Changes in Stress-Strain Index and Corneal Biomechanics in Granular Corneal Dystrophy
Abstract
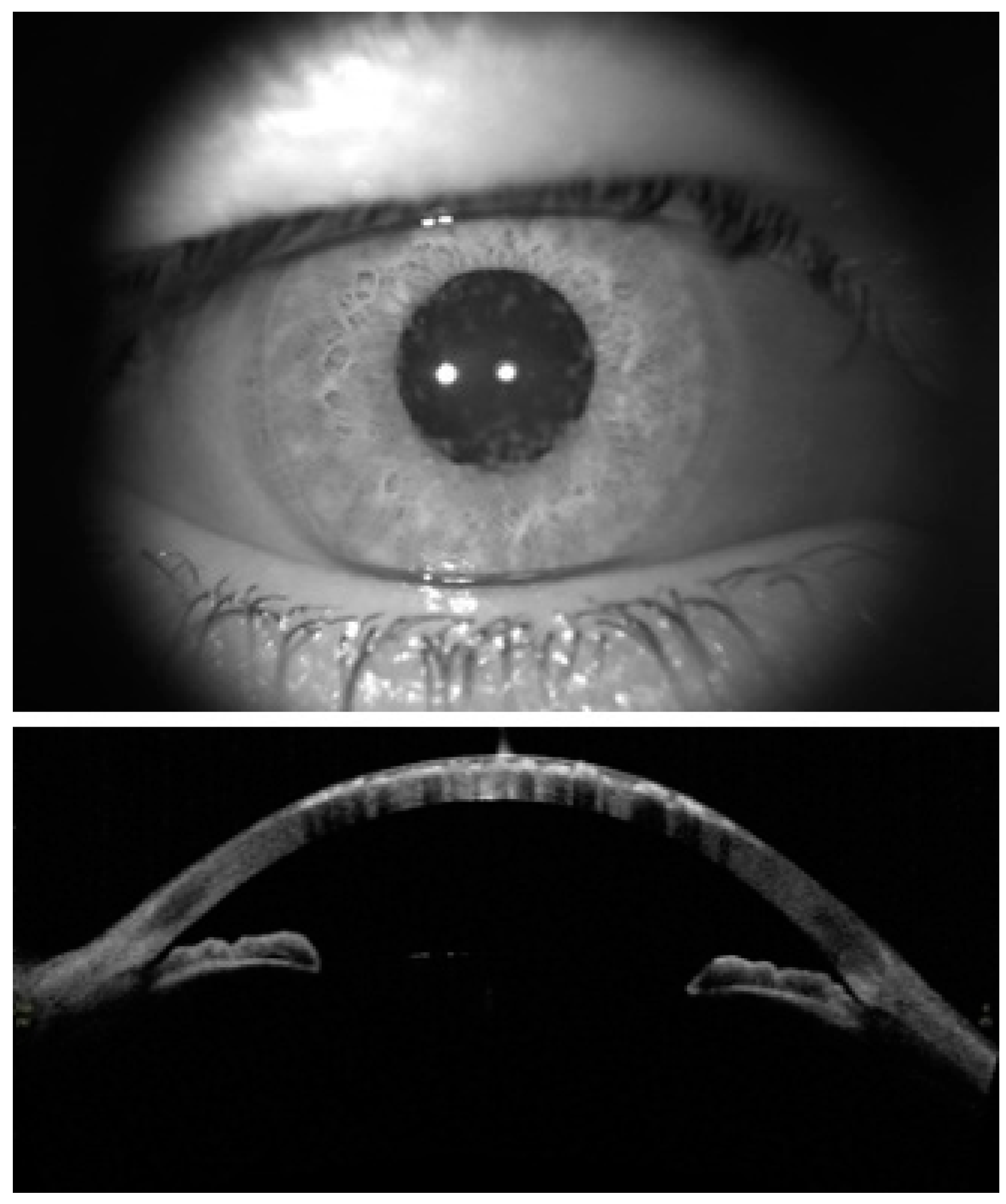
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourges, J.-L. Corneal dystrophies. J. Fr. D’ophtalmol. 2017, 40, e177–e192. [Google Scholar] [CrossRef] [PubMed]
- Poulaki, V.; Colby, K. (Eds.) Genetics of Anterior and Stromal Corneal Dystrophies, Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2008. [Google Scholar]
- Santos, L.N.; Fernandes, B.F.; de Moura, L.R.; Cheema, D.P.; Maloney, S.; Logan, P.; Burnier, M.N., Jr. Histopathologic study of corneal stromal dystrophies: A 10-year experience. Cornea 2007, 26, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.K.; Naumann, G. The frequency of corneal dystrophies requiring keratoplasty in Europe and the USA. Cornea 1987, 6, 209–211. [Google Scholar] [CrossRef]
- Musch, D.C.; Niziol, L.M.; Stein, J.D.; Kamyar, R.M.; Sugar, A. Prevalence of corneal dystrophies in the United States: Estimates from claims data. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6959–6963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feizi, S.; Pakravan, M.; Baradaran-Rafiee, A.-R.; Yazdani, S. Granular corneal dystrophy manifesting after radial keratotomy. Cornea 2007, 26, 1267–1269. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.M.; Gaster, R.N.; Pratt, M.V. Unusual superficial confluent form of granular corneal dystrophy. Ophthalmology 1983, 90, 1507–1511. [Google Scholar] [CrossRef]
- Haddad, R.; Font, R.L.; Fine, B. Unusual superficial variant of granular dystrophy of the cornea. Am. J. Ophthalmol. 1977, 83, 213–218. [Google Scholar] [CrossRef]
- Kamiya, K.; Shimizu, K.; Ohmoto, F. The changes in corneal biomechanical parameters after phototherapeutic keratectomy in eyes with granular corneal dystrophy. Eye 2009, 23, 1790–1795. [Google Scholar] [CrossRef] [Green Version]
- del Buey, M.A.; Cristóbal, J.A.; Ascaso, F.J.; Lavilla, L.; Lanchares, E. Biomechanical properties of the cornea in Fuchs’ corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3199–3202. [Google Scholar] [CrossRef]
- Feizi, S.; Karjou, Z.; Abbasi, H.; Javadi, M.A.; Azari, A.A. Characterization of in vivo biomechanical properties in macular corneal dystrophy. Am. J. Ophthalmol. 2020, 215, 8–13. [Google Scholar] [CrossRef]
- Piñero, D.P.; Alcón, N. In vivo characterization of corneal biomechanics. J. Cataract Refract. Surg. 2014, 40, 870–887. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J. Concepts and misconceptions in corneal biomechanics. J. Cataract Refract. Surg. 2014, 40, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.Q.; Patel, D.V.; McGhee, C.N. Biomechanical responses of healthy and keratoconic corneas measured using a noncontact scheimpflug-based tonometer. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Langenbucher, A.; Seitz, B. Excimer Laser Phototherapeutic Keratectomy for Granular and Lattice Corneal Dystrophy: A Comparative Study; Slack Incorporated Thorofare: San Francisco, CA, USA, 2005. [Google Scholar]
- Fagerholm, P. Phototherapeutic keratectomy: 12 years of experience. Acta Ophthalmol. Scand. 2003, 81, 19–32. [Google Scholar] [CrossRef]
- Stasi, K.; Chuck, R.S. Update on phototherapeutic keratectomy. Curr. Opin. Ophthalmol. 2009, 20, 272–275. [Google Scholar] [CrossRef]
- Rathi, V.M.; Vyas, S.P.; Sangwan, V.S. Phototherapeutic keratectomy. Indian J. Ophthalmol. 2012, 60, 5. [Google Scholar] [CrossRef]
- Szentmáry, N.; Seitz, B.; Langenbucher, A.; Schlötzer-Schrehardt, U.; Hofmann-Rummelt, C.; Naumann, G.O. Histologic and ultrastructural changes in corneas with granular and macular dystrophy after excimer laser phototherapeutic keratectomy. Cornea 2006, 25, 257–263. [Google Scholar] [CrossRef]
- Seitz, B.; Behrens, A.; Fischer, M.; Langenbucher, A.; Naumann, G.O. Morphometric analysis of deposits in granular and lattice corneal dystrophy: Histopathologic implications for phototherapeutic keratectomy. Cornea 2004, 23, 380–385. [Google Scholar] [CrossRef]
- Dogru, M.; Katakami, C.; Nishida, T.; Yamanaka, A. Alteration of the ocular surface with recurrence of granular/avellino corneal dystrophy after phototherapeutic keratectomy: Report of five cases and literature review. Ophthalmology 2001, 108, 810–817. [Google Scholar] [CrossRef]
- Miyata, K.; Takahashi, T.; Tomidokoro, A.; Ono, K.; Oshika, T. Iatrogenic keratectasia after phototherapeutic keratectomy. Br. J. Ophthalmol. 2001, 85, 238. [Google Scholar] [CrossRef]
- Moshirfar, M.; Motlagh, M.N.; Murri, M.S.; Momeni-Moghaddam, H.; Ronquillo, Y.C.; Hoopes, P.C. Advances in biomechanical parameters for screening of refractive surgery candidates: A review of the literature, part III. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 219. [Google Scholar] [PubMed]
- McMonnies, C.W. Assessing corneal hysteresis using the ocular response analyzer. Optom. Vis. Sci. 2012, 89, E343–E349. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-Z.; Lam, A.K. The roles of cornea and axial length in corneal hysteresis among emmetropes and high myopes: A pilot study. Curr. Eye Res. 2015, 40, 282–289. [Google Scholar] [CrossRef]
- Huseynova, T.; Waring IV, G.O.; Roberts, C.; Krueger, R.R.; Tomita, M. Corneal biomechanics as a function of intraocular pressure and pachymetry by dynamic infrared signal and Scheimpflug imaging analysis in normal eyes. Am. J. Ophthalmol. 2014, 157, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Maeda, N.; Ikuno, Y.; Asai, T.; Hara, C.; Nishida, K. Factors associated with corneal deformation responses measured with a dynamic Scheimpflug analyzer. Investig. Ophthalmol. Vis. Sci. 2017, 58, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Ambrósio, R., Jr.; Heidari, H.R.; Maddah, N.; Danesh, Z.; Sabzi, F. Diagnostic Ability of Corneal Shape and Biomechanical Parameters for Detecting Frank Keratoconus. Cornea 2018, 37, 1025–1034. [Google Scholar] [CrossRef]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Roberts, C.J.; Maddah, N.; Ambrósio, R., Jr.; Hosseini, S.R. Corneal biomechanical parameters in keratoconus eyes with abnormal elevation on the back corneal surface only versus both back and front surfaces. Sci. Rep. 2021, 11, 11971. [Google Scholar] [CrossRef]
- Eliasy, A.; Chen, K.J.; Vinciguerra, R.; Lopes, B.T.; Abass, A.; Vinciguerra, P.; Ambrósio, R., Jr.; Roberts, C.J.; Elsheikh, A. Determination of Corneal Biomechanical Behavior in-vivo for Healthy Eyes Using CorVis ST Tonometry: Stress-Strain Index. Front. Bioeng. Biotechnol. 2019, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Elsheikh, A.; Wang, D.; Brown, M.; Rama, P.; Campanelli, M.; Pye, D. Assessment of corneal biomechanical properties and their variation with age. Curr. Eye Res. 2007, 32, 11–19. [Google Scholar] [CrossRef]
- Ethier, C.R.; Johnson, M.; Ruberti, J. Ocular biomechanics and biotransport. Annu. Rev. Biomed. Eng. 2004, 6, 249–273. [Google Scholar] [CrossRef]
- Valbon, B.F.; Ambrósio, R., Jr.; Fontes, B.M.; Luz, A.; Roberts, C.J.; Alves, M.R. Ocular biomechanical metrics by CorVis ST in healthy Brazilian patients. J. Refract. Surg. 2014, 30, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Wang, D.; Pye, D. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. 2007, 23, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, R.; Nakakura, S.; Tabuchi, H.; Murata, H.; Nakao, Y.; Ihara, N.; Rimayanti, U.; Aihara, M.; Kiuchi, Y. The Relationship between Corvis ST Tonometry Measured Corneal Parameters and Intraocular Pressure, Corneal Thickness and Corneal Curvature. PLoS ONE 2015, 10, e0140385. [Google Scholar] [CrossRef] [PubMed]
- Kenia, V.P.; Kenia, R.V.; Pirdankar, O.H. Short term changes in corneal stress-strain index and other corneal biomechanical parameters post-laser in situ keratomileusis. Indian J. Ophthalmol. 2021, 69, 2650–2656. [Google Scholar]
- Vinciguerra, R.; Ambrósio, R., Jr.; Roberts, C.J.; Azzolini, C.; Vinciguerra, P. Biomechanical Characterization of Subclinical Keratoconus Without Topographic or Tomographic Abnormalities. J. Refract. Surg. 2017, 33, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Heravian, J.; Ansari, A.; Shayanfar, H.; Moshirfar, M. Detection ability of corneal biomechanical parameters for early diagnosis of ectasia. Eye 2022. [Google Scholar] [CrossRef]
- Tanikawa, A.; Soma, T.; Miki, A.; Koh, S.; Kitaguchi, Y.; Maeda, N.; Oie, Y.; Kawasaki, S.; Nishida, K. Assessment of the corneal biomechanical features of granular corneal dystrophy type 2 using dynamic ultra-high-speed Scheimpflug imaging. Graefes Arch Clin. Exp. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Wu, M.; Han, J.; Wang, X.; Shao, T.; Wang, Y. The alterations of corneal biomechanics in adult patients with corneal dystrophy. Eye 2022. [Google Scholar] [CrossRef]
- Dawson, D.G.; Grossniklaus, H.E. Biomechanical and wound healing characteristics of corneas after excimer laser keratorefractive surgery: Is there a difference between advanced surface ablation and sub-Bowman’s keratomileusis? J. Refract. Surg. 2008, 24, S90. [Google Scholar]
- Igarashi, S.; Makita, Y.; Hikichi, T.; Mori, F.; Hanada, K.; Yoshida, A. Association of keratoconus and Avellino corneal dystrophy. Br. J. Ophthalmol. 2003, 87, 367–368. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, M.; Sakimoto, T.; Sawa, M.; Katami, M. Familial case of keratoconus with corneal granular dystrophy. Jpn. J. Ophthalmol. 1998, 42, 385–388. [Google Scholar] [CrossRef]
- Wollensak, G.; Green, W.R.; Temprano, J. Keratoconus associated with corneal granular dystrophy in a patient of Italian origin. Cornea 2002, 21, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; D’Ath, P.J.; Parmar, D.N.; Sykakis, E. Keratoconus and granular dystrophy. Case Rep. 2014, 2014, bcr2014205584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadidi, K.; Mosavi, S.A.; Morovvati, S. Familial case of keratoconus with corneal granular dystrophy in a family of Iranian origin. J. Clin. Exp. Ophthalmol. 2014, 5, 2. [Google Scholar]
- Vinciguerra, R.; Ambrósio Jr, R.; Elsheikh, A.; Roberts, C.J.; Lopes, B.; Morenghi, E.; Azzolini, C.; Vinciguerra, P. Detection of keratoconus with a new biomechanical index. J. Refract. Surg. 2016, 32, 803–810. [Google Scholar] [CrossRef] [PubMed]
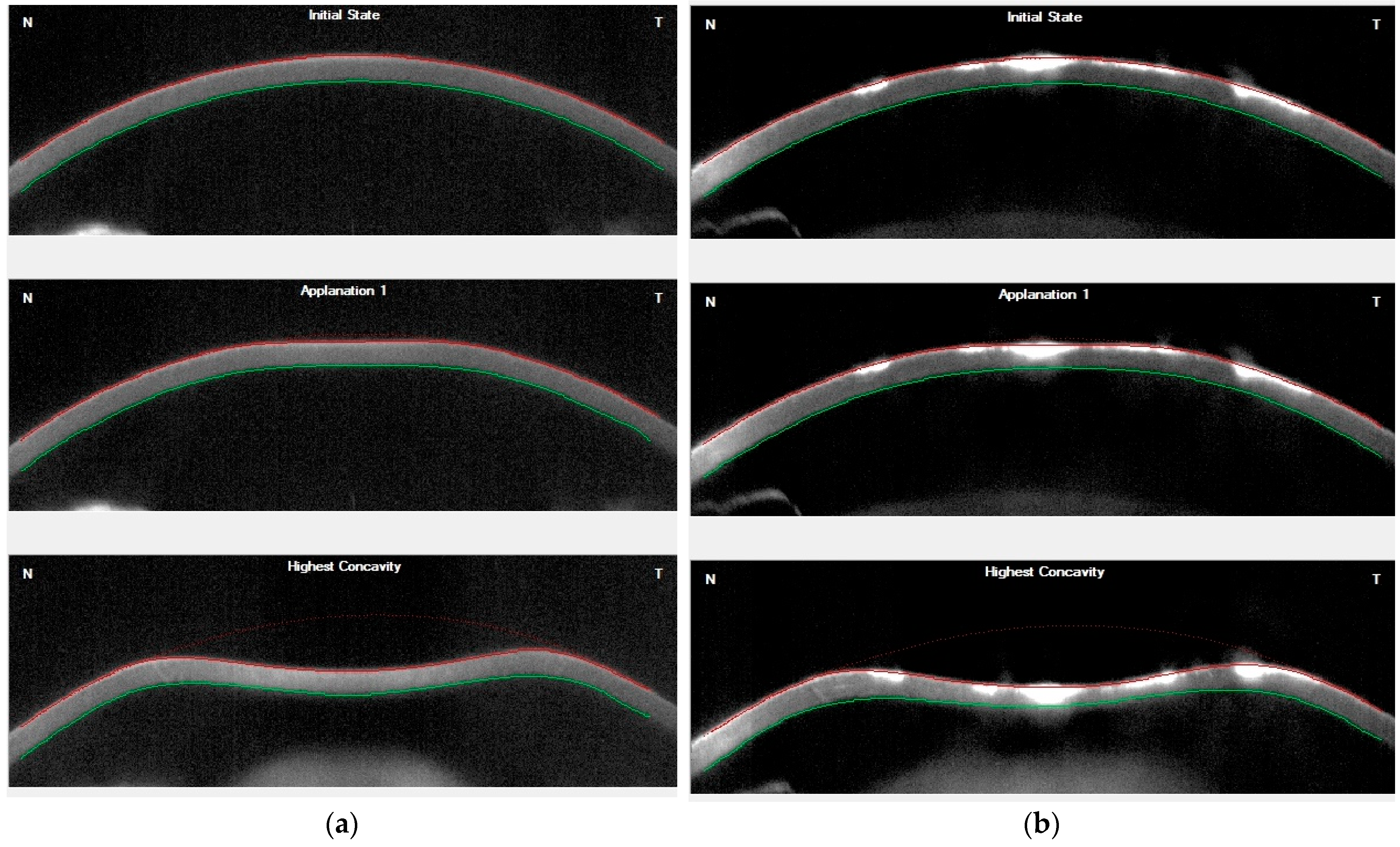
| Abbreviation (Unit) | Complete Name | Description of Specific Corvis ST Parameters |
|---|---|---|
| AL1 (mm) | Applanation length at the first flattening | Cord diameter of the flattened cornea at the first applanation |
| AL2 (mm) | Applanation length at the second flattening | Cord diameter of the flattened cornea at the second applanation |
| A1V (m/s) | First applanation velocity | Inward velocity of the cornea at the first applanation |
| A2V (m/s) | Second applanation velocity | Outward velocity of the cornea at the second applanation |
| A1T (ms) | Time at the first applanation | Time from initiation of air puff until the first applanation |
| A2T (ms) | Time at the second applanation | Time from initiation of air puff until the second applanation |
| A1DA (mm) | Deformation amplitude at the first applanation | Corneal displacement from the natural state until the first applanation |
| A2DA (mm) | Deformation amplitude at the second applanation | Corneal displacement at the second applanation |
| HC, PD (mm) | Highest concavity, Peak distance | Distance between the two peaks of the cornea at the highest concavity phase |
| HC, Radius (mm) | Highest concavity radius | Radius of curvature of the cornea at the highest concavity phase |
| HC-T (ms) | Highest concavity time | Time from initiation of air puff until highest concavity |
| HC, DA (mm) | Highest concavity, Deformation amplitude | Corneal displacement at the highest concavity phase |
| SPA1 (mm Hg/mm) | Stiffness parameter at the first applanation | Resultant pressure [adjusted pressure at A1 (adj AP1)-biomechanically compensated IOP (bIOP)] divided by deflection amplitude at A1 |
| IR (mm−1) | Integrated inverse radius | Area under the inverse concave radius curve |
| DARMax1 | Deformation amplitude ratio at 1 mm | Ratio between DA at the apex and the average of DAs at 1 mm around the center in temporal and nasal directions |
| DAR Max2 | Deformation amplitude ratio at 2 mm | Ratio between DA at the apex and the average of DAs at 2 mm around the center in temporal and nasal directions |
| SSI | Stress-stain index | A parameter to predict the biomechanical behavior of the cornea in terms of the material properties used in its structure |
| CCT (µm) | Central corneal thickness | Corneal thickness at the corneal apex |
| IOPnct (mmHg) | Non-corrected IOP | Intraocular pressure non-corrected based on the corneal characteristic and age |
| bIOP (mmHg) | Biomechanically corrected intraocular pressure | Estimates IOP based on an algorithm that reduces the confounding effects of corneal characteristics and aging |
| Variables | Mean ± SD (95% CI) | p-Value | |
|---|---|---|---|
| GCD (n = 12 Eyes) | Control (n = 20 Eyes) | ||
| AL1 (mm) | 2.55 ± 0.24 (2.45 to 2.65) | 2.58 ± 0.21 (2.48 to 2.68) | 0.704 |
| AL2 (mm) | 3.06 ± 0.49 (2.64 to 3.49) | 3.46 ± 1.09 (3.05 to 3.87) | 0.193 |
| AV1 (m/s) | 0.15 ± 0.02 (0.14 to 0.15) | 0.13 ± 0.02 (0.12 to 0.13) | <0.001 |
| AV2 (m/s) | −0.26 ± 0.06 (−0.28 to −0.25) | −0.27 ± 0.05 (−0.29 to −0.25) | 0.872 |
| AT1 (ms) | 7.33 ± 0.66 (7.26 to 7.39) | 7.47 ± 0.36 (7.41 to 7.53) | 0.002 |
| AT2 (ms) | 21.02 ± 0.61 (20.89 to 21.14) | 21.08 ± 0.29 (20.96 to 21.21) | 0.471 |
| A1DA (mm) | 0.15 ± 0.02 (0.14 to 0.15) | 0.14 ± 0.01 (0.14 to 0.15) | 0.108 |
| A2DA (mm) | 0.43 ± 0.05 (0.40 to 0.46) | 0.40 ± 0.07 (0.37 to 0.43) | 0.144 |
| HC, PD (mm) | 4.99 ± 0.45 (4.90 to 5.08) | 4.99 ± 0.27 (4.90 to 5.07) | 0.955 |
| HC, R (mm) | 7.42 ± 0.76 (6.99 to 7.85) | 8.20 ± 1.08 (7.79 to 8.62) | 0.014 |
| HC-T (ms) | 16.59 ± 0.51 (16.38 to 16.80) | 16.60 ± 0.35 (16.39 to 16.81) | 0.948 |
| HC, DA (mm) | 1.05 ± 0.16 (1.01 to 1.09) | 1.00 ± 0.11 (0.97 to 1.04) | 0.123 |
| SP-A1 (mm Hg/mm) | 104.03 ± 19.29 (99.35 to 108.72) | 110.45 ± 17.13 (105.90 to 115.01) | 0.061 |
| IR (mm−1) | 7.48 ± 1.01 (7.18 to 7.78) | 6.80 ± 1.22 (6.51 to 7.09) | 0.003 |
| DAR Max2 | 3.77 ± 0.42 (3.66 to 3.89) | 3.65 ± 0.49 (3.54 to 3.76) | 0.154 |
| DAR Max1 | 1.51 ± 0.06 (1.49 to 1.53) | 1.50 ± 0.06 (1.48 to 1.51) | 0.259 |
| SSI | 1.09 ± 0.20 (1.00 to 1.17) | 1.21 ± 0.17 (1.13 to 1.30) | 0.04 |
| CBI | 0.33 ± 0.03 (0.31 to 0.35) | 0.02 ± 0.03 (0.01 to 0.03) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, H.; Momeni-Moghaddam, H.; Jadidi, K.; Pirhadi, S.; Moshirfar, M. Changes in Stress-Strain Index and Corneal Biomechanics in Granular Corneal Dystrophy. J. Clin. Med. 2022, 11, 6571. https://doi.org/10.3390/jcm11216571
Heidari H, Momeni-Moghaddam H, Jadidi K, Pirhadi S, Moshirfar M. Changes in Stress-Strain Index and Corneal Biomechanics in Granular Corneal Dystrophy. Journal of Clinical Medicine. 2022; 11(21):6571. https://doi.org/10.3390/jcm11216571
Chicago/Turabian StyleHeidari, Hamidreza, Hamed Momeni-Moghaddam, Khosrow Jadidi, Shiva Pirhadi, and Majid Moshirfar. 2022. "Changes in Stress-Strain Index and Corneal Biomechanics in Granular Corneal Dystrophy" Journal of Clinical Medicine 11, no. 21: 6571. https://doi.org/10.3390/jcm11216571





