Comaneci-Assisted Coiling of Wide-Necked Intracranial Aneurysm: A Single-Center Preliminary Experience
Abstract
:1. Introduction
2. Methods
2.1. Antiplatelet and Antithrombotic Management
2.2. Endovascular Procedure
3. Results
3.1. Population
3.2. Complications
3.3. Follow-Up
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molyneux, A.J.; Kerr, R.S.; Yu, L.-M.; Clarke, M.; Sneade, M.; A Yarnold, J.; Sandercock, P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Diao, Y.-H.; Cai, S.-F.; Yang, X.-Y. Endovascular coiling versus microsurgical clipping for ruptured intracranial aneurysms: A meta-analysis and systematic review. Chin. Neurosurg. J. 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ling, X.; Petersen, J.D.; Liu, J.; Xiao, A.; Huang, J. Clipping versus coiling for aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis of prospective studies. Neurosurg. Rev. 2021, 45, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Mascitelli, J.R.; Lawton, M.T.; Hendricks, B.K.; Nakaji, P.; Zabramski, J.M.; Spetzler, R.F. Analysis of Wide-Neck Aneurysms in the Barrow Ruptured Aneurysm Trial. Neurosurgery 2018, 85, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.S.; Molyneux, A.; Coon, A.L.; Saatci, I.; Szikora, I.; Baltacioglu, F.; Sultan, A.; Hoit, D.; Almandoz, J.E.D.; Elijovich, L.; et al. The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: Final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) Study. J. Neurointerv. Surg. 2019, 11, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Bhogal, P.; Lylyk, I.; Chudyk, J.; Perez, N.; Bleise, C.; Lylyk, P. The Contour—Early Human Experience of a Novel Aneurysm Occlusion Device. Clin. Neuroradiol. 2020, 31, 147–154. [Google Scholar] [CrossRef]
- Chancellor, B.; Raz, E.; Shapiro, M.; Tanweer, O.; Nossek, E.; A Riina, H.; Nelson, P.K. Flow Diversion for Intracranial Aneurysm Treatment: Trials Involving Flow Diverters and Long-Term Outcomes. Neurosurgery 2019, 86, S36–S45. [Google Scholar] [CrossRef]
- Becske, T.; Potts, M.B.; Shapiro, M.; Kallmes, D.F.; Brinjikji, W.; Saatci, I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; Moran, C.J.; et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J. Neurosurg. 2017, 127, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-W.; Park, S.; Shin, H.; Koh, J. Complications in Stent-Assisted Endovascular Therapy of Ruptured Intracranial Aneurysms and Relevance to Antiplatelet Administration: A Systematic Review. Am. J. Neuroradiol. 2015, 36, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Bodily, K.; Cloft, H.; Lanzino, G.; Fiorella, D.; White, P.; Kallmes, D. Stent-Assisted Coiling in Acutely Ruptured Intracranial Aneurysms: A Qualitative, Systematic Review of the Literature. Am. J. Neuroradiol. 2011, 32, 1232–1236. [Google Scholar] [CrossRef]
- Pierot, L.; Cognard, C.; Spelle, L.; Moret, J. Safety and Efficacy of Balloon Remodeling Technique during Endovascular Treatment of Intracranial Aneurysms: Critical Review of the Literature. Am. J. Neuroradiol. 2011, 33, 12–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinjikji, W.; Cloft, H.; Kallmes, D. Difficult Aneurysms for Endovascular Treatment: Overwide or Undertall? Am. J. Neuroradiol. 2009, 30, 1513–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moret, J.; Cognard, C.; Weill, A.; Castaings, L.; Rey, A. La technique de reconstruction dans le traitement des anévrismes intra-crâniens à collet large. Résultats angiographiques et cliniques à long terme. A propos de 56 cas [Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases]. J. Neuroradiol. 1997, 24, 30–44. [Google Scholar] [PubMed]
- Tumialán, L.M.; Zhang, Y.J.; Cawley, C.M.; Dion, J.E.; Tong, F.C.; Barrow, D.L. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms: A cautionary report. J. Neurosurg. 2008, 108, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
- Sluzewski, M.; Van Rooij, W.J.; Beute, G.N.; Nijssen, P.C. Balloon-assisted coil embolization of intracranial aneurysms: Incidence, complications, and angiography results. J. Neurosurg. 2006, 105, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Lawson, A.L.D.; Chandran, A.; Puthuran, M.; Goddard, T.; Nahser, H.; Patankar, T. Republished: Initial experience of coiling cerebral aneurysms using the new Comaneci device. J. Neurointerv. Surg. 2015, 8, e32. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Weber, A.; Carolus, A.; Drescher, F.; Götz, F.; Weber, W. Coiling of wide-necked carotid artery aneurysms assisted by a temporary bridging device (Comaneci): Preliminary experience. J. Neurointerv. Surg. 2016, 9, 1039–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taqi, M.A.; Raz, E.; Vechera, A.; Shapiro, M.; Gupta, R.; Haynes, J.; Taussky, P.; Grandhi, R.; Riina, H.A.; Nelson, P.K.; et al. Early Experience with Comaneci, a Newly FDA-Approved Controllable Assist Device for Wide-Necked Intracranial Aneurysm Coiling. Cerebrovasc. Dis. 2021, 50, 464–471. [Google Scholar] [CrossRef]
- Sirakov, S.S.; Sirakov, A.; Hristov, H.; Raychev, R. Coiling of ruptured, wide-necked basilar tip aneurysm using double Comaneci technique. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef]
- Wessels, L.; De Vries, J.; Boogaarts, H.D. The Comaneci Device for Entering an Acute-Angled Small Feeder during Cerebral Arteriovenous Malformation Embolization: A Novel Technique to Support Microcatheterization. Neurointervention 2020, 15, 79–83. [Google Scholar] [CrossRef]
- Thiery, L.; Carle, X.; Testud, B.; Boulouis, G.; Habert, P.; Tradi, F.; Reyre, A.; Lehmann, P.; Dory-Lautrec, P.; Stellmann, J.-P.; et al. Distal cerebral vasospasm treatment following aneurysmal subarachnoid hemorrhage using the Comaneci device: Technical feasibility and single-center preliminary results. J. Neurointerv. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Giorgianni, A.; Agosti, E.; Terrana, A.V.; Vinacci, G.; Nativo, L.; Valvassori, L.; Locatelli, D.; Lanzino, G. Comaneci-Assisted Coiling Embolization of a Posttraumatic Carotid-Cavernous Fistula. World Neurosurg. 2020, 141, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Starke, R.; Koltz, M.; Jabbour, P.; Tjoumakaris, S.; Dumont, A.; Rosenwasser, R.; Singhal, S.; Gonzalez, L. Stent-Assisted Coiling Versus Balloon Remodeling of Wide-Neck Aneurysms: Comparison of Angiographic Outcomes. Am. J. Neuroradiol. 2013, 34, 1987–1992. [Google Scholar] [CrossRef] [Green Version]
- Cottier, J.-P.; Pasco, A.; Gallas, S.; Gabrillargues, J.; Cognard, C.; Drouineau, J.; Brunereau, L.; Herbreteau, D. Utility of Balloon-assisted Guglielmi Detachable Coiling in the Treatment of 49 Cerebral Aneurysms: A Retrospective, Multicenter Study. Am. J. Neuroradiol. 2001, 22, 345–351. [Google Scholar] [PubMed]
- Sirakov, A.; Minkin, K.; Penkov, M.; Ninov, K.; Karakostov, V.; Sirakov, S. Comaneci-Assisted Coiling as a Treatment Option for Acutely Ruptured Wide Neck Cerebral Aneurysm: Case Series of 118 Patients. Neurosurgery 2020, 87, 1148–1156. [Google Scholar] [CrossRef]
- Molina-Nuevo, J.D.; López-Martínez, L.; Pedrosa-Jiménez, M.J.; Juliá-Molla, E.; Hernández-Fernández, F. Comaneci device-assisted embolization of wide-necked carotid aneurysms with an unfavorable ratio. BMC Neurol. 2020, 20, 384. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, Y.; Shen, L.; Ni, Y.; Ji, Q. Comparison of Stent-Assisted Coiling and Balloon-Assisted Coiling in the Treatment of Ruptured Wide-Necked Intracranial Aneurysms in the Acute Period. World Neurosurg. 2016, 96, 316–321. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Wang, Y.; Bai, P.; Wang, H.-Z.; Sun, T.; Yu, H.-L. Stent-assisted coiling and balloon-assisted coiling in the management of intracranial aneurysms: A systematic review & meta-analysis. J. Neurol. Sci. 2016, 364, 160–166. [Google Scholar] [CrossRef]
- Consoli, A.; Vignoli, C.; Renieri, L.; Rosi, A.; Chiarotti, I.; Nappini, S.; Limbucci, N.; Mangiafico, S. Assisted coiling of saccular wide-necked unruptured intracranial aneurysms: Stent versus balloon. J. Neurointerv. Surg. 2014, 8, 52–57. [Google Scholar] [CrossRef]
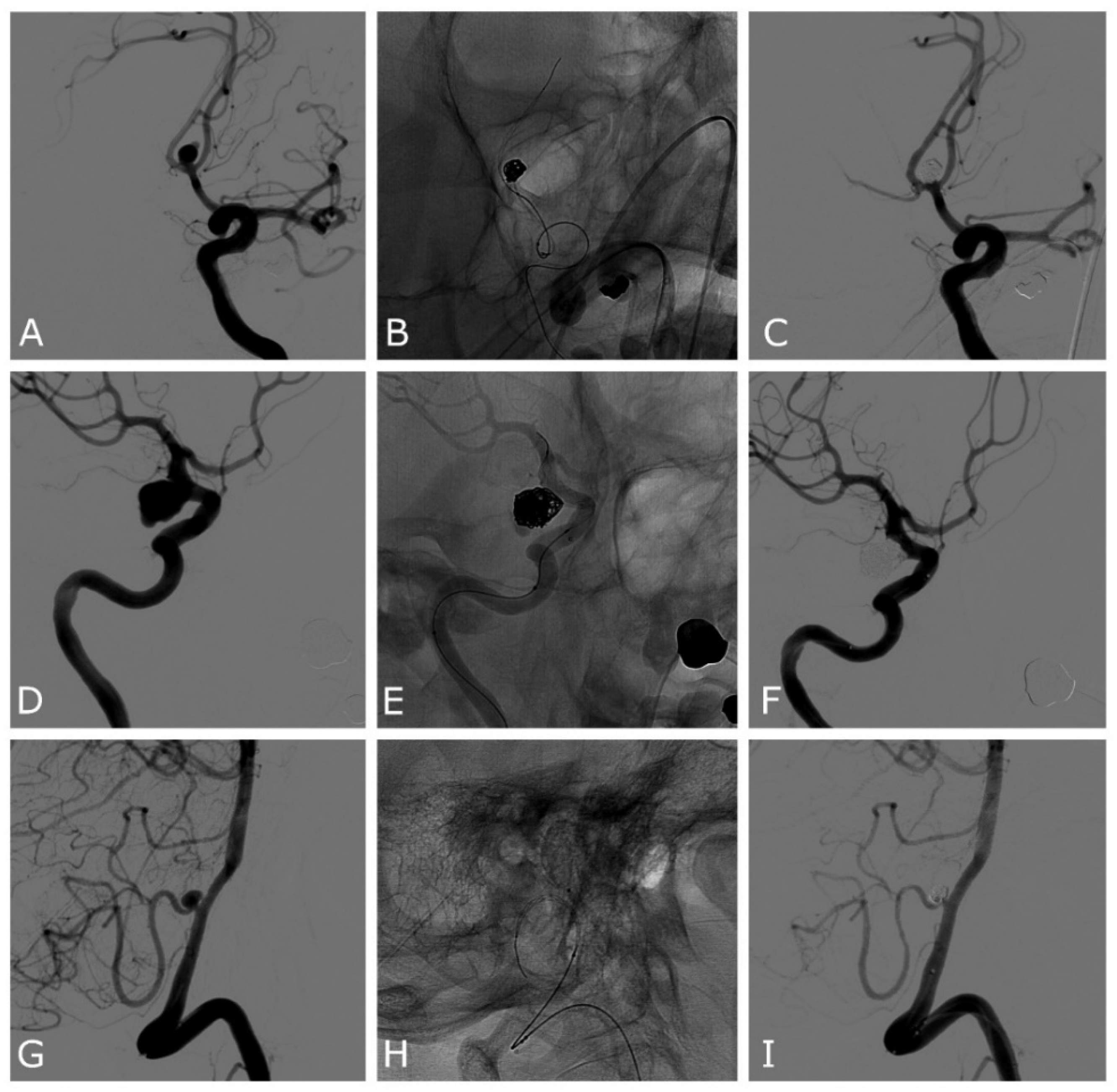
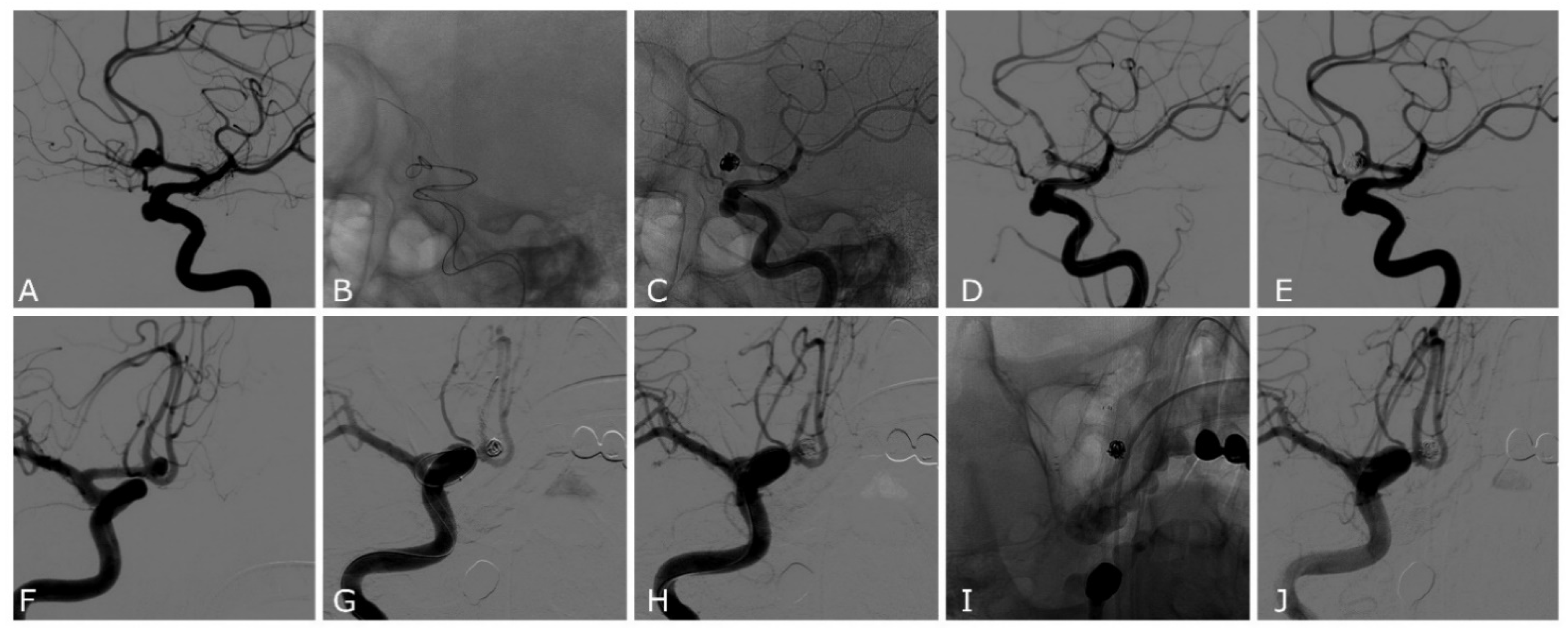
| Case | Age | Sex | Location | SAH | Max Size (Height, Width, Neck) mm | Device | Complications | Rescue Treatment | mRR | mRs at 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | F | PCOM | No | 4.2, 7, 4 | Comaneci | - | I | 0 | |
| 2 | 65 | M | PCOM | Yes | 3.1, 4, 3 | 17 | - | I | 0 | |
| 3 | 70 | F | PICA | Yes | 3.5, 2, 1.5 | Comaneci | - | I | 0 | |
| 4 | 86 | F | PCOM | Yes | 6.4, 4.3, 5.5 | Comaneci | Intra-Comaneci and parent artery clot formation | Stent | II | 6 |
| 5 | 59 | F | ACOM | Yes | 10, 6.2, 5.5 | 17 | - | II | 0 | |
| 6 | 65 | F | PCOM | No | 6.7, 3.9, 3 | Petit | - | II | 0 | |
| 7 | 51 | F | MCA | No | 2, 2.2, 2 | Petit | - | II | 5 | |
| 8 | 65 | M | ACOM | Yes | 5.7, 3.7, 3.5 | 17 | - | 0 | ||
| 9 | 77 | F | PCOM | Yes | 8, 10.3, 5.9 | Comaneci | Intra-Comaneci clot formation | I | 0 | |
| 10 | 65 | F | ACOM | Yes | 3, 2.4, 2 | 17 | - | I | 5 | |
| 11 | 45 | M | ACOM | Yes | 5.5, 3.6, 2.8 | 17 | Intra-Comaneci clot formation | IIIb | 1 | |
| 12 | 62 | F | ACOM | Yes | 9, 4.5, 3 | 17 | Intra-Comaneci clot formation | Stent | IIIb | 0 |
| 13 | 55 | M | ACOM | Yes | 14.5, 15.6, 8.2 | 17 | - | I | 0 | |
| 14 | 61 | M | ACOM | Yes | 3, 5.9, 5 | 17 | Intra-Comaneci clot formation | II | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinacci, G.; Celentano, A.; Agosti, E.; Terrana, A.V.; Vizzari, F.A.; Nativo, L.; Baruzzi, F.; Tabano, A.; Locatelli, D.; Giorgianni, A. Comaneci-Assisted Coiling of Wide-Necked Intracranial Aneurysm: A Single-Center Preliminary Experience. J. Clin. Med. 2022, 11, 6650. https://doi.org/10.3390/jcm11226650
Vinacci G, Celentano A, Agosti E, Terrana AV, Vizzari FA, Nativo L, Baruzzi F, Tabano A, Locatelli D, Giorgianni A. Comaneci-Assisted Coiling of Wide-Necked Intracranial Aneurysm: A Single-Center Preliminary Experience. Journal of Clinical Medicine. 2022; 11(22):6650. https://doi.org/10.3390/jcm11226650
Chicago/Turabian StyleVinacci, Gabriele, Angelica Celentano, Edoardo Agosti, Alberto Vito Terrana, Francesco Alberto Vizzari, Luca Nativo, Fabio Baruzzi, Antonio Tabano, Davide Locatelli, and Andrea Giorgianni. 2022. "Comaneci-Assisted Coiling of Wide-Necked Intracranial Aneurysm: A Single-Center Preliminary Experience" Journal of Clinical Medicine 11, no. 22: 6650. https://doi.org/10.3390/jcm11226650
APA StyleVinacci, G., Celentano, A., Agosti, E., Terrana, A. V., Vizzari, F. A., Nativo, L., Baruzzi, F., Tabano, A., Locatelli, D., & Giorgianni, A. (2022). Comaneci-Assisted Coiling of Wide-Necked Intracranial Aneurysm: A Single-Center Preliminary Experience. Journal of Clinical Medicine, 11(22), 6650. https://doi.org/10.3390/jcm11226650






