Comparison of Graft Materials in Multilayer Reconstruction with Nasoseptal Flap for High-Flow CSF Leak during Endoscopic Skull Base Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Graft Preparation
2.3. Reconstruction Techniques
2.4. Data Collections and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Surgical Outcomes and Intergroup Differences
3.3. Risk Factor Analysis for Postoperative CSF Leak or Meningitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavallo, L.M.; Messina, A.; Esposito, F.; de Divitiis, O.; Dal Fabbro, M.; de Divitiis, E.; Cappabianca, P. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J. Neurosurg. 2007, 107, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Hadad, G.; Bassagasteguy, L.; Carrau, R.L.; Mataza, J.C.; Kassam, A.; Snyderman, C.H.; Mintz, A. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope 2006, 116, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Murai, H.; Hasegawa, Y.; Hanazawa, T.; Yamakami, I.; Saeki, N. Endoscopic endonasal skull base reconstruction using a nasal septal flap: Surgical results and comparison with previous reconstructions. Neurosurg. Rev. 2010, 33, 235–241. [Google Scholar] [CrossRef]
- Fiorindi, A.; Gioffre, G.; Boaro, A.; Billeci, D.; Frascaroli, D.; Sonego, M.; Longatti, P. Banked Fascia Lata in Sellar Dura Reconstruction after Endoscopic Transsphenoidal Skull Base Surgery. J. Neurol. Surg. B Skull Base 2015, 76, 303–309. [Google Scholar] [PubMed] [Green Version]
- Horiguchi, K.; Nishioka, H.; Fukuhara, N.; Yamaguchi-Okada, M.; Yamada, S. A new multilayer reconstruction using nasal septal flap combined with fascia graft dural suturing for high-flow cerebrospinal fluid leak after endoscopic endonasal surgery. Neurosurg. Rev. 2016, 39, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.W.; Mariani, L. Sellar reconstruction with resorbable vicryl patches, gelatin foam, and fibrin glue in transsphenoidal surgery: A 10-year experience with 376 patients. J. Neurosurg. 2000, 93, 762–765. [Google Scholar] [CrossRef] [Green Version]
- Frank, G.; Pasquini, E.; Doglietto, F.; Mazzatenta, D.; Sciarretta, V.; Farneti, G.; Calbucci, F. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery 2006, 59, ONS75–ONS83. [Google Scholar] [CrossRef] [PubMed]
- Kulwin, C.; Schwartz, T.H.; Cohen-Gadol, A.A. Endoscopic extended transsphenoidal resection of tuberculum sellae meningiomas: Nuances of neurosurgical technique. Neurosurg. Focus 2013, 35, E6. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Navarro, V.; Anand, V.K.; Schwartz, T.H. Gasket seal closure for extended endonasal endoscopic skull base surgery: Efficacy in a large case series. World Neurosurg. 2013, 80, 563–568. [Google Scholar] [CrossRef]
- Youngerman, B.E.; Kosty, J.A.; Gerges, M.M.; Tabaee, A.; Kacker, A.; Anand, V.K.; Schwartz, T.H. Acellular dermal matrix as an alternative to autologous fascia lata for skull base repair following extended endoscopic endonasal approaches. Acta Neurochir. 2020, 162, 863–873. [Google Scholar] [CrossRef]
- Gaynor, B.G.; Benveniste, R.J.; Lieberman, S.; Casiano, R.; Morcos, J.J. Acellular dermal allograft for sellar repair after transsphenoidal approach to pituitary adenomas. J. Neurol. Surg. B Skull Base 2013, 74, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jeon, C.; Kong, D.S.; Park, K.; Kim, J.H. Clinical efficacy of radiation-sterilized allografts for sellar reconstruction after transsphenoidal surgery. J. Korean Neurosurg. Soc. 2011, 50, 503–506. [Google Scholar] [CrossRef]
- Esposito, F.; Dusick, J.R.; Fatemi, N.; Kelly, D.F. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper. Neurosurg. 2007, 60, ONS-295–ONS-304. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.W.; Gardner, P.A.; Zanation, A.M. International consensus statement on endoscopic skull-base surgery: Executive summary. Int Forum Allergy Rhinol. 2019, 9, S127–S144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eloy, J.A.; Patel, S.K.; Shukla, P.A.; Smith, M.L.; Choudhry, O.J.; Liu, J.K. Triple-layer reconstruction technique for large cribriform defects after endoscopic endonasal resection of anterior skull base tumors. Int. Forum Allergy Rhinol. 2013, 3, 204–211. [Google Scholar] [CrossRef]
- Hu, F.; Gu, Y.; Zhang, X.; Xie, T.; Yu, Y.; Sun, C.; Li, W. Combined use of a gasket seal closure and a vascularized pedicle nasoseptal flap multilayered reconstruction technique for high-flow cerebrospinal fluid leaks after endonasal endoscopic skull base surgery. World Neurosurg. 2015, 83, 181–187. [Google Scholar] [CrossRef]
- Xuejian, W.; Fan, H.; Xiaobiao, Z.; Yong, Y.; Ye, G.; Tao, X.; Junqi, G. Endonasal endoscopic skull base multilayer reconstruction surgery with nasal pedicled mucosal flap to manage high flow CSF leakage. Turk. Neurosurg. 2013, 23, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Munich, S.A.; Fenstermaker, R.A.; Fabiano, A.J.; Rigual, N.R. Cranial base repair with combined vascularized nasal septal flap and autologous tissue graft following expanded endonasal endoscopic neurosurgery. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2013, 74, 101–108. [Google Scholar] [CrossRef]
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R.; et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 2010, 22, 1–143. [Google Scholar]
- Li, Z.; Ji, T.; Huang, G.D.; Guo, J.; Yang, J.H.; Li, W.P. A Stratified Algorithm for Skull Base Reconstruction with Endoscopic Endonasal Approach. J. Craniofac. Surg. 2018, 29, 193–198. [Google Scholar] [CrossRef]
- Prickett, K.K.; Wise, S.K.; Delgaudio, J.M. Choice of graft material and postoperative healing in endoscopic repair of cerebrospinal fluid leak. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 457–461. [Google Scholar] [CrossRef]
- Brown, S.L.; Govier, F.E. Cadaveric versus autologous fascia lata for the pubovaginal sling: Surgical outcome and patient satisfaction. J. Urol. 2000, 164, 1633–1637. [Google Scholar] [CrossRef]
- Almeida, S.H.; Gregório, E.P.; Rodrigues, M.A.; Grando, J.P.; Moreira, H.A.; Fraga, F.C. Banked cadaveric fascia lata: 3-year follow-up. Transpl. Proc. 2004, 36, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.Z.; Brown, S.; Anand, V.K.; Schwartz, T.H. "Gasket-seal" watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery 2008, 62, ONSE342–ONSE343. [Google Scholar] [CrossRef]
- Luginbuhl, A.J.; Campbell, P.G.; Evans, J.; Rosen, M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope 2010, 120, 876–880. [Google Scholar] [CrossRef] [Green Version]
- Abiri, A.; Abiri, P.; Goshtasbi, K.; Lehrich, B.M.; Sahyouni, R.; Hsu, F.P.K.; Cadena, G.; Kuan, E.C. Endoscopic Anterior Skull Base Reconstruction: A Meta-Analysis and Systematic Review of Graft Type. World Neurosurg. 2020, 139, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.L.; Medary, M.B.; Dureza, C.D.; Bellotte, J.B.; Flannagan, P.P.; Oh, M.Y.; Fukushima, T. Dural repair using acellular human dermis: Experience with 200 cases: Technique assessment. Neurosurgery 2000, 46, 1391–1396. [Google Scholar] [CrossRef]
- Citardi, M.J.; Cox, A.J., 3rd; Bucholz, R.D. Acellular dermal allograft for sellar reconstruction after transsphenoidal hypophysectomy. Am. J. Rhinol. 2000, 14, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Taufique, Z.M.; Bhatt, N.; Zagzag, D.; Lebowitz, R.A.; Lieberman, S.M. Revascularization of AlloDerm Used during Endoscopic Skull Base Surgery. J. Neurol. Surg. B Skull Base 2019, 80, 46–50. [Google Scholar] [CrossRef]
- Germani, R.M.; Vivero, R.; Herzallah, I.R.; Casiano, R.R. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am. J. Rhinol. 2007, 21, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.R.; Dean, R.L.; Hurley, D.B.; Chuang, J.; Citardi, M.J. Endoscopic reconstruction of anterior and middle cranial fossa defects using acellular dermal allograft. Laryngoscope 2003, 113, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Aurora, A.; Iannotti, J.P. Allograft Fascia Lata as an Augmentation Device for Musculoskeletal Repairs; Cleveland Clinic: Cleveland, OH, USA, 2008. [Google Scholar]
- Lee, M.J.; Oh, J.Y.; Choung, H.K.; Kim, N.J.; Sung, M.S.; Khwarg, S.I. Frontalis sling operation using silicone rod compared with preserved fascia lata for congenital ptosis a three-year follow-up study. Ophthalmology 2009, 116, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Kim, H.Y.; Kim, S.H.; Min, J.Y.; Nam, D.H.; Park, K.; Dhong, H.J.; Kim, J.H. Challenging reconstructive techniques for skull base defect following endoscopic endonasal approaches. Acta Neurochir. 2011, 153, 807–813. [Google Scholar] [CrossRef] [PubMed]
| All Patients | |
|---|---|
| Number of patients | 193 |
| Age, year (range) | 48.9 (4–84) |
| Sex, male | 86 (44.6) |
| BMI, kg/m2 | 24.4 ± 4.0 |
| Pathology | |
| Craniopharyngioma | 69 (35.8) |
| Meningioma (tuberculum sellae) | 43 (22.3) |
| Pituitary adenoma | 37 (19.2) |
| Chordoma | 11 (5.7) |
| Meningioma (olfactory groove) | 5 (2.6) |
| Etc * | 28 (14.5) |
| Tumor size, mm | 30.4 ± 10.0 |
| Time of surgery, min | 284.2 ± 94.2 |
| Previous radiation therapy | 9 (4.7) |
| Previous gamma knife radiosurgery | 10 (5.2) |
| Revision surgery | 24 (12.4) |
| Defect location | |
| Suprasella | 162 (83.9) |
| Posterior fossa | 13 (6.7) |
| Anterior skull base | 18 (9.3) |
| Graft | |
| Acellular dermal matrix | 48 (24.9) |
| Homologous graft | 102 (52.8) |
| Autologous graft | 43 (22.3) |
| Postoperative lumbar drain | 111 (57.5) |
| Follow-up period, years | 3.5 ± 1.9 |
| Postoperative CSF leak | 23 (11.9) |
| Postoperative meningitis | 8 (4.1) |
| Acellular Dermal Matrix | Banked Fascia Lata | Autologous Fascia Lata | p-Value | |
|---|---|---|---|---|
| Number of patients | 48 | 102 | 43 | |
| Age, year (range) * | 48.5 (9–84) | 49.5 (6–81) | 48.1 (4–77) | 0.95 |
| Sex, male † | 17 (35.4) | 48 (47.1) | 21 (48.8) | 0.33 |
| BMI, kg/m2 * | 23.8 ± 3.2 | 24.3 ± 4.2 | 25.4 ± 4.4 | 0.10 |
| Tumor size, mm * | 31.0 ± 9.9 | 29.6 ± 9.6 | 31.6 ± 10.9 | 0.42 |
| Time of surgery, min * | 268.7 ± 92.3 | 288.3 ± 92.1 | 291.7 ± 101.3 | 0.63 |
| Previous RT ‡ | 1 (2.1) | 4 (3.9) | 4 (9.3) | 0.31 |
| Previous GKS ‡ | 2 (4.2) | 6 (5.9) | 2 (4.7) | 1.00 |
| Revision surgery † | 4 (8.3) | 11 (10.8) | 9 (20.9) | 0.15 |
| Defect location ‡ | 0.52 | |||
| Suprasella | 44 (91.7) | 82 (80.4) | 36 (83.7) | |
| Posterior fossa | 1 (2.1) | 9 (8.8) | 3 (7.0) | |
| Anterior skull base | 3 (6.3) | 11 (10.8) | 4 (9.3) | |
| Postoperative L-drain † | 18 (37.5) | 66 (64.7) | 27 (62.8) | 0.005 |
| L-drain duration (days) * | 4.9 ± 1.4 | 4.9 ± 2.4 | 4.5 ± 2.3 | 0.57 |
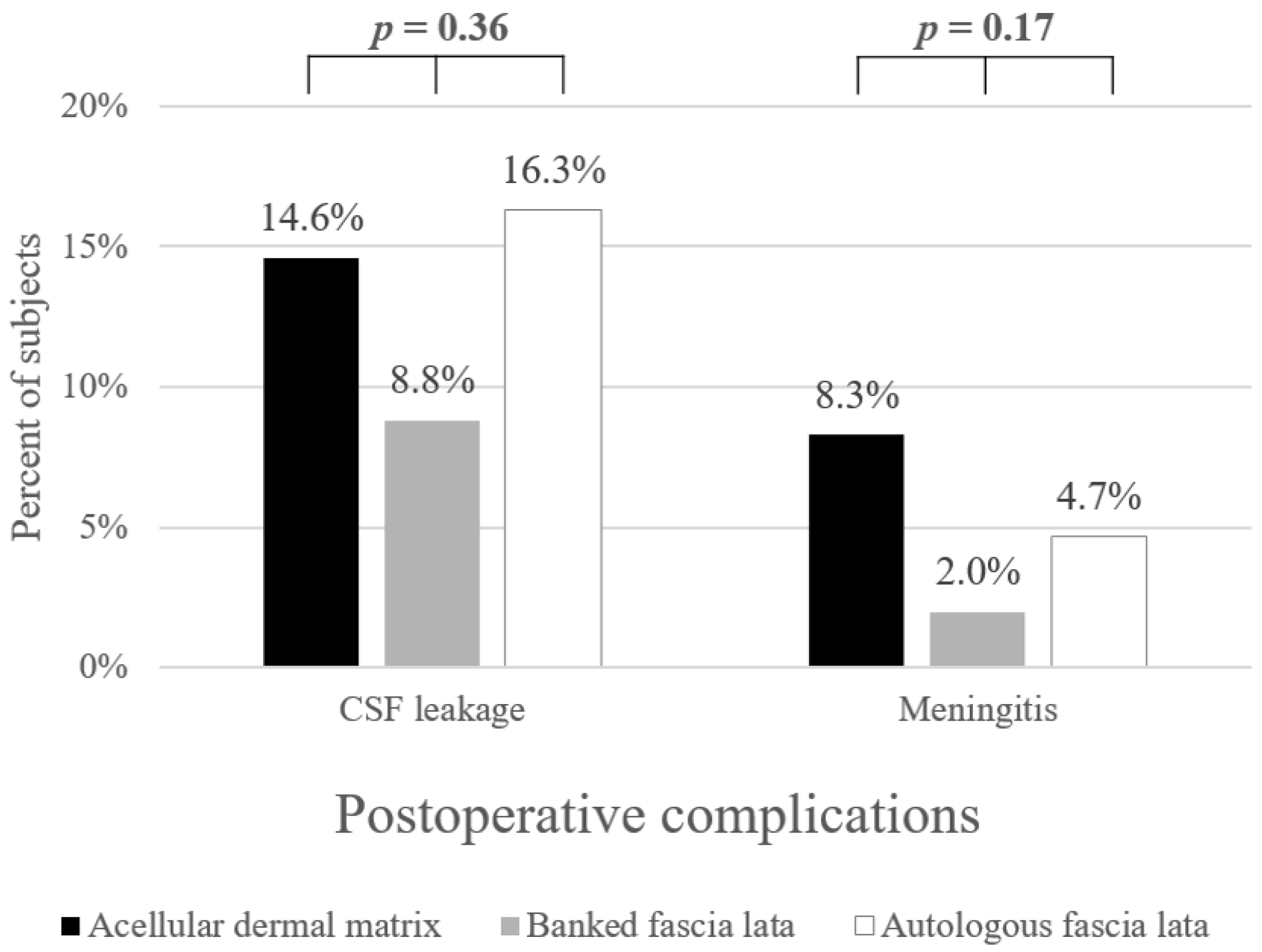
| Postoperative CSF leakage † | 7 (14.6) | 9 (8.8) | 7 (16.3) | 0.36 |
| Postoperative meningitis ‡ | 4 (8.3) | 2 (2.0) | 2 (4.7) | 0.17 |
| Yes (23) | No (170) | p-Value * | |
|---|---|---|---|
| Age, year (range) | 45.5 (8–77) | 49.4 (4–84) | 0.50 |
| Sex, male, % | 14 (60.9) | 72 (42.4) | 0.09 |
| BMI, kg/m2 | 24.9 ± 3.7 | 24.4 ± 4.1 | 0.39 |
| Pathology, yes, % | |||
| Craniopharyngioma | 9 (39.1) | 60 (35.3) | 0.72 |
| Meningioma (tuberculum sellae) | 5 (21.7) | 38 (22.4) | 0.95 |
| Pituitary adenoma | 5 (21.7) | 32 (18.8) | 0.78 |
| Chordoma | 1 (4.3) | 10 (5.9) | 1.00 |
| Meningioma (olfactory groove) | 1 (4.3) | 4 (2.4) | 0.47 |
| Etc | 2 (8.7) | 26 (15.3) | 0.54 |
| Tumor size, mm | 32.2 ± 11.6 | 30.1 ± 9.7 | 0.41 |
| Time of surgery, min | 251.1 ± 88.3 | 288.7 ± 94.3 | 0.10 |
| Previous RT, yes, % | 0 (0) | 9 (5.3) | 0.60 |
| Previous GKS, yes, % | 1 (4.3) | 9 (5.3) | 1.00 |
| Revision surgery, yes, % | 5 (21.7) | 19 (11.2) | 0.17 |
| Defect location, yes, % | 0.81 | ||
| Suprasella | 21 (91.3) | 141 (82.9) | |
| Posterior fossa | 1 (4.3) | 12 (7.1) | |
| Anterior skull base | 1 (4.3) | 17 (10.0) | |
| Graft, yes, % | 0.36 | ||
| Acellular dermal matrix | 7 (30.4) | 41 (24.1) | |
| Allograft | 9 (39.1) | 93 (54.7) | |
| Autograft | 7 (30.4) | 36 (21.2) | |
| Postoperative lumbar drain, yes, % | 12 (52.2) | 99 (58.2) | 0.58 |
| Yes (8) | No (185) | p-Value * | |
|---|---|---|---|
| Age, year (range) | 39.4 (8–63) | 49.4 (4–84) | 0.16 |
| Sex, male, % | 4 (50) | 82 (44.3) | 1.00 |
| BMI, kg/m2 | 23.6 ± 2.8 | 24.5 ± 4.1 | 0.59 |
| Pathology, yes, % | |||
| Craniopharyngioma | 4 (50) | 65 (35.1) | 0.46 |
| Meningioma (tuberculum sellae) | 2 (25) | 41 (22.2) | 1.00 |
| Pituitary adenoma | 2 (25) | 35 (18.9) | 0.65 |
| Chordoma | 0 (0) | 11 (5.9) | 1.00 |
| Meningioma (olfactory groove) | 0 (0) | 5 (2.7) | 1.00 |
| Etc | 0 (0) | 28 (15.1) | 0.61 |
| Tumor size, mm | 29.3 ± 6.4 | 30.4 ± 10.1 | 0.96 |
| Time of surgery, min | 237.4 ± 80.4 | 286.2 ± 94.4 | 0.21 |
| Previous RT, yes, % | 0 (0) | 9 (4.9) | 1.00 |
| Previous GKS, yes, % | 0 (0) | 10 (5.4) | 1.00 |
| Revision surgery, yes, % | 2 (25.0) | 22 (11.9) | 0.26 |
| Defect location, yes, % | 1.00 | ||
| Suprasella | 8 (100) | 154 (83.2) | |
| Posterior fossa | 0 (0) | 13 (7.0) | |
| Anterior skull base | 0 (0) | 18 (9.7) | |
| Graft, yes, % | 0.17 | ||
| Acellular dermal matrix | 4 (50.0) | 44 (23.8) | |
| Allograft | 2 (25.0) | 100 (54.1) | |
| Autograft | 2 (25.0) | 41 (22.2) | |
| Postoperative lumbar drain, yes, % | 4 (50.0) | 107 (57.8) | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.K.; Kong, D.-S.; Nam, D.-H.; Hong, S.D. Comparison of Graft Materials in Multilayer Reconstruction with Nasoseptal Flap for High-Flow CSF Leak during Endoscopic Skull Base Surgery. J. Clin. Med. 2022, 11, 6711. https://doi.org/10.3390/jcm11226711
Kim BK, Kong D-S, Nam D-H, Hong SD. Comparison of Graft Materials in Multilayer Reconstruction with Nasoseptal Flap for High-Flow CSF Leak during Endoscopic Skull Base Surgery. Journal of Clinical Medicine. 2022; 11(22):6711. https://doi.org/10.3390/jcm11226711
Chicago/Turabian StyleKim, Byung Kil, Doo-Sik Kong, Do-Hyun Nam, and Sang Duk Hong. 2022. "Comparison of Graft Materials in Multilayer Reconstruction with Nasoseptal Flap for High-Flow CSF Leak during Endoscopic Skull Base Surgery" Journal of Clinical Medicine 11, no. 22: 6711. https://doi.org/10.3390/jcm11226711
APA StyleKim, B. K., Kong, D.-S., Nam, D.-H., & Hong, S. D. (2022). Comparison of Graft Materials in Multilayer Reconstruction with Nasoseptal Flap for High-Flow CSF Leak during Endoscopic Skull Base Surgery. Journal of Clinical Medicine, 11(22), 6711. https://doi.org/10.3390/jcm11226711





