Prevalence and Treatment Outcomes of Arrhythmias in Patients with Single Ventricle Physiology over the Age of 40 Years
Abstract
:1. Introduction
2. Materials and Methods
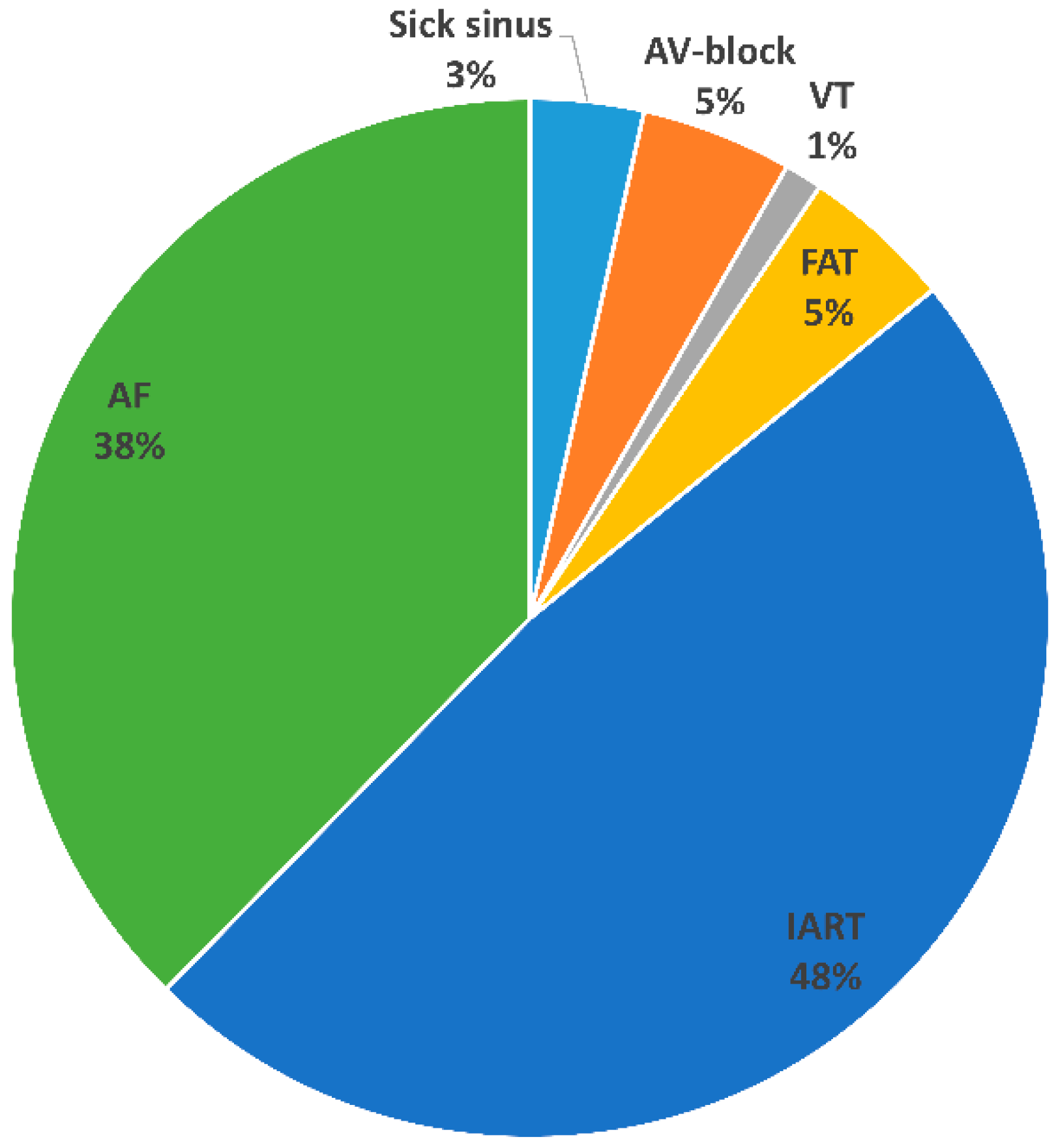
3. Results
3.1. Electrophysiological Study with Ablation
3.2. Direct Current Cardioversion
3.3. Combined Intervention (EPS and DCCV)
3.4. Medical Therapy
3.5. Device Therapy
3.6. Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult with Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, Cir0000000000000696. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Poirier, N.; Mercier, L.A. Univentricular heart. Circulation 2007, 115, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujol, C.; Schiele, S.; Maurer, S.J.; Hock, J.; Fritz, C.; Hager, A.; Ewert, P.; Tutarel, O. Patients with Single-Ventricle Physiology over the Age of 40 Years. J. Clin. Med. 2020, 9, 4085. [Google Scholar] [CrossRef]
- Bolger, A.P.; Sharma, R.; Li, W.; Leenarts, M.; Kalra, P.R.; Kemp, M.; Coats, A.J.S.; Anker, S.D.; Gatzoulis, M.A. Neurohormonal Activation and the Chronic Heart Failure Syndrome in Adults with Congenital Heart Disease. Circulation 2002, 106, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, e51–e156. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transpl. 2019, 38, 879–901. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.K. The spectrum of long-term electrophysiologic abnormalities in patients with univentricular hearts. Congenit. Heart Dis. 2009, 4, 310–317. [Google Scholar] [CrossRef]
- Ghai, A.; Harris, L.; Harrison, D.A.; Webb, G.D.; Siu, S.C. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J. Am. Coll. Cardiol. 2001, 37, 585–592. [Google Scholar] [CrossRef]
- Carins, T.A.; Shi, W.Y.; Iyengar, A.J.; Nisbet, A.; Forsdick, V.; Zannino, D.; Gentles, T.; Radford, D.J.; Justo, R.; Celermajer, D.S.; et al. Long-term outcomes after first-onset arrhythmia in Fontan physiology. J. Thorac. Cardiovasc. Surg. 2016, 152, 1355–1363.e1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, E.A.; Lu, M.; Berul, C.I.; Etheridge, S.P.; Idriss, S.F.; Margossian, R.; Reed, J.H.; Prakash, A.; Sleeper, L.A.; Vetter, V.L.; et al. Arrhythmias in a contemporary fontan cohort: Prevalence and clinical associations in a multicenter cross-sectional study. J. Am. Coll. Cardiol. 2010, 56, 890–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labombarda, F.; Hamilton, R.; Shohoudi, A.; Aboulhosn, J.; Broberg, C.S.; Chaix, M.A.; Cohen, S.; Cook, S.; Dore, A.; Fernandes, S.M.; et al. Increasing Prevalence of Atrial Fibrillation and Permanent Atrial Arrhythmias in Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 70, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Martín de Miguel, I.; Ávila, P. Atrial Fibrillation in Congenital Heart Disease. Eur. Cardiol. 2021, 16, e06. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Madrid, A.; Paul, T.; Abrams, D.; Aziz, P.F.; Blom, N.A.; Chen, J.; Chessa, M.; Combes, N.; Dagres, N.; Diller, G.; et al. Arrhythmias in congenital heart disease: A position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018, 20, 1719–1753. [Google Scholar] [CrossRef]
- Klehs, S.; Schneider, H.E.; Backhoff, D.; Paul, T.; Krause, U. Radiofrequency Catheter Ablation of Atrial Tachycardias in Congenital Heart Disease: Results with Special Reference to Complexity of Underlying Anatomy. Circ. Arrhythm. Electrophysiol. 2017, 10, e005451. [Google Scholar] [CrossRef]
- Grubb, C.S.; Lewis, M.; Whang, W.; Biviano, A.; Hickey, K.; Rosenbaum, M.; Garan, H. Catheter Ablation for Atrial Tachycardia in Adults with Congenital Heart Disease: Electrophysiological Predictors of Acute Procedural Success and Post-Procedure Atrial Tachycardia Recurrence. JACC Clin. Electrophysiol. 2019, 5, 438–447. [Google Scholar] [CrossRef]
- Egbe, A.C.; Connolly, H.M.; Niaz, T.; McLeod, C.J. Outcome of direct current cardioversion for atrial arrhythmia in adult Fontan patients. Int. J. Cardiol 2016, 208, 115–119. [Google Scholar] [CrossRef]
- Bouchardy, J.; Therrien, J.; Pilote, L.; Ionescu-Ittu, R.; Martucci, G.; Bottega, N.; Marelli, A.J. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009, 120, 1679–1686. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.; Whang, W.; Biviano, A.; Hickey, K.; Garan, H.; Rosenbaum, M. Predictors and rates of recurrence of atrial arrhythmias following catheter ablation in adults with congenital heart disease. Congenit. Heart Dis. 2019, 14, 207–212. [Google Scholar] [CrossRef]
- Kürer, C.C.; Tanner, C.S.; Vetter, V.L. Electrophysiologic findings after Fontan repair of functional single ventricle. J. Am. Coll. Cardiol. 1991, 17, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Khairy, P.; Fernandes, S.M.; Mayer, J.E., Jr.; Triedman, J.K.; Walsh, E.P.; Lock, J.E.; Landzberg, M.J. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008, 117, 85–92. [Google Scholar] [CrossRef] [PubMed]
| All | with Arrhythmias | without Arrhythmias | p | |
|---|---|---|---|---|
| n (%) | 49 (100.0) | 29 (59.2) | 20 (40.8) | |
| Age (years) | 49.2 ± 6.4 | 47.5 ± 4.6 | 51.6 ± 7.8 | 0.02 |
| Female, n (%) | 19 (38.8) | 11 (37.9) | 8 (40) | 0.88 |
| Congenital heart defect, n (%) | ||||
| Double-inlet left ventricle | 23 (46.9) | 9 (31) | 14 (70) | 0.007 |
| Tricuspid atresia | 20 (40.8) | 17 (58.6) | 3 (15) | 0.002 |
| Miscellaneous ‡ | 6 (12.2) | 3 (10.3) | 3 (15) | 0.62 |
| Type of cardiac surgery, n (%) | ||||
| Atrio-pulmonary connection | 20 (40.8) | 15 (51.7) | 5 (25) | 0.06 |
| TCPC | 11 (22.4) | 7 (24.1) | 4 (20) | 0.8 |
| Other surgical intervention † | 9 (18.4) | 4 (13.8) | 5 (25) | 0.32 |
| No surgical interventions (native) | 9 (18.4) | 3 (10.3) * | 6 (30) ** | 0.08 |
| Age at first Fontan (years) | 45 (91.8) | 16.3 ± 7.1 | 24.3 ± 10.9 | 0.02 |
| Ventricle morphology, n (%) | 0.7 | |||
| Left | 45 (91.8) | 27 (93.1) | 18 (90) | |
| Right | 4 (8.1) | 2 (6.9) | 2 (10) | |
| Cyanosis, n (%) | 27 (55.1) | 14 (48.3) | 13 (65) | 0.25 |
| PHVD | 8 (16.3) | 6 (19.3) | 2 (10) | 0.32 |
| Cardiac medication, n (%) | ||||
| ACE-inhibitors/AT-Blocker | 8 (16.3) | 5 (17.2) | 3 (15) | 0.84 |
| Beta-blocker | 34 (69.4) | 24 (82.8) | 10 (50) | 0.01 |
| MRA | 30 (61.2) | 21 (72.4) | 9 (45) | 0.06 |
| Digoxin | 14 (28.6) | 8 (27.6) | 6 (30) | 0.85 |
| Amiodarone | 17 (58.6) | 17 (58.6) | 0 | <0.0001 |
| Diuretics | 36 (73.4) | 23 (79.3) | 13 (65) | 0.27 |
| Anticoagulation, n (%) | 0.03 | |||
| None | 10 (20.4) | 2 (6.9) | 8 (40) | |
| Vitamin K-Antagonist | 36 (73.5) | 25 (86.2) | 11 (55) | |
| Direct oral anticoagulants | 1 (2.0) | 1 (3.4) | 0 | |
| ASA | 1 (2.0) | 0 | 1 (5) | |
| ASA + Clopidogrel | 1 (2.0) | 1 (3.4) | 0 | |
| Advanced PAH therapies, n (%) | 8 (16.3) | 4 (13.8) | 4 (20) | 0.47 |
| NYHA class, n (%) | 0.25 | |||
| I | 11 (22.4) | 5 (17.2) | 6 (30) | |
| II | 29 (59.2) | 20 (68.9) | 9 (45) | |
| III | 9 (18.4) | 4 (13.8) | 5 (25) | |
| Ventricular function, n (%) | ||||
| Normal | 11 (22.4) | 6 (20.7) | 5 (25) | 0.72 |
| Mild | 17 (34.7) | 8 (27.6) | 9 (45) | 0.2 |
| Moderate | 17 (34.6) | 11 (37.9) | 6 (30) | 0.57 |
| Severe | 4 (8.2) | 4 (13.8) | 0 | 0.08 |
| Patient No. | CHD | Type of Surgery | Sex | Type of Arrhythmia | Procedure | Acute Success | Complications/Anatomical Features |
|---|---|---|---|---|---|---|---|
| 1 | TA | native CHD | female | AF | Isolation PVs; cavo-pulmonary isthmus ablation, left atrial lines | yes | no |
| AF | Re-isolation PV | yes | no | ||||
| AF | Re-isolation PVs | yes | no | ||||
| 2 | TA | Atrio-pulmonary connection | female | IART | CT isthmus ablation; ablation from tricuspid ring to scar tissue in the RA-PA connection | yes | no |
| IART + FAT | Ablation IART around tricuspid valve; overdrive pacing of FAT in PA zone | yes | no | ||||
| 3 | DILV | Atrio-pulmonary connection | male | typical atrial flutter + IART | ablation CT isthmus; ablation IART at the atrial septum | yes | no |
| 4 | single ventricle | Atrio-pulmonary connection | female | IART + AF | ablation line from CT isthmus to left inferior PV; Isolation of PVs; | no | no/azygos continuation |
| IART | ablation line from CT isthmus to left inferior PV | yes | no/azygos continuation | ||||
| 5 | TA | Atrio-pulmonary connection | male | 1. IART 2. IART | 1. ablation around RA-PA conduit 2. ablation line between conduit and lateral atriotomy | no | no |
| multiple IARTs | mechanical termination of IART; ablation at anterolateral conduit side | no | no | ||||
| IART | ablation around RA-RV conduit and lateral RA side | yes | no | ||||
| IART (2 forms) | 1. septal ablation, cranio-posterior side of RA-RV conduit 2. ablation around right PVs | no | no/retrograde access (aorta) for PVs ablation | ||||
| 6 | TA | Atrio-pulmonary connection | female | IART | ablation at CS ostium | yes | no |
| 7 | TA | Atrio-pulmonary connection | male | IART | ablation antero-inferior RA | yes | no |
| 8 | criss-cross heart | Shunts | male | multiple IARTs (3 different) | 1. Ablation in anterior-cranial zone of Björk tunnel 2. Ablation in posterior- cranial zone of Björk tunnel 3. IART not ablatable, ECV | no | no |
| IART | widened ablation in RA | yes | hypotension requiring noradrenalin and O2 supply | ||||
| 9 | TA | TCPC | male | unstable IART | CT isthmus ablation; lateral line ablation around RA scar | yes | no |
| unstable IART | ablation in posterior RA, at transition with SVC | yes | no |
| Patients. | Type of Surgery | Arrhythmias | ||||
|---|---|---|---|---|---|---|
| Atrial fibrillation | IART | Focal atrial tachycardia | Ventricular tachycardia | Total number shocks/patient | ||
| Patient 1 | Native | 4 | 4 | |||
| Patient 2 | Native | 3 | 3 | |||
| Patient 3 | TCPC conversion | 3 | 3 | |||
| Patient 4 | TCPC conversion | 1 | 1 | |||
| Patient 5 | TCPC directly | 1 | 1 | |||
| Patient 6 | TCPC directly | 3 | 3 | |||
| Patient 7 | TCPC directly | 3 | 1 | 4 | ||
| Patient 8 | Atrio-pulmonary connection | 1 | 1 | |||
| Patient 9 | Atrio-pulmonary connection | 2 | 2 | |||
| Patient 10 | Atrio-pulmonary connection | 2 | 2 | |||
| Patient 11 | Atrio-pulmonary connection | 2 | 3 | 1 | 6 | |
| Patient 12 | Atrio-pulmonary connection | 2 | 2 | |||
| Patient 13 | Atrio-pulmonary connection | 1 | 1 | |||
| Patient 14 | Atrio-pulmonary connection | 3 | 1 | 2 | 6 | |
| Patient 15 | Atrio-pulmonary connection | 2 | 2 | |||
| Patient 16 | Atrio-pulmonary connection | 3 | 3 | |||
| Patient 17 | Atrio-pulmonary connection | 1 | 1 | |||
| Patient 18 | Atrio-pulmonary connection | 1 | 2 | 3 | ||
| Patient 19 | Shunts | 1 | 1 | |||
| Patient 20 | Shunts | 3 | 3 |
| Univariate HR (95% C.I.) | p | Multivariate HR (95% C.I.) | p | |
|---|---|---|---|---|
| Age | 0.57 (0.43–0.76) | <0.0001 | 0.58 (0.43–0.78) | <0.0001 |
| Age at first Fontan | 0.93 (0.84–1.02) | 0.13 | ||
| Sex | 1.03 (0.39–2.72) | 0.95 | ||
| Type CHD | 1.29 (0.63–2.63) | 0.49 | ||
| Cirrhosis | 1.7 (0.55–5.48) | 0.34 | ||
| Impaired renal function | 0.99 (0.38–2.62) | 0.98 | ||
| Atrio-pulmonary connection | 1.75 (0.63–4.88) | 0.28 | ||
| Native CHD | 0.33 (0.043–2.55) | 0.29 | ||
| Severely impaired ventricular function | 17.03 (1.54–188.5) | 0.021 | 8.57 (0.75–97.82) | 0.084 |
| PHVD | 1.33 (0.47–3.77) | 0.59 | ||
| Cyanosis | 0.68 (0.26–1.83) | 0.45 | ||
| Arrhythmia before age 40 | 1.54 (0.38–6.3) | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol, C.; Hessling, G.; Telishevska, M.; Schiele, S.; Deisenhofer, I.; Ewert, P.; Tutarel, O. Prevalence and Treatment Outcomes of Arrhythmias in Patients with Single Ventricle Physiology over the Age of 40 Years. J. Clin. Med. 2022, 11, 6568. https://doi.org/10.3390/jcm11216568
Pujol C, Hessling G, Telishevska M, Schiele S, Deisenhofer I, Ewert P, Tutarel O. Prevalence and Treatment Outcomes of Arrhythmias in Patients with Single Ventricle Physiology over the Age of 40 Years. Journal of Clinical Medicine. 2022; 11(21):6568. https://doi.org/10.3390/jcm11216568
Chicago/Turabian StylePujol, Claudia, Gabriele Hessling, Marta Telishevska, Sandra Schiele, Isabel Deisenhofer, Peter Ewert, and Oktay Tutarel. 2022. "Prevalence and Treatment Outcomes of Arrhythmias in Patients with Single Ventricle Physiology over the Age of 40 Years" Journal of Clinical Medicine 11, no. 21: 6568. https://doi.org/10.3390/jcm11216568





