1.5 T MR-Guided Daily Adapted SBRT on Lymph Node Oligometastases from Prostate Cancer
Abstract
:1. Introduction
2. Material and Methods
- age ≥ 18 years.
- histological diagnosis of PCa
- performance status ECOG (Eastern Cooperative Oncology Group Criteria) ≤ 2
- oligometastatic or oligoprogressive disease of the lymph nodes (≤5 nodal disease sites) from PCa diagnosed with PET-choline or PET-PSMA
- primary tumor controlled (prostate cancer locally treated with surgery/RT/other or not in radiological progression at the time of SBRT)
- a minimum follow-up period of 3 months after SBRT
- general contraindications for 1.5 T MR
- claustrophobia
2.1. Treatment Procedure
2.2. Endpoints, Follow-Up, and Statistics
3. Results
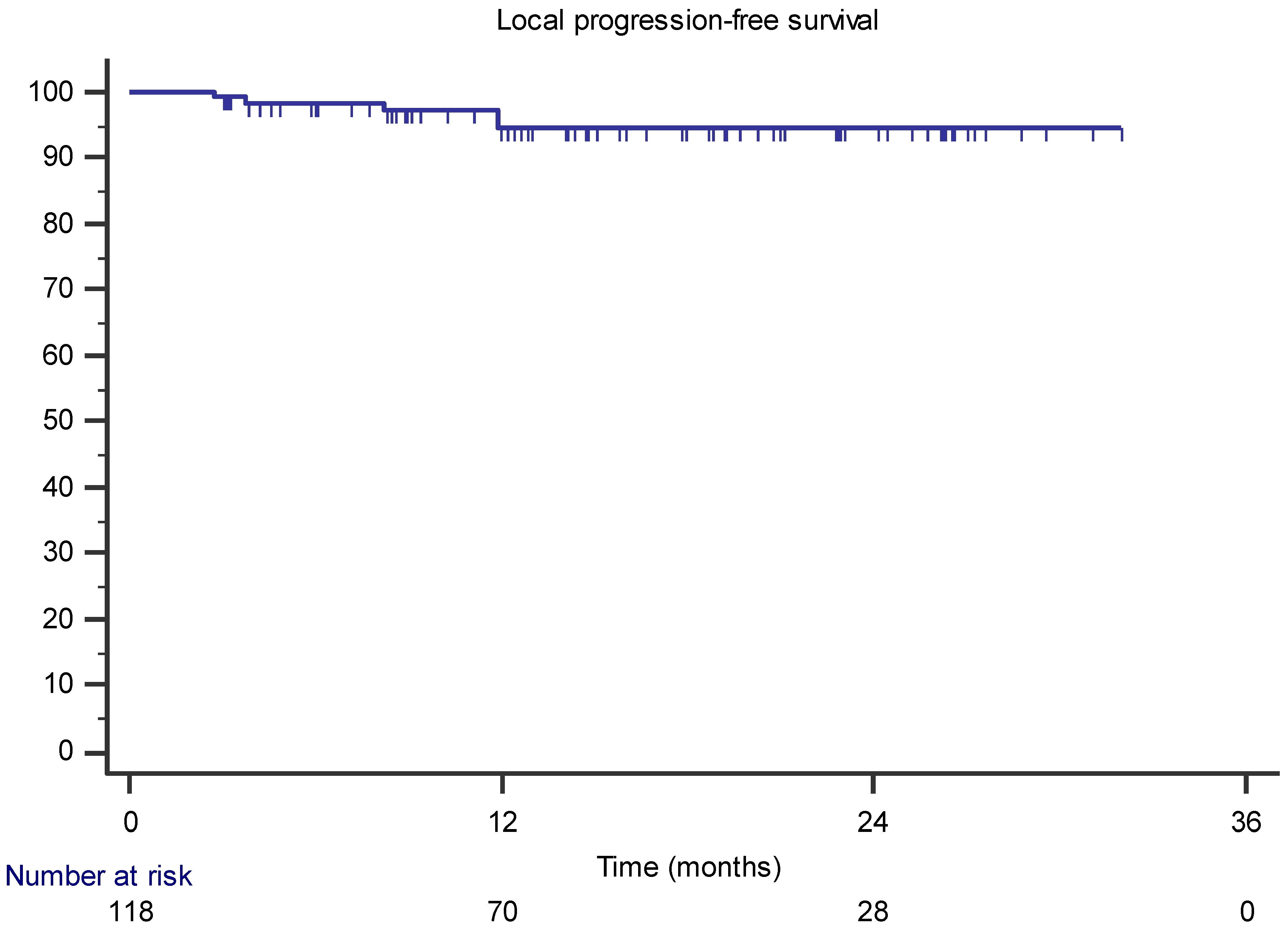
3.1. Local Control, Survival, and Toxicity
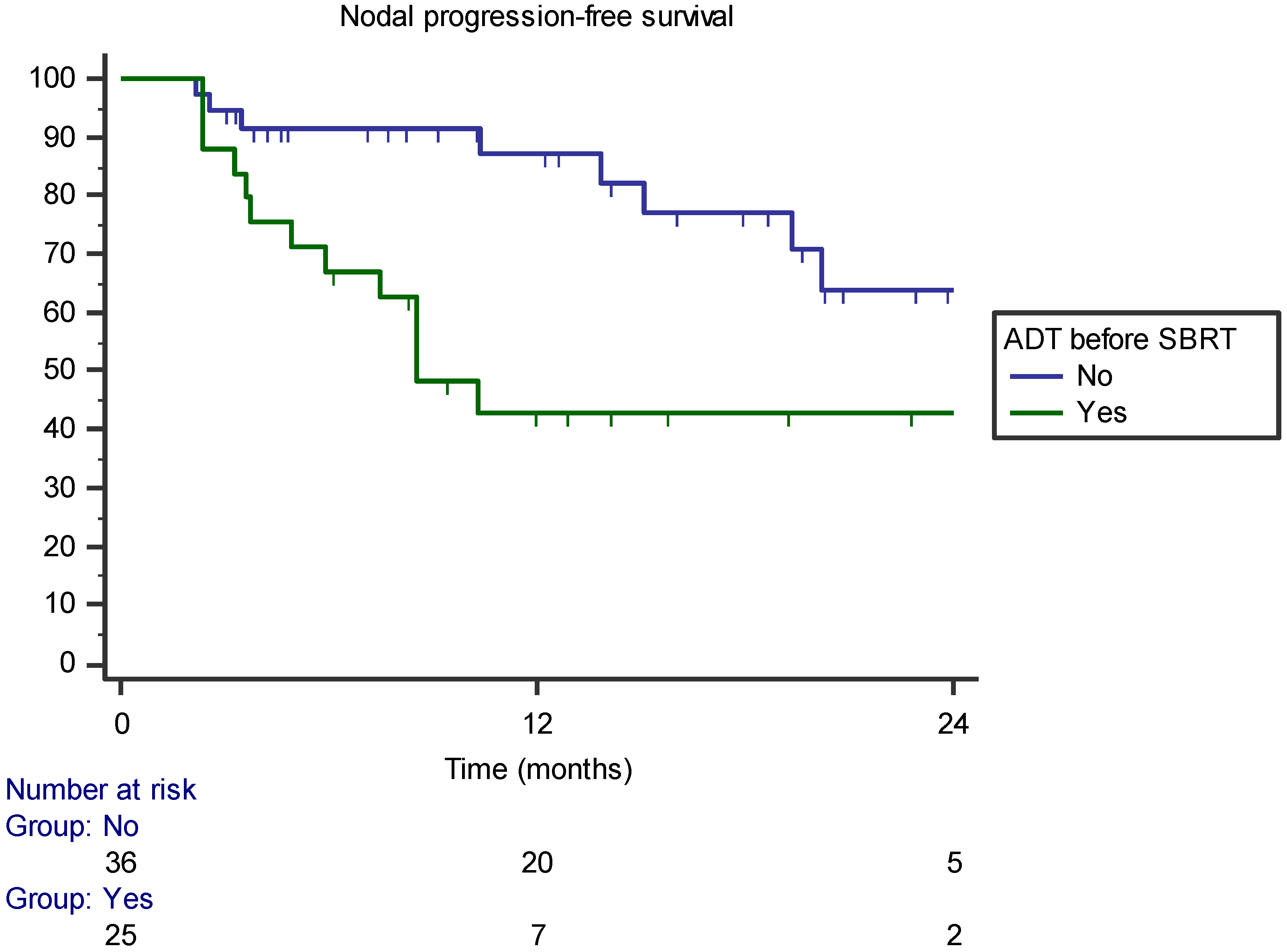
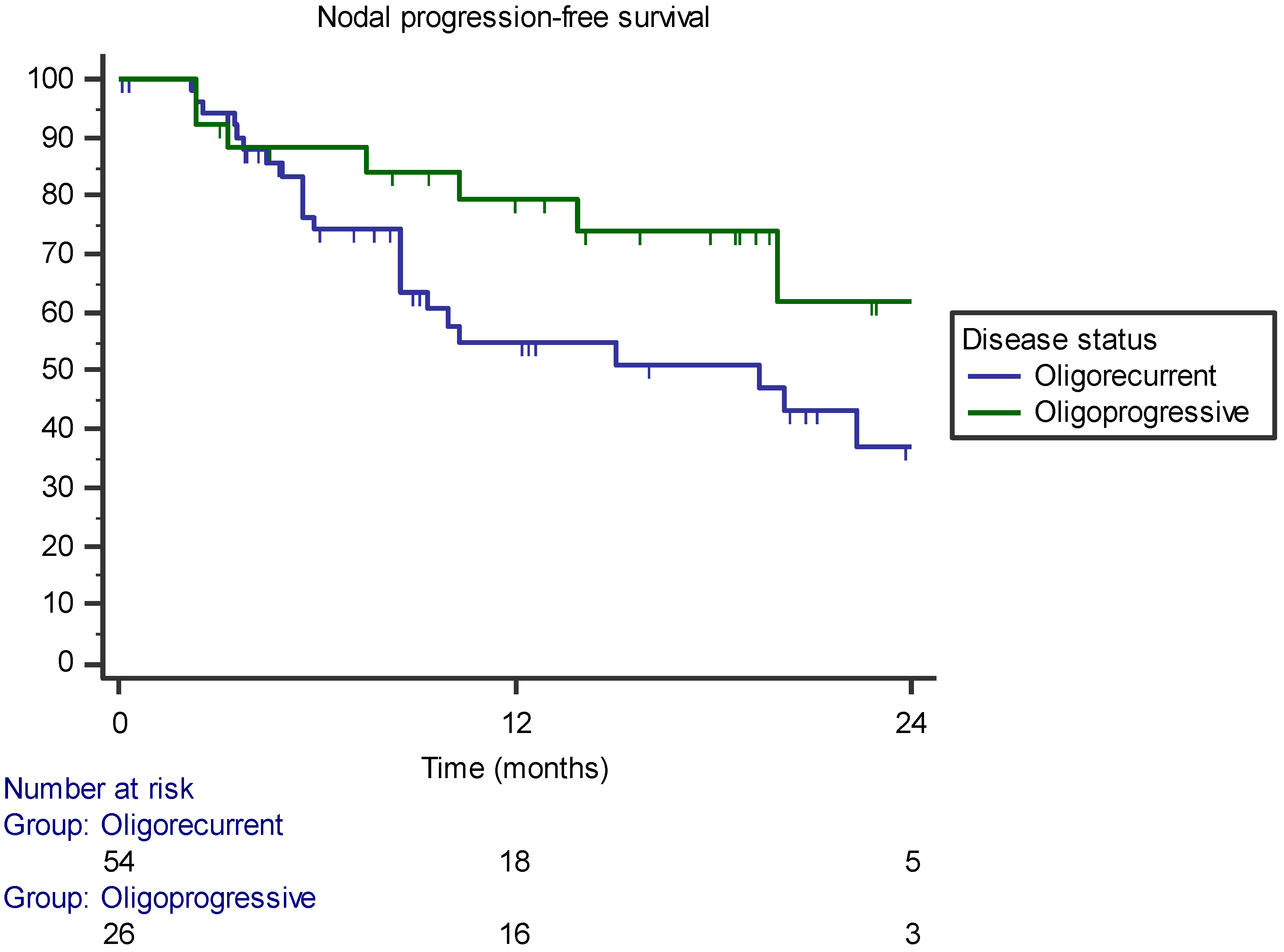
3.2. Pattern of Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Sweeney, C.J.; Tombal, B. “Gotta Catch ’em All”, or Do We? Pokemet Approach to Metastatic Prostate Cancer. Eur. Urol. 2017, 72, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2020, 79, 243–262. [Google Scholar] [CrossRef]
- Botrel, T.E.A.; Clark, O.; Pompeo, A.C.L.; Bretas, F.F.H.; Sadi, M.V.; Ferreira, U.; Dos Reis, R.B. Efficacy and Safety of Combined Androgen Deprivation Therapy (ADT) and Docetaxel Compared with ADT Alone for Metastatic Hormone-Naive Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0157660. [Google Scholar] [CrossRef]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur. Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef]
- Ost, P.; Jereczek-Fossa, B.; Van As, N.; Zilli, T.; Tree, A.; Henderson, D.; Orecchia, R.; Casamassima, F.; Surgo, A.; Miralbell, R.; et al. Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin. Oncol. 2016, 28, e115–e120. [Google Scholar] [CrossRef]
- Nicosia, L.; Franzese, C.; Mazzola, R.; Franceschini, D.; Rigo, M.; D’Agostino, G.; Corradini, S.; Alongi, F.; Scorsetti, M. Recurrence pattern of stereotactic body radiotherapy in oligometastatic prostate cancer: A multi-institutional analysis. Strahlenther. Onkol. 2019, 196, 213–221. [Google Scholar] [CrossRef]
- Triggiani, L.; Alongi, F.; Buglione, M.; Detti, B.; Santoni, R.; Bruni, A.; Maranzano, E.; Lohr, F.; D’Angelillo, R.; Magli, A.; et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: New evidence from a multicentric study. Br. J. Cancer 2017, 116, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, A.; Bonetti, M.; Buwenge, M.; Macchia, G.; Deodato, F.; Cilla, S.; Galietta, E.; Strigari, L.; Cellini, F.; Tagliaferri, L.; et al. Stereotactic radiotherapy of nodal oligometastases from prostate cancer: A prisma-compliant systematic review. Clin. Exp. Metastasis 2022, 39, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, R.; Francolini, G.; Triggiani, L.; Napoli, G.; Cuccia, F.; Nicosia, L.; Livi, L.; Magrini, S.M.; Salgarello, M.; Alongi, F. Metastasis-directed Therapy (SBRT) Guided by PET-CT 18F-CHOLINE versus PET-CT 68Ga-PSMA in Castration-sensitive Oligorecurrent Prostate Cancer: A Comparative Analysis of Effectiveness. Clin. Genitourin. Cancer 2020, 19, 230–236. [Google Scholar] [CrossRef]
- Cuccia, F.; Rigo, M.; Gurrera, D.; Nicosia, L.; Mazzola, R.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Attinà, G.; Pastorello, E.; et al. Mitigation on bowel loops daily variations by 1.5-T MR-guided daily-adaptive SBRT for abdomino-pelvic lymph-nodal oligometastases. J. Cancer Res. Clin. Oncol. 2021, 147, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Schegerer, A.A.; Lechel, U.; Ritter, M.; Weisser, G.; Fink, C.; Brix, G. Dose and Image Quality of Cone-Beam Computed Tomography as Compared with Conventional Multislice Computed Tomography in Abdominal Imaging. Investig. Radiol. 2014, 49, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Hörner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Weykamp, F.; Herder-Wagner, C.; Regnery, S.; Hoegen, P.; Renkamp, C.K.; Liermann, J.; Rippke, C.; Koerber, S.A.; König, L.; Buchele, C.; et al. Stereotactic body radiotherapy of lymph node metastases under MR-guidance: First clinical results and patient-reported outcomes. Strahlenther. und Onkol. 2021, 198, 56–65. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Werensteijn-Honingh, A.M.; Intven, M.P.; Eppinga, W.S.; Hes, J.; Snoeren, L.M.; Sikkes, G.G.; Hooijdonk, C.G.G.-V.; Raaymakers, B.W.; et al. Target coverage and dose criteria based evaluation of the first clinical 1.5T MR-linac SBRT treatments of lymph node oligometastases compared with conventional CBCT-linac treatment. Radiother. Oncol. 2020, 146, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Ruggieri, R.; Rigo, M.; Naccarato, S.; Gurrera, D.; Figlia, V.; Mazzola, R.; Ricchetti, F.; Nicosia, L.; Giaj-Levra, N.; Cuccia, F.; et al. Adaptive SBRT by 1.5 T MR-linac for prostate cancer: On the accuracy of dose delivery in view of the prolonged session time. Phys. Med. 2020, 80, 34–41. [Google Scholar] [CrossRef]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Romero, A.M.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Shahi, J.; Peng, J.; Donovan, E.; Vansantvoort, J.; Wong, R.; Tsakiridis, T.; Quan, K.; Parpia, S.; Swaminath, A. Overall and chemotherapy-free survival following stereotactic body radiation therapy for abdominopelvic oligometastases. J. Med. Imaging Radiat. Oncol. 2020, 64, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Sicignano, G.; Rigo, M.; Figlia, V.; Cuccia, F.; De Simone, A.; Giaj-Levra, N.; Mazzola, R.; Naccarato, S.; Ricchetti, F.; et al. Daily dosimetric variation between image-guided volumetric modulated arc radiotherapy and MR-guided daily adaptive radiotherapy for prostate cancer stereotactic body radiotherapy. Acta Oncol. 2020, 60, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.; Bossi-Zanetti, I.; Mauro, R.; Beltramo, G.; Fariselli, L.; Bianchi, L.; Fodor, C.I.; Fossati, P.; Baroni, G.; Orecchia, R. CyberKnife robotic image-guided stereotactic radiotherapy for oligometastic cancer. Strahlenther. Onkol. 2013, 189, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Franceschini, D.; Perrone-Congedi, F.; Casamassima, F.; Gerardi, M.; Rigo, M.; Mazzola, R.; Perna, M.; Scotti, V.; Fodor, A.; et al. A multicenter LArge retrospectIve daTabase on the personalization of stereotactic ABlative radiotherapy use in lung metastases from colon-rectal cancer: The LaIT-SABR study. Radiother. Oncol. 2021, 166, 92–99. [Google Scholar] [CrossRef]
- Videtic, G.M.; Paulus, R.; Singh, A.K.; Chang, J.Y.; Parker, W.; Olivier, K.R.; Timmerman, R.D.; Komaki, R.R.; Urbanic, J.J.; Stephans, K.L.; et al. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients with Stage I Peripheral Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. 2018, 103, 1077–1084. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Lépinoy, A.; Silva, Y.E.; Martin, E.; Bertaut, A.; Quivrin, M.; Aubignac, L.; Cochet, A.; Créhange, G. Salvage extended field or involved field nodal irradiation in 18F-fluorocholine PET/CT oligorecurrent nodal failures from prostate cancer. Eur. J. Pediatr. 2018, 46, 40–48. [Google Scholar] [CrossRef]
- Pinkawa, M.; Aebersold, D.M.; Böhmer, D.; Flentje, M.; Ghadjar, P.; Schmidt-Hegemann, N.-S.; Höcht, S.; Hölscher, T.; Müller, A.-C.; Niehoff, P.; et al. Radiotherapy in nodal oligorecurrent prostate cancer. Strahlenther. Onkol. 2021, 197, 575–580. [Google Scholar] [CrossRef]

| Mean age (years) (range) | 71 (54–87) |
| ECOG PS | |
| • 0 | 62 |
| • 1 | 1 |
| Initial treatment | |
| • RP +/− ADT | 51 (81%) |
| • RT +/− ADT | 6 (9.4%) |
| • HIFU/other | 2 (3.2%) |
| • ADT | 4 (6.4%) |
| Initial stage | |
| • T2 | 17 (27%) |
| • T3 | 32 (51%) |
| • T4 | 1 (1.5%) |
| • Unknown | 13 (20.5%) |
| Initial PSA (ng/mL) (range) | 19.1 (3.5–140) |
| Initial Gleason | |
| • 6 | 9 (14%) |
| • 7 | 22 (35%) |
| • >8 | 28 (44.5%) |
| • Unknown | 4 (6.5%) |
| PSA before SBRT (ng/mL) (range) | 2.23 (0.22–36.84) |
| Oligometastatic status | |
| • Oligoreccurent | 40 (63.5%) |
| • Oligoprogressive | 23 (36.5%) |
| Imaging pre-SBRT | |
| • Choline-PET | 12 (19%) |
| • PSMA-PET | 51 (81%) |
| N° of treated lesions (per patient) | |
| • 1 | 41 (65%) |
| • 2 | 13 (20.5%) |
| • 3 | 7 (11.5%) |
| • 4 | 2 (3%) |
| Total SBRT course | |
| • 1 | 55 (87%) |
| • 2 | 7 (11.5%) |
| • 3 | 1 (1.5%) |
| Lesion diameter (mm) (total = 118) | 6 |
| Range (mm) | 2–26 |
| Lymph node site | |
| Pelvic | 88 (74.5%) |
| Extrapelvic | 30 (25.5%) |
| SBRT regimen | |
| Median dose (range) | 35 (15–40) |
| Median dose/fractions (range) | 7 (5–21) |
| Median BED (α/β = 1.5) | 198.33 (65–315) |
| Covariates | LPFS | NPFS | PFS |
|---|---|---|---|
| p | |||
| BED 198.33 | 0.344 | 0.968 | 0.08 |
| PTV margin | 0.427 | 0.496 | 0.674 |
| PSA 0.82 | 0.573 | 0.604 | 0.494 |
| PSA 0.7 | 0.17 | 0.783 | 0.379 |
| PSA 0.9 | 0.573 | 0.604 | 0.494 |
| Gleason | 0.267 | 0.608 | 0.795 |
| ADT concomitant | 0.07 | - | - |
| ADT before SBRT | 0.171 | 0.014 | 0.002 |
| Oligorecurrent versus oligoprogressive | - | 0.068 | 0.158 |
| Pelvic versus extrapelvic | 0.004 | 0.076 | 0.233 |
| Covariates | LPFS | NPFS | PFS |
|---|---|---|---|
| PSA before SBRT < 0.7 | p = 0.149 (HR 0.134, 95% CI 0.009–2.060) | - | - |
| Concomitant ADT | p = 0.872 (HR 0.805, 95% CI 0–1.705) | - | - |
| ADT before SBRT | p = 0.503 (HR 2.665, 95% CI 0.151–46.966) | p = 0.002 (HR 0.199, 95% CI 0.073–0.549) | p = 0.01 (HR 0.259, 95% CI 0.117–0.574) |
| Pelvic versus extrapelvic lymph node | p = 0.923 (HR 0, 95% CI 0–8.826) | p = 0.51 (HR 1.492, 95% CI 0.454–4.895) | |
| Oligorecurrent versus oligoprogressive | - | p = 0.018 (HR 3.679, 95% CI 1.247–10.851) | p = 0.106 (HR 1.975, 95% CI 0.865–4.511) |
| BED | - | - | p = 0.442 (HR 1.784, 95% CI 0.408–7.811) |
| Nodal recurrence | |
| Some nodal level | 6 (31.5) |
| Different nodal level | 11 (58) |
| Both | 2 (10.5) |
| Global disease recurrence | |
| Nodal | 15 (50) |
| Bone | 7 (23.4) |
| Node plus bone | 4 (13.3) |
| Visceral | 1 (3.3) |
| Prostate bed | 1 (3.3) |
| Biochemical | 2 (6.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, L.; Trapani, G.; Rigo, M.; Giaj-Levra, N.; Mazzola, R.; Pastorello, E.; Ricchetti, F.; Cuccia, F.; Figlia, V.; Fiorini, M.; et al. 1.5 T MR-Guided Daily Adapted SBRT on Lymph Node Oligometastases from Prostate Cancer. J. Clin. Med. 2022, 11, 6658. https://doi.org/10.3390/jcm11226658
Nicosia L, Trapani G, Rigo M, Giaj-Levra N, Mazzola R, Pastorello E, Ricchetti F, Cuccia F, Figlia V, Fiorini M, et al. 1.5 T MR-Guided Daily Adapted SBRT on Lymph Node Oligometastases from Prostate Cancer. Journal of Clinical Medicine. 2022; 11(22):6658. https://doi.org/10.3390/jcm11226658
Chicago/Turabian StyleNicosia, Luca, Giovanna Trapani, Michele Rigo, Niccolò Giaj-Levra, Rosario Mazzola, Edoardo Pastorello, Francesco Ricchetti, Francesco Cuccia, Vanessa Figlia, Matilde Fiorini, and et al. 2022. "1.5 T MR-Guided Daily Adapted SBRT on Lymph Node Oligometastases from Prostate Cancer" Journal of Clinical Medicine 11, no. 22: 6658. https://doi.org/10.3390/jcm11226658





