The Role of Immediate Post-Procedural Cone-Beam Computed Tomography (CBCT) in Predicting the Early Radiologic Response of Hepatocellular Carcinoma (HCC) Nodules to Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. DEB-TACE Procedure
2.3. CBCT Image Protocol
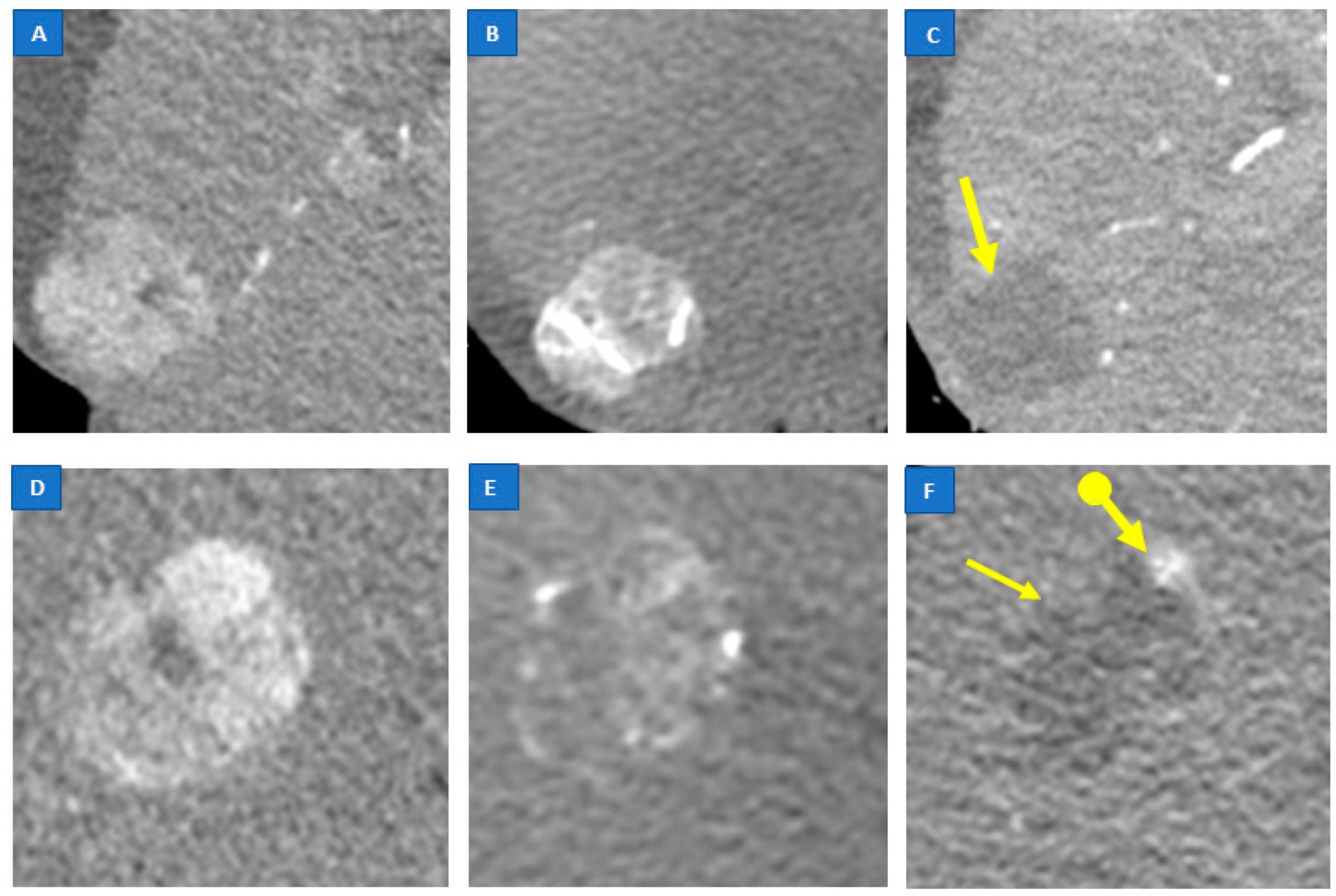
2.4. Analysis of CBCT Imaging
2.5. Preoperative and Follow-Up Imaging
2.6. Statistical Analysis
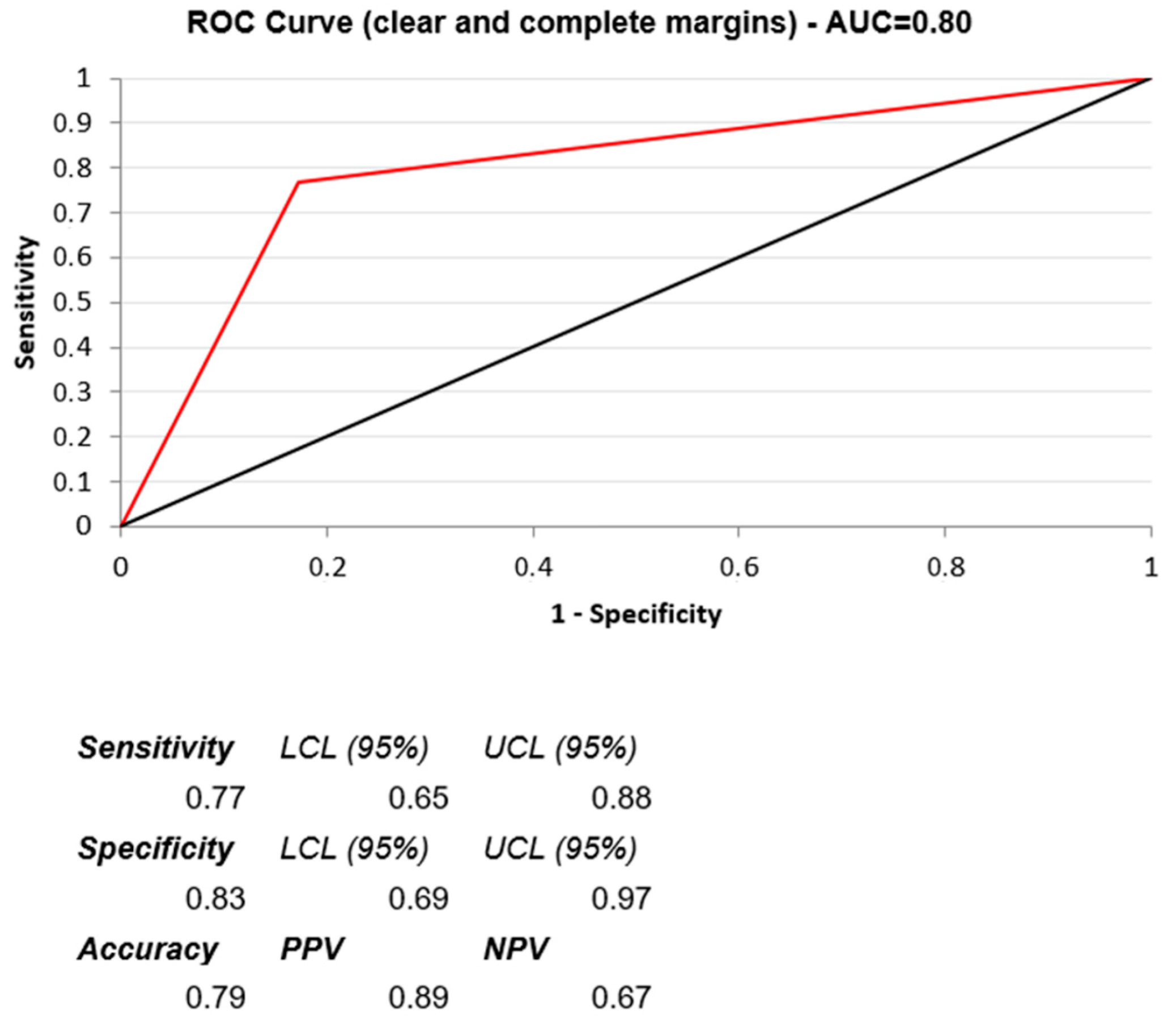
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fábrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation Barcelona Clinic Liver Cancer (BCLC) Staging System. The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Burrel, M.; Reig, M.; Forner, A.; Barrufet, M.; de Lope, C.R.; Tremosini, S.; Ayuso, C.; Llovet, J.M.; Real, M.I.; Bruix, J. Survival of Patients with Hepatocellular Carcinoma Treated by Transarterial Chemoembolisation (TACE) Using Drug Eluting Beads. Implications for Clinical Practice and Trial Design. J. Hepatol. 2012, 56, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, I.; Florio, F.; Golfieri, R.; Grosso, M.; Lauretti, D.L.; Cioni, R. Trends in Utilization of Transarterial Treatments for Hepatocellular Carcinoma: Results of a Survey by the Italian Society of Interventional Radiology. Cardiovasc. Intervent. Radiol. 2014, 37, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Pomfret, E.A.; Washburn, K.; Wald, C.; Nalesnik, M.A.; Douglas, D.; Russo, M.; Roberts, J.; Reich, D.J.; Schwartz, M.E.; Mieles, L.; et al. Report of a National Conference on Liver Allocation in Patients with Hepatocellular Carcinoma in the United States. Liver Transpl. 2010, 16, 262–278. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Hashimoto, M.; Hashimoto, N.; Ikuno, M.; Okumura, K.; Yoshida, M.; Matsui, O. Comparison of Local Control in Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma ≤6 Cm with or without Intraprocedural Monitoring of the Embolized Area Using Cone-Beam Computed Tomography. Cardiovasc. Intervent. Radiol. 2014, 37, 388–395. [Google Scholar] [CrossRef]
- Iwazawa, J.; Ohue, S.; Hashimoto, N.; Muramoto, O.; Mitani, T. Survival after C-Arm CT-Assisted Chemoembolization of Unresectable Hepatocellular Carcinoma. Eur. J. Radiol. 2012, 81, 3985–3992. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, I.J.; Kim, H.B.; Park, B.; Kim, B.H.; Park, J.-W.; Kim, C.-M. Efficacy and Safety of Transarterial Chemoembolisation with Cone-Beam CT in Patients with Hepatocellular Carcinoma within the Milan Criteria: A Retrospective Cohort Study. Clin. Radiol. 2019, 74, 407.e19–407.e28. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.-J.; Park, M.-S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete Response at First Chemoembolization Is Still the Most Robust Predictor for Favorable Outcome in Hepatocellular Carcinoma. J. Hepatol. 2015, 62, 1304–1310. [Google Scholar] [CrossRef]
- Xia, D.; Wang, Q.; Bai, W.; Wang, E.; Wang, Z.; Mu, W.; Sun, J.; Huang, M.; Yin, G.; Li, H.; et al. Optimal Time Point of Response Assessment for Predicting Survival Is Associated with Tumor Burden in Hepatocellular Carcinoma Receiving Repeated Transarterial Chemoembolization. Eur. Radiol. 2022, 32, 5799–5810. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (MRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jeon, U.B.; Lee, J.W.; Choo, K.S.; Kim, C.W.; Kim, S.; Lee, T.H.; Jeong, Y.J.; Kang, D.H. Iodized Oil Uptake Assessment with Cone-Beam CT in Chemoembolization of Small Hepatocellular Carcinomas. World J. Gastroenterol. 2009, 15, 5833–5837. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-H.; Wang, L.-G.; Bao, H.-W.; Lou, J.-L.; Cai, L.-X.; Wu, C.; Chen, L.-M.; Zheng, S.-S. Usefulness of C-Arm Angiographic Computed Tomography for Detecting Iodized Oil Retention during Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma. J. Int. Med. Res. 2010, 38, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, R.; Duran, R.; Zhao, Y.; Yenokyan, G.; Chapiro, J.; Schernthaner, R.; Radaelli, A.; Lin, M.; Geschwind, J.-F. Intraprocedural 3D Quantification of Lipiodol Deposition on Cone-Beam CT Predicts Tumor Response after Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Cardiovasc. Intervent. Radiol. 2015, 38, 1548–1556. [Google Scholar] [CrossRef]
- Syha, R.; Grözinger, G.; Grosse, U.; Maurer, M.; Zender, L.; Horger, M.; Nikolaou, K.; Ketelsen, D. Parenchymal Blood Volume Assessed by C-Arm-Based Computed Tomography in Immediate Posttreatment Evaluation of Drug-Eluting Bead Transarterial Chemoembolization in Hepatocellular Carcinoma. Investig. Radiol. 2016, 51, 121–126. [Google Scholar] [CrossRef]
- O’Donohoe, R.L.; Kavanagh, R.G.; Cahalane, A.M.; Houlihan, D.D.; McCann, J.W.; Ryan, E.R. C-Arm Cone-Beam CT Parenchymal Blood Volume Imaging for Transarterial Chemoembolization of Hepatocellular Carcinoma: Implications for Treatment Planning and Response. Eur. Radiol. Exp. 2019, 3, 21. [Google Scholar] [CrossRef]
- Müller, K.; Datta, S.; Gehrisch, S.; Ahmad, M.; Mohammed, M.A.A.; Rosenberg, J.; Hwang, G.L.; Louie, J.D.; Sze, D.Y.; Kothary, N. The Role of Dual-Phase Cone-Beam CT in Predicting Short-Term Response after Transarterial Chemoembolization for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2017, 28, 238–245. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver. European Organisation for Research and Treatment of Cancer EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Purcell, Y.; Sartoris, R.; Paradis, V.; Vilgrain, V.; Ronot, M. Influence of Pretreatment Tumor Growth Rate on Objective Response of Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. J. Gastroenterol. Hepatol. 2020, 35, 305–313. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, A.-H.; Wang, W.; Wu, Y.-H.; Sun, Q.-L.; Shu, C. Radiological Appearance of Hepatocellular Carcinoma Predicts the Response to Trans-Arterial Chemoembolization in Patients Undergoing Liver Transplantation. BMC Cancer 2019, 19, 1041. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, M.H.; Choi, S.Y.; Yi, B.H.; Lee, H.K. Magnetic Resonance Imaging Features Predictive of an Incomplete Response to Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma: A STROBE-Compliant Study. Medicine 2019, 98, e15592. [Google Scholar] [CrossRef]
- Fronda, M.; Doriguzzi Breatta, A.; Gatti, M.; Calandri, M.; Maglia, C.; Bergamasco, L.; Righi, D.; Faletti, R.; Fonio, P. Quantitative Assessment of HCC Wash-out on CT Is a Predictor of Early Complete Response to TACE. Eur. Radiol. 2021, 31, 6578–6588. [Google Scholar] [CrossRef] [PubMed]
- Vesselle, G.; Quirier-Leleu, C.; Velasco, S.; Charier, F.; Silvain, C.; Boucebci, S.; Ingrand, P.; Tasu, J.-P. Predictive Factors for Complete Response of Chemoembolization with Drug-Eluting Beads (DEB-TACE) for Hepatocellular Carcinoma. Eur. Radiol. 2016, 26, 1640–1648. [Google Scholar] [CrossRef]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Piratvisuth, T. Safety Margin of Embolized Area Can Reduce Local Recurrence of Hepatocellular Carcinoma after Superselective Transarterial Chemoembolization. Clin. Mol. Hepatol. 2019, 25, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Orlacchio, A.; Roma, S.; dell’Olio, V.; Crociati, S.; Lenci, I.; Francioso, S. Role of Cone-Beam CT in the Intraprocedural Evaluation of Chemoembolization of Hepatocellular Carcinoma. J. Oncol. 2021, 2021, 8856998. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Lucatelli, P.; De Rubeis, G.; Basilico, F.; Ginanni Corradini, L.; Corona, M.; Bezzi, M.; Catalano, C. Sequential Dual-Phase Cone-Beam CT Is Able to Intra-Procedurally Predict the One-Month Treatment Outcome of Multi-Focal HCC, in Course of Degradable Starch Microsphere TACE. Radiol. Med. 2019, 124, 1212–1219. [Google Scholar] [CrossRef]

| Variable | Complete Response (CR+), n = 52 | No Complete Response (CR−), n = 29 | Univariate p Value | Multivariate BLR p Value | Multivariate BLR Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Age mean | 69 | 70 | 0.44 | ||
| Males | 42 (80.8%) | 26 (89.7%) | 0.37 | ||
| Etiology | 0.715 | ||||
| 26 (50%) | 14 (48.3%) | |||
| 4 (7.7%) | 3 (10.3%) | |||
| 5 (9.6%) | 2 (6.9%) | |||
| 13 (25%) | 3 (10.3%) | |||
| 4 (7.7%) | 7 (24.1%) | |||
| Naive/not naive lesions ratio | 43/9 | 19/10 | 0.11 | ||
| Lesions with Dmax < 30 mm | 48 (92.3%) | 20 (69%) | 0.01 | 0.03 | 6.3 (1.2–32) |
| Intensity score * | 0.0009 | 0.001 | 6 (2–18) | ||
| 0 | 0 | 2 (6.9%) | |||
| 1 | 17 (32.7%) | 19 (65.5%) | |||
| 2 | 35 (67.3%) | 8 (27.6%) | |||
| Homogeneity score | 0.401 | ||||
| 0 | 37 (71.2%) | 25 (86.2%) | |||
| 1 | 15 (28.8%) | 4 (13.8%) | |||
| Margin evaluation score | 0.003 | <0.0001 | 17 (5–55) | ||
| 0 | 12 (23.1%) | 24 (82.8%) | |||
| 1 | 40 (76.9%) | 5 (17.2%) | |||
| Embolizing agents | 0.23 | ||||
| 33/47 (70.2%) | 14/47 (29.8%) | |||
| 19/34 (55.9%) | 15/34 (44.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronda, M.; Mistretta, F.; Calandri, M.; Ciferri, F.; Nardelli, F.; Bergamasco, L.; Fonio, P.; Doriguzzi Breatta, A. The Role of Immediate Post-Procedural Cone-Beam Computed Tomography (CBCT) in Predicting the Early Radiologic Response of Hepatocellular Carcinoma (HCC) Nodules to Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE). J. Clin. Med. 2022, 11, 7089. https://doi.org/10.3390/jcm11237089
Fronda M, Mistretta F, Calandri M, Ciferri F, Nardelli F, Bergamasco L, Fonio P, Doriguzzi Breatta A. The Role of Immediate Post-Procedural Cone-Beam Computed Tomography (CBCT) in Predicting the Early Radiologic Response of Hepatocellular Carcinoma (HCC) Nodules to Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE). Journal of Clinical Medicine. 2022; 11(23):7089. https://doi.org/10.3390/jcm11237089
Chicago/Turabian StyleFronda, Marco, Francesco Mistretta, Marco Calandri, Fernanda Ciferri, Floriana Nardelli, Laura Bergamasco, Paolo Fonio, and Andrea Doriguzzi Breatta. 2022. "The Role of Immediate Post-Procedural Cone-Beam Computed Tomography (CBCT) in Predicting the Early Radiologic Response of Hepatocellular Carcinoma (HCC) Nodules to Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE)" Journal of Clinical Medicine 11, no. 23: 7089. https://doi.org/10.3390/jcm11237089
APA StyleFronda, M., Mistretta, F., Calandri, M., Ciferri, F., Nardelli, F., Bergamasco, L., Fonio, P., & Doriguzzi Breatta, A. (2022). The Role of Immediate Post-Procedural Cone-Beam Computed Tomography (CBCT) in Predicting the Early Radiologic Response of Hepatocellular Carcinoma (HCC) Nodules to Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE). Journal of Clinical Medicine, 11(23), 7089. https://doi.org/10.3390/jcm11237089






