical Activity. Am. J. Cardiol. 2016, 119, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Pepera, G.; Tribali, M.-S.; Batalik, L.; Petrov, I.; Papathanasiou, J. Epidemiology, risk factors and prognosis of cardiovascular disease in the Coronavirus Disease 2019 (COVID-19) pandemic era: A systematic review. Rev. Cardiovasc. Med. 2022, 23, 28. [Google Scholar] [CrossRef]
- Stefanakis, M.; Batalik, L.; Papathanasiou, J.; Dipla, L.; Antoniou, V.; Pepera, G. Exercise-based cardiac rehabilitation programs in the era of COVID-19: A critical review. Rev. Cardiovasc. Med. 2021, 22, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Nauman, J.; Franklin, B.A.; Nes, B.M.; Sallis, R.E.; Sawada, S.S.; Marinović, J.; Stensvold, D.; Lavie, C.J.; Tari, A.R.; Wisløff, U. Association Between Personal Activity Intelligence and Mortality: Population-Based China Kadoorie Biobank Study. Mayo Clin. Proc. 2021, 97, 668–681. [Google Scholar] [CrossRef]
- Nes, B.M.; Gutvik, C.R.; Lavie, C.J.; Nauman, J.; Wisløff, U. Personalized Activity Intelligence (PAI) for Prevention of Cardiovascular Disease and Promotion of Physical Activity. Am. J. Med. 2017, 130, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Nauman, J.; Nes, B.M.; Zisko, N.; Revdal, A.; Myers, J.; Kaminsky, L.A.; Wisløff, U. Personal Activity Intelligence (PAI): A new standard in activity tracking for obtaining a healthy cardiorespiratory fitness level and low cardiovascular risk. Prog. Cardiovasc. Dis. 2019, 62, 179–185. [Google Scholar] [CrossRef]
- Kieffer, S.K.; Nauman, J.; Syverud, K.; Selboskar, H.; Lydersen, S.; Ekelund, U.; Wisløff, U. Association between Personal Activity Intelligence (PAI) and body weight in a population free from cardiovascular disease—The HUNT study. Lancet Reg. Health Eur. 2021, 5, 100091. [Google Scholar] [CrossRef]
- Nauman, J.; Sui, X.; Lavie, C.J.; Wen, C.P.; Laukkanen, J.A.; Blair, S.N.; Dunn, P.; Arena, R.; Wisløff, U. Personal activity intelligence and mortality—Data from the Aerobics Center Longitudinal Study. Prog. Cardiovasc. Dis. 2020, 64, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nauman, J.; Arena, R.; Zisko, N.; Sui, X.; Lavie, C.J.; Laukkanen, J.A.; Blair, S.N.; Dunn, P.; Nes, B.M.; Tari, A.R.; et al. Temporal changes in personal activity intelligence and mortality: Data from the aerobics center longitudinal study. Prog. Cardiovasc. Dis. 2020, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, S.K.; Croci, I.; Wisløff, U.; Nauman, J. Temporal Changes in a Novel Metric of Physical Activity Tracking (Personal Activity Intelligence) and Mortality: The HUNT Study, Norway. Prog. Cardiovasc. Dis. 2018, 62, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieffer, S.K.; Zisko, N.; Coombes, J.; Nauman, J.; Wisløff, U. Personal Activity Intelligence and Mortality in Patients with Cardiovascular Disease: The HUNT Study. Mayo Clin. Proc. 2018, 93, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Zisko, N.; Skjerve, K.N.; Tari, A.R.; Sandbakk, S.B.; Wisløff, U.; Nes, B.M.; Nauman, J. Personal Activity Intelligence (PAI), Sedentary Behavior and Cardiovascular Risk Factor Clustering—The HUNT Study. Prog. Cardiovasc. Dis. 2017, 60, 89–95. [Google Scholar] [CrossRef]
- Tari, A.R.; Selbæk, G.; Franklin, B.A.; Bergh, S.; Skjellegrind, H.; Sallis, R.E.; Bosnes, I.; Stordal, E.; Ziaei, M.; Lydersen, S.; et al. Temporal changes in personal activity intelligence and the risk of incident dementia and dementia related mortality: A prospective cohort study (HUNT). eClinicalMedicine 2022, 52, 101607. [Google Scholar] [CrossRef]
- Yang, G.H.; Stroup, D.F.; Thacker, S.B. National public health surveillance in China: Implications for public health in China and the United States. Biomed. Environ. Sci. 1997, 10, 1–13. [Google Scholar]
- Liu, S.; Wu, X.; Lopez, A.D.; Wang, L.; Cai, Y.; Page, A.; Yin, P.; Liu, Y.; Xiaoling, W.; Liu, J.; et al. An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull. World Health Organ. 2015, 94, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lee, L.; Chen, J.; Collins, R.; Wu, F.; Guo, Y.; Linksted, P.; Peto, R. Cohort Profile: The Kadoorie Study of Chronic Disease in China (KSCDC). Int. J. Epidemiol. 2005, 34, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Chen, J.; Collins, R.; Guo, Y.; Peto, R.; Wu, F.; Li, L.; on behalf of the China Kadoorie Biobank (CKB) collaborative group. China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011, 40, 1652–1666. [Google Scholar] [CrossRef] [Green Version]
- Lewington, S.; Li, L.; Sherliker, P.; Guo, Y.; Millwood, I.; Bian, Z.; Whitlock, G.; Yang, L.; Collins, R.; Chen, J.; et al. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: The China Kadoorie Biobank. J. Hypertens. 2012, 30, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med. Sci. Sport. Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, D.A.; Du, H.; Clarke, R.; Guo, Y.; Yang, L.; Bian, Z.; Chen, Y.; Millwood, I.; Yu, C.; He, P.; et al. Association of Physical Activity With Risk of Major Cardiovascular Diseases in Chinese Men and Women. JAMA Cardiol. 2017, 2, 1349–1358. [Google Scholar] [CrossRef]
- Tu, H.; Liao, X.; Schuller, K.; Cook, A.; Fan, S.; Lan, G.; Lu, Y.; Yuan, Z.; Moore, J.B.; Maddock, J.E. Insights from an observational assessment of park-based physical activity in Nanchang, China. Prev. Med. Rep. 2015, 2, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Smith, C.F.; Wilkins, E.; Rayner, M.; et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, M.; O’Donovan, G.; Stamatakis, E. Association between physical activity and sub-types of cardiovascular disease death causes in a general population cohort. Eur. J. Epidemiol. 2018, 34, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Joseph, G.; Marott, J.L.; Torp-Pedersen, C.; Biering-Sørensen, T.; Nielsen, G.; Christensen, A.-E.; Johansen, M.B.; Schnohr, P.; Sogaard, P.; Mogelvang, R. Dose-Response Association Between Level of Physical Activity and Mortality in Normal, Elevated, and High Blood Pressure. Hypertension 2019, 74, 1307–1315. [Google Scholar] [CrossRef]
- Sluik, D.; Buijsse, B.; Muckelbauer, R.; Kaaks, R.; Teucher, B.; Johnsen, N.F.; Tjønneland, A.; Overvad, K.; Østergaard, J.N.; Amiano, P.; et al. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch. Intern. Med. 2012, 172, 1285–1295. [Google Scholar] [CrossRef]
- Liu, J.; Qi, J.; Yin, P.; Liu, Y.; You, J.; Lin, L.; Zhou, M.; Wang, L. Cardiovascular Disease Mortality—China, 2019. China CDC Wkly. 2021, 3, 323–326. [Google Scholar] [CrossRef]
- Patel, M.S.; Asch, D.A.; Volpp, K.G. Wearable Devices as Facilitators, Not Drivers, of Health Behavior Change. JAMA 2015, 313, 459–460. [Google Scholar] [CrossRef]
- Coombes, J.S.; Keating, S.E.; Mielke, G.I.; Fassett, R.G.; Coombes, B.K.; O’Leary, K.P.; Cox, E.R.; Burton, N.W. Personal Activity Intelligence (PAI) e-Health Program in People with Type 2 Diabetes: A Pilot Randomized Controlled Trial. Med. Sci. Sports Exerc. 2021, 54, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.L.; Hing, W.; Coombes, J.S.; Gough, S.; Climstein, M.; Adsett, G.; Jayasinghe, R.; Furness, J. Effect of personal activity intelligence (PAI) monitoring in the maintenance phase of cardiac rehabilitation: A mixed methods evaluation. BMC Sports Sci. Med. Rehabil. 2021, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary Patterns and the Risk of Coronary Heart Disease in Women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [Green Version]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef]
- Antoniou, V.; Davos, C.H.; Kapreli, E.; Batalik, L.; Panagiotakos, D.B.; Pepera, G. Effectiveness of Home-Based Cardiac Rehabilitation, Using Wearable Sensors, as a Multicomponent, Cutting-Edge Intervention: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3772. [Google Scholar] [CrossRef]
- Saeidi, M.; Soroush, A.; Komasi, S.; Heydarpour, B.; Carrozzino, D.; Fulcheri, M.; Marchettini, P.; Rabboni, M.; Compare, A. Coronary artery bypass graft patients’ perception about the risk factors of illness: Educational necessities of second prevention. Ann. Card. Anaesth. 2017, 20, 303–308. [Google Scholar] [CrossRef]
| Inactive (n = 324,166) | ≤50 (n = 10,393) | 51–99 (n = 8420) | ≥100 (n = 100,813) | p-Value a | |
|---|---|---|---|---|---|
| Female sex, no. (%) | 199,490 (61.5) | 6183 (59.5) | 5355 (63.6) | 55,235 (54.8) | <0.001 |
| Age, mean (SD), y | 49.9 (10.1) | 50.7 (10.1) | 51.3 (10.4) | 54.3 (10.6) | <0.001 |
| Urban residence, no. (%) | 116,708 (36.0) | 7222 (69.5) | 4958 (58.9) | 64,227 (63.7) | <0.001 |
| Body mass index, no. (%) | |||||
| <18.5 | 13,541 (4.2) | 296 (2.9) | 219 (2.6) | 3331 (3.3) | |
| 18.5–24.9 | 207,932 (64.1) | 6159 (59.3) | 5156 (61.2) | 61,030 (60.5) | |
| 25.0–29.9 | 90,390 (27.9) | 3445 (33.2) | 2680 (31.8) | 31,960 (31.7) | |
| ≥30.0 | 12,303 (3.8) | 493 (4.7) | 365 (4.3) | 4492 (4.5) | <0.001 |
| Systolic blood pressure, mean (SD), mmHg | |||||
| 130.3 (20.9) | 128.2 (20.8) | 129.7 (21.5) | 131.1 (21.0) | <0.001 | |
| Diastolic blood pressure, mean (SD), mmHg | |||||
| 77.8 (11.1) | 77.0 (11.2) | 77.1 (11.3) | 77.3 (11.0) | <0.001 | |
| Education level, no. (%) | |||||
| No formal education | 66,015 (20.4) | 731 (7.0) | 1088 (12.9) | 12,295 (12.2) | |
| Primary school | 107,386 (33.1) | 2051 (19.7) | 1976 (23.5) | 28,579 (28.4) | |
| Middle or high school | 137,522 (42.2) | 6058 (58.3) | 4219 (50.1) | 50,375 (50.0) | |
| College or university | 13,243 (4.1) | 1553 (14.9) | 1137 (13.5) | 9564 (9.5) | <0.001 |
| Smoking status, no. (%) | |||||
| Never | 224,025 (69.1) | 7366 (70.9) | 6170 (73.3) | 67,623 (67.1) | |
| Former | 17,907 (5.5) | 745 (7.2) | 593 (7.0) | 8741 (8.7) | |
| Current | 82,234 (25.4) | 2282 (22.0) | 1657 (19.7) | 24,449 (24.2) | <0.001 |
| Regular alcohol intake, no. (%) | |||||
| Yes | 47,952 (14.8) | 1592 (15.3) | 1142 (13.6) | 15,565 (15.4) | <0.001 |
| Household income, yuan/yr, no. (%) b | |||||
| <10,000 | 93,839 (29.0) | 2045 (19.7) | 2271 (27.0) | 23,524 (23.3) | |
| 10,000–19,999 | 94,746 (29.2) | 3139 (30.2) | 2354 (28.0) | 28,857 (28.6) | |
| 20,000–34,999 | 78,084 (24.1) | 2821 (27.1) | 2007 (23.8) | 28,784 (28.6) | |
| ≥35,000 | 57,497 (17.7) | 2388 (23.0) | 1788 (21.2) | 19,648 (19.5) | <0.001 |
| Self-rated health status, no. (%) | |||||
| Excellent | 60,024 (18.5) | 2012 (19.4) | 1575 (18.7) | 19,405 (19.3) | |
| Good/Fair | 235,365 (72.6) | 7526 (72.4) | 6071 (72.1) | 74,414 (73.8) | |
| Poor | 28,777 (8.9) | 855 (8.2) | 774 (9.2) | 6994 (6.9) | <0.001 |
| Family history of CVD, no. (%) | |||||
| Yes | 63,128 (19.5) | 2518 (24.2) | 2018 (24.0) | 21,731 (21.6) | <0.001 |
| PAI | Person-Years | Deaths | HR | (95% CI) a | HR | (95% CI) b |
|---|---|---|---|---|---|---|
| Inactive | 2,653,654 | 1949 | 1.00 | (Ref.) | 1.00 | (Ref.) |
| ≤50 | 81,979 | 76 | 1.01 | (0.80–1.27) | 1.02 | (0.81–1.29) |
| 51–99 | 67,421 | 63 | 0.95 | (0.74–1.23) | 0.99 | (0.76–1.27) |
| ≥100 | 805,145 | 962 | 0.89 | (0.82–0.98) | 0.91 | (0.83–1.00) |
| <100 | 2,803,054 | 2088 | 1.00 | (Ref.) | 1.00 | (Ref.) |
| ≥100 | 805,145 | 962 | 0.90 | (0.82–0.98) | 0.91 | (0.83–1.00) |
| PAI | Person-Years | Deaths | HR | (95% CI) a | HR | (95% CI) b |
|---|---|---|---|---|---|---|
| Inactive | 2,653,654 | 1226 | 1.00 | (Ref.) | 1.00 | (Ref.) |
| ≤50 | 81,979 | 43 | 1.05 | (0.77–1.42) | 1.02 | (0.75–1.40) |
| 51–99 | 67,421 | 38 | 0.96 | (0.69–1.33) | 0.98 | (0.71–1.37) |
| ≥100 | 805,145 | 501 | 0.93 | (0.83–1.05) | 0.94 | (0.83–1.05) |
| <100 | 2,803,054 | 1307 | 1.00 | (Ref.) | 1.00 | (Ref.) |
| ≥100 | 805,145 | 501 | 0.94 | (0.84–1.05) | 0.93 | (0.83–1.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammer, P.; Tari, A.R.; Franklin, B.A.; Wen, C.-P.; Wisløff, U.; Nauman, J. Personal Activity Intelligence and Ischemic Heart Disease in a Healthy Population: China Kadoorie Biobank Study. J. Clin. Med. 2022, 11, 6552. https://doi.org/10.3390/jcm11216552
Hammer P, Tari AR, Franklin BA, Wen C-P, Wisløff U, Nauman J. Personal Activity Intelligence and Ischemic Heart Disease in a Healthy Population: China Kadoorie Biobank Study. Journal of Clinical Medicine. 2022; 11(21):6552. https://doi.org/10.3390/jcm11216552
Chicago/Turabian StyleHammer, Pål, Atefe R. Tari, Barry A. Franklin, Chi-Pang Wen, Ulrik Wisløff, and Javaid Nauman. 2022. "Personal Activity Intelligence and Ischemic Heart Disease in a Healthy Population: China Kadoorie Biobank Study" Journal of Clinical Medicine 11, no. 21: 6552. https://doi.org/10.3390/jcm11216552
APA StyleHammer, P., Tari, A. R., Franklin, B. A., Wen, C.-P., Wisløff, U., & Nauman, J. (2022). Personal Activity Intelligence and Ischemic Heart Disease in a Healthy Population: China Kadoorie Biobank Study. Journal of Clinical Medicine, 11(21), 6552. https://doi.org/10.3390/jcm11216552





