Reticulated Platelets and Their Relationship with Endothelial Progenitor Cells during the Acute Phase of ST-Elevation Myocardial Infarction
Abstract
:1. Introduction
2. Methods
2.1. Blood Sampling
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Lai, R.; Zhang, Y.; Shi, D. The Prognostic Value of Reticulated Platelets in Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2020, 7, 578041. [Google Scholar] [PubMed]
- Heinisch, P.P.; Bello, C.; Emmert, M.Y.; Carrel, T.; Dreßen, M.; Hörer, J.; Winkler, B.; Luedi, M.M. Endothelial progenitor cells as biomarkers of cardiovascular pathologies: A narrative review. Cells 2022, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Leshem-Lev, D.; Omelchenko, A.; Perl, L.; Kornowski, R.; Battler, A.; Lev, E.I. Exposure to platelets promotes functional properties of endothelial progenitor cells. J. Thromb. Thrombolysis 2010, 30, 398–403. [Google Scholar] [PubMed]
- Raz, O.; Lev, D.L.; Battler, A.; Lev, E.I. Pathways mediating the interaction between endothelial progenitor cells (EPCs) and platelets. PLoS ONE 2014, 9, e95156. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Honma, T.; Katoh, A.; Sasaki, K.; Shimada, T.; Oike, Y.; Imaizumi, T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001, 103, 2776–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojakowski, W.; Tendera, M.; Michałowska, A.; Majka, M.; Kucia, M.; Maślankiewicz, K.; Wyderka, R.; Ochała, A.; Ratajczak, M.Z. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation 2004, 110, 3213–3220. [Google Scholar]
- Marfella, R.; Rizzo, M.R.; Siniscalchi, M.; Paolisso, P.; Barbieri, M.; Sardu, C.; Savinelli, A.; Angelico, N.; Del Gaudio, S.; Esposito, N.; et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: Effects on myocardial salvage. Int. J. Cardiol. 2013, 168, 3954–3962. [Google Scholar]
- D’Onofrio, N.; Sardu, C.; Paolisso, P.; Minicucci, F.; Gragnano, F.; Ferraraccio, F.; Panarese, I.; Scisciola, L.; Mauro, C.; Rizzo, M.R.; et al. MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J. Cell. Physiol. 2020, 235, 1438–1452. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- McCabe, D.J.H.; Harrison, P.; Sidhu, P.S.; Brown, M.M.; Machin, S.J. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br. J. Haematol. 2004, 126, 861–869. [Google Scholar] [CrossRef]
- Lakkis, N.; Dokainish, H.; Abuzahra, M.; Tsyboulev, V.; Jorgensen, J.; Ponce De Leon, A.; Saleem, A. Reticulated platelets in acute coronary syndrome: A marker of platelet activity. J. Am. Coll. Cardiol. 2004, 44, 2091–2093. [Google Scholar] [CrossRef] [Green Version]
- Ault, K.A.; Rinder, H.M.; Mitchell, J.; Carmody, M.B.; Vary, C.P.; Hillman, R.S. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am. J. Clin. Pathol. 1992, 98, 637–646. [Google Scholar] [CrossRef]
- Perl, L.; Matatov, Y.; Koronowski, R.; Lev, E.I.; Solodky, A. Prognostic significance of reticulated platelet levels in diabetic patients with stable coronary artery disease. Platelets 2020, 31, 1012–1018. [Google Scholar] [CrossRef]
- Anetsberger, A.; Blobner, M.; Haller, B.; Schmid, S.; Umgelter, K.; Hager, T.; Langgartner, C.; Kochs, E.F.; Laugwitz, K.-L.; Jungwirth, B.; et al. Immature platelets as a novel biomarker for adverse cardiovascular events in patients after non-cardiac surgery. Thromb. Haemost. 2017, 117, 1887–1895. [Google Scholar] [CrossRef]
- Perl, L.; Lerman-Shivek, H.; Rechavia, E.; Vaduganathan, M.; Leshem-Lev, D.; Zemer-Wassercug, N.; Dadush, O.; Codner, P.; Bental, T.; Battler, A.; et al. Response to prasugrel and levels of circulating reticulated platelets in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.-J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Mazzaferri, E.; et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef]
- Coenen, D.M.; Heinzmann, A.C.A.; Karel, M.F.A.; Cosemans, J.M.E.M.; Koenen, R.R. The multifaceted contribution of platelets in the emergence and aftermath of acute cardiovascular events. Atherosclerosis 2021, 319, 132–141. [Google Scholar] [CrossRef]
- Lee, P.S.S.; Poh, K.K. Endothelial progenitor cells in cardiovascular diseases. World J. Stem. Cells 2014, 6, 355–366. [Google Scholar] [CrossRef]
- Alexandru, N.; Safciuc, F.; Constantin, A.; Nemecz, M.; Tanko, G.; Filippi, A.; Dragan, E.; Bãdilã, E.; Georgescu, A. Platelets of healthy origins promote functional improvement of atherosclerotic endothelial progenitor cells. Front. Pharmacol. 2019, 10, 424. [Google Scholar] [CrossRef]
- Teraguchi, I.; Imanishi, T.; Ozaki, Y.; Tanimoto, T.; Kitabata, H.; Ino, Y.; Ishibashi, K.; Komukai, K.; Hirata, K.; Akasaka, T. Impact of stress hyperglycemia on myocardial salvage following successfully recanalized primary acute myocardial infarction. Circ. J. 2012, 76, 2690–2696. [Google Scholar] [CrossRef] [Green Version]
- Alexandru, N.; Andrei, E.; Dragan, E.; Georgescu, A. Interaction of platelets with endothelial progenitor cells in the experimental atherosclerosis: Role of transplanted endothelial progenitor cells and platelet microparticles. Biol. Cell 2015, 107, 189–204. [Google Scholar] [CrossRef] [PubMed]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
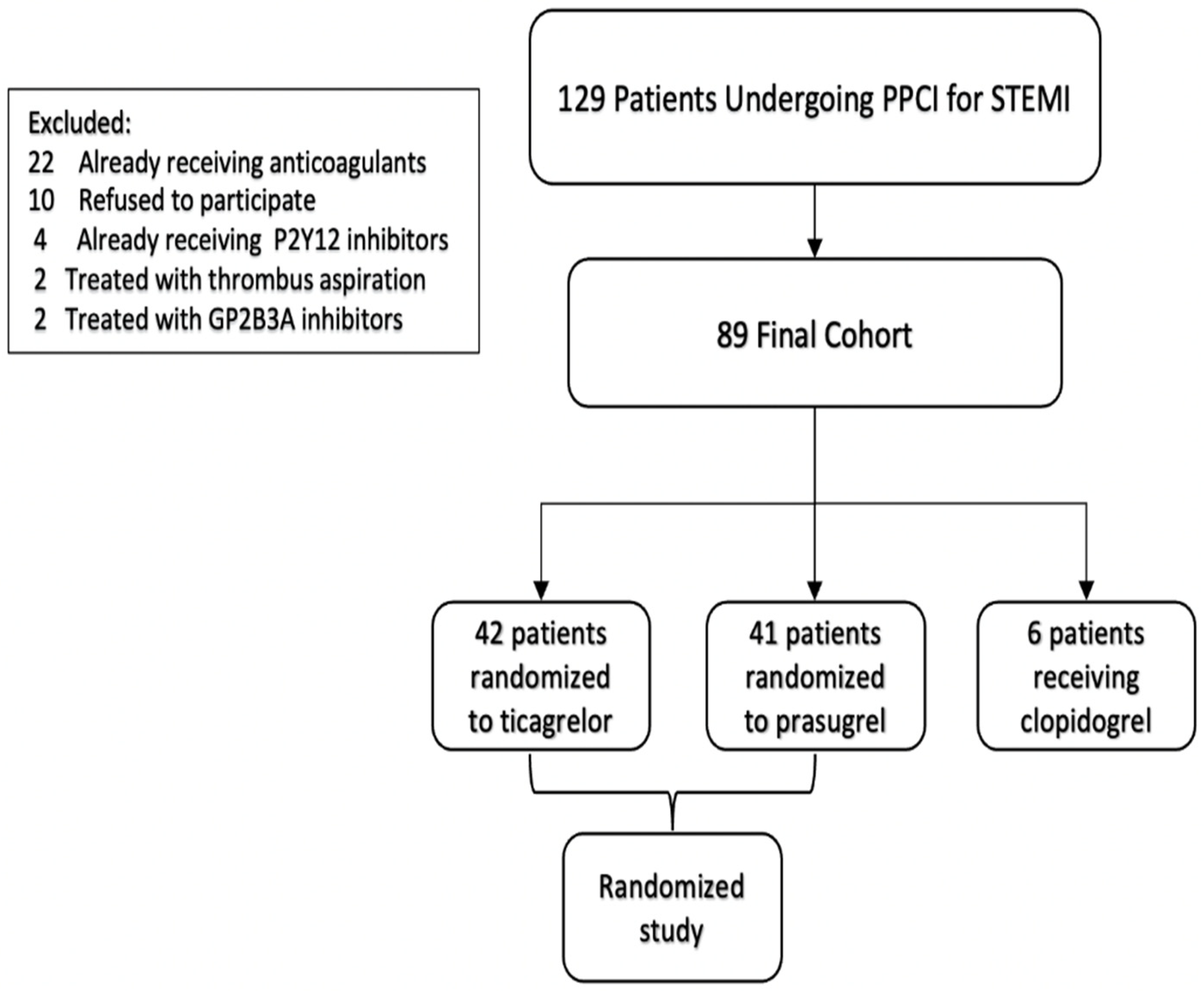
| Variable | All Patients n = 89 | Prasugrel n = 41 | Ticagrelor n = 42 | Clopidogrel n = 6 | p Value |
|---|---|---|---|---|---|
| Age (years) | 61.9 ± 11.0 | 61.5 ± 11.3 | 61.3 ± 10.9 | 63.4 ± 11.8 | 0.48 |
| Female sex (%) | 30 (33.7) | 11 (27) | 16 (38) | 3 (50) | 0.14 |
| BMI (kg/m2) | 28.2 ± 4.3 | 28.7 ± 4.4 | 28.1 ± 4.5 | 27.9 ± 4.7 | 0.26 |
| Smoking (%) | 36 (40.4) | 19 (46) | 14 (33) | 3 (50.0) | 0.22 |
| Diabetes mellitus (%) | 35 (39.3) | 16 (39) | 16 (38) | 3 (50.0) | 0.42 |
| Hypertension (%) | 48 (53.9) | 20 (49) | 24 (57) | 4 (66.7) | 0.12 |
| PVD (%) | 6 (6.7) | 2 (5) | 3 (7.1) | 1 (16.7) | 0.27 |
| CAD (%) | 27 (30.3) | 12 (29.3) | 13 (31) | 2 (33.3) | 0.81 |
| COPD (%) | 14 (15.7) | 7 (17) | 6 (14) | 1 (16.7) | 0.70 |
| LVEF (%) | 49.1 ± 7.9 | 48.8 ± 8.1 | 50.4 ± 6.8 | 48.8 ± 8.1 | 0.34 |
| Aspirin (%) | 38 (42.7) | 15 (36.6) | 18 (42.9) | 3 (50.0) | 0.22 |
| Statin (%) | 51 (57.3) | 23 (56.1) | 24 (57.1) | 4 (67.7) | 0.62 |
| Beta blocker (%) | 21 (23.6) | 10 (24.4) | 9 (21.4) | 2 (33.3) | 0.75 |
| ACEI (%) | 31 (34.8) | 14 (34.1) | 15 (35.7) | 2 (33.3) | 0.88 |
| Test | Levels |
|---|---|
| Hemoglobin (g/dl) | 14.3 ± 1.8 |
| Platelet count (K/micl) | 244.708 |
| Mean platelet volume (fl) | 9.672 |
| White blood cell count (K/micl) | 10.427 |
| Creatinine (mg/dL) | 0.912 |
| Glucose (mg/dL) | 165.461 |
| Troponin T (median, (IQR)) (ng/L) | 1205.5 (622.4–2857.2) |
| PRU Baseline | 248.2 ± 48.8 |
| PRU T1 | 18.9 ± 12.4 |
| CFU Baseline (%) | 1.22 ± 0.81 |
| CFU T1 (%) | 1.62 ± 0.90 |
| CD34+ Baseline (%) | 1.09 ± 0.83 |
| CD34+ T1 (%) | 1.96 ± 1.24 |
| CD133+ Baseline (%) | 0.88 ± 0.45 |
| CD133+ T1 (%) | 1.83 ± 1.17 |
| MTT Baseline (%) | 0.13 ± 0.08 |
| MTT T1 (%) | 0.54 ± 0.27 |
| RP Baseline (%) | 0.66 ± 0.23 |
| RP T1 (%) | 0.54 ± 0.28 |
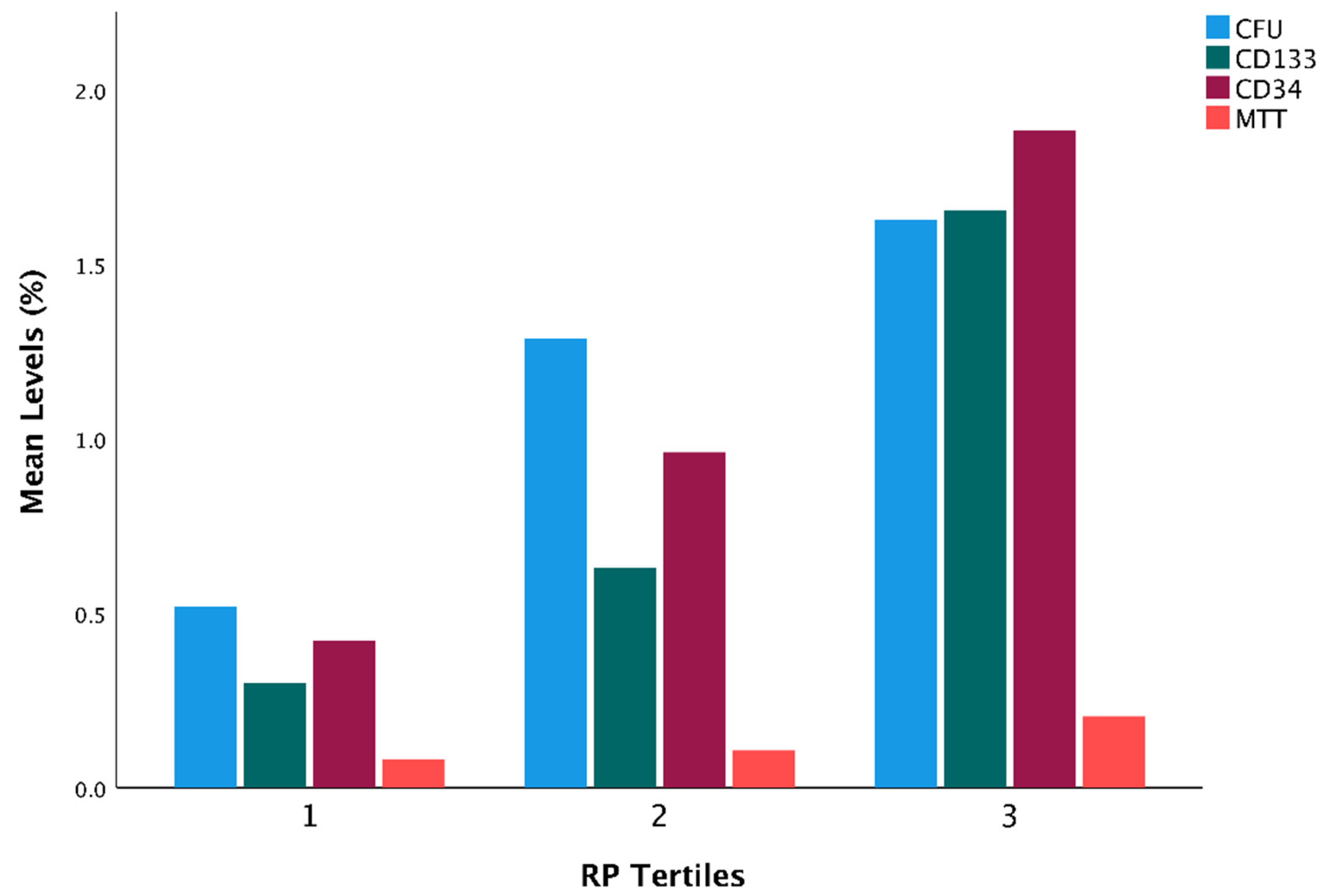
| Measurement | RP Tertile 1 | RP Tertile 2 | RP Tertile 3 | p Value |
|---|---|---|---|---|
| CFU | 0.52 | 1.29 | 1.63 | <0.01 |
| CD133+ (%) | 0.30 | 0.63 | 1.65 | 0.02 |
| CD34+ (%) | 0.42 | 0.96 | 1.88 | <0.01 |
| MTT (%) | 0.08 | 0.11 | 0.20 | 0.04 |
| RP | MTT | CFU | CD34+ | CD133+ | ||
|---|---|---|---|---|---|---|
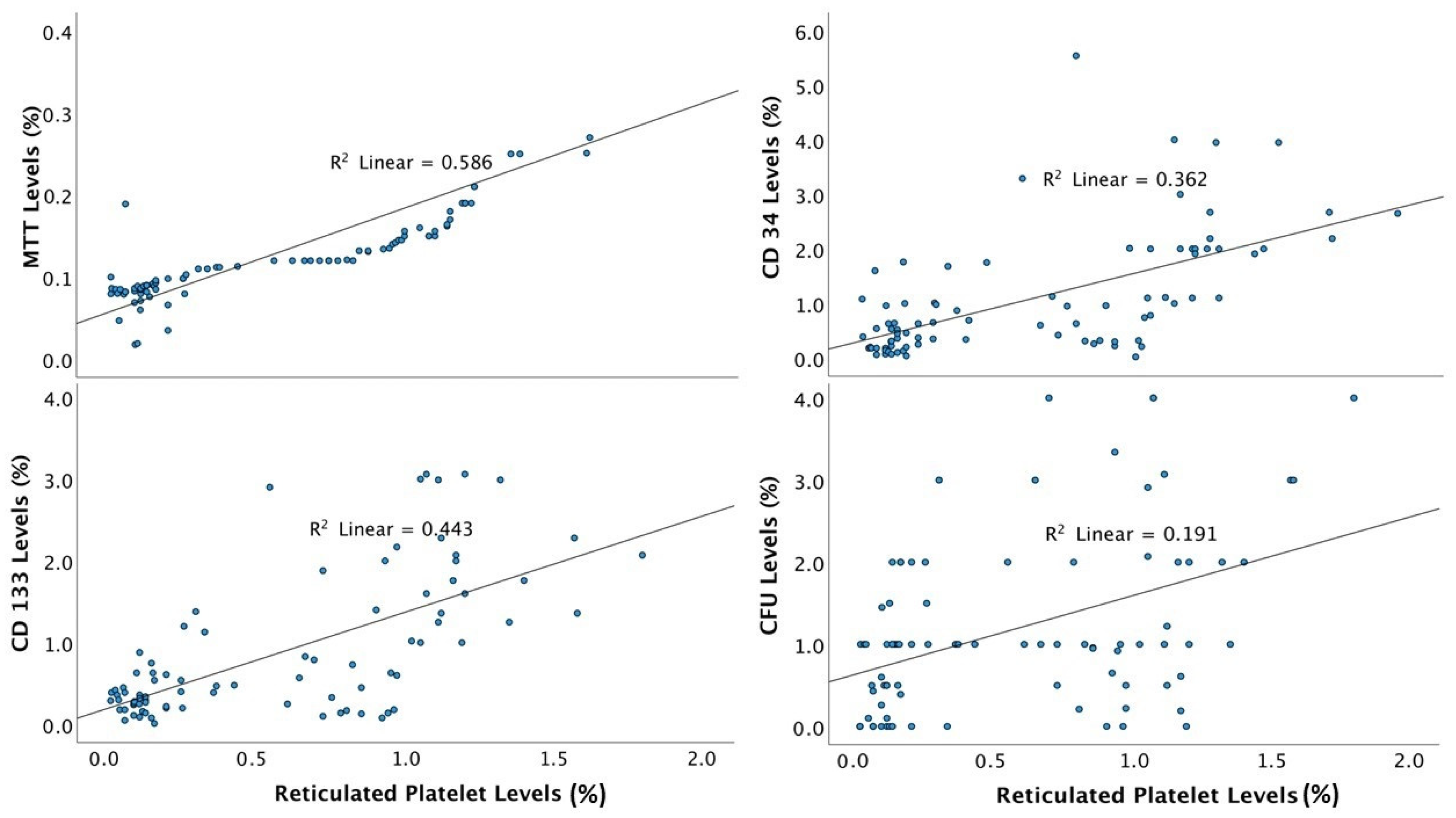
| RP | Pearson Correlation | 1 | 0.766 | 0.437 | 0.602 | 0.666 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| n | 89 | 89 | 85 | 85 | 84 | |
| MTT | Pearson Correlation | 0.766 | 1 | 0.401 | 0.537 | 0.519 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| n | 89 | 89 | 85 | 85 | 84 | |
| CFU | Pearson Correlation | 0.437 | 0.401 | 1 | 0.324 | 0.461 |
| p value | <0.001 | <0.001 | 0.003 | <0.001 | ||
| n | 85 | 85 | 85 | 81 | 81 | |
| CD34+ | Pearson Correlation | 0.602 | 0.537 | 0.324 | 1 | 0.694 |
| p value | <0.001 | <0.001 | 0.003 | <0.001 | ||
| n | 85 | 85 | 81 | 85 | 83 | |
| CD133+ | Pearson Correlation | 0.666 | 0.519 | 0.461 | 0.694 | 1 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| n | 84 | 84 | 81 | 83 | 84 | |
| RP | ||
|---|---|---|
| CD34+ | Pearson Correlation | 0.260 |
| p value | 0.073 | |
| CD133+ | Pearson Correlation | 0.133 |
| p value | 0.796 | |
| MTT | Pearson Correlation | 0.465 |
| p value | <0.001 | |
| CFU | Pearson Correlation | 0.206 |
| p value | 0.159 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schamroth Pravda, N.; Kheifets, M.; Wiessman, M.; Leshem-Lev, D.; Vaknin Assa, H.; Kornowski, R.; Talmor-Barkan, Y.; Perl, L. Reticulated Platelets and Their Relationship with Endothelial Progenitor Cells during the Acute Phase of ST-Elevation Myocardial Infarction. J. Clin. Med. 2022, 11, 6597. https://doi.org/10.3390/jcm11216597
Schamroth Pravda N, Kheifets M, Wiessman M, Leshem-Lev D, Vaknin Assa H, Kornowski R, Talmor-Barkan Y, Perl L. Reticulated Platelets and Their Relationship with Endothelial Progenitor Cells during the Acute Phase of ST-Elevation Myocardial Infarction. Journal of Clinical Medicine. 2022; 11(21):6597. https://doi.org/10.3390/jcm11216597
Chicago/Turabian StyleSchamroth Pravda, Nili, Mark Kheifets, Maya Wiessman, Dorit Leshem-Lev, Hana Vaknin Assa, Ran Kornowski, Yeela Talmor-Barkan, and Leor Perl. 2022. "Reticulated Platelets and Their Relationship with Endothelial Progenitor Cells during the Acute Phase of ST-Elevation Myocardial Infarction" Journal of Clinical Medicine 11, no. 21: 6597. https://doi.org/10.3390/jcm11216597






