A New Pharmacological Vitreolysis through the Supplement of Mixed Fruit Enzymes for Patients with Ocular Floaters or Vitreous Hemorrhage-Induced Floaters
Abstract
:1. Introduction
2. Methods
2.1. Design
2.2. Subjects
2.3. Materials
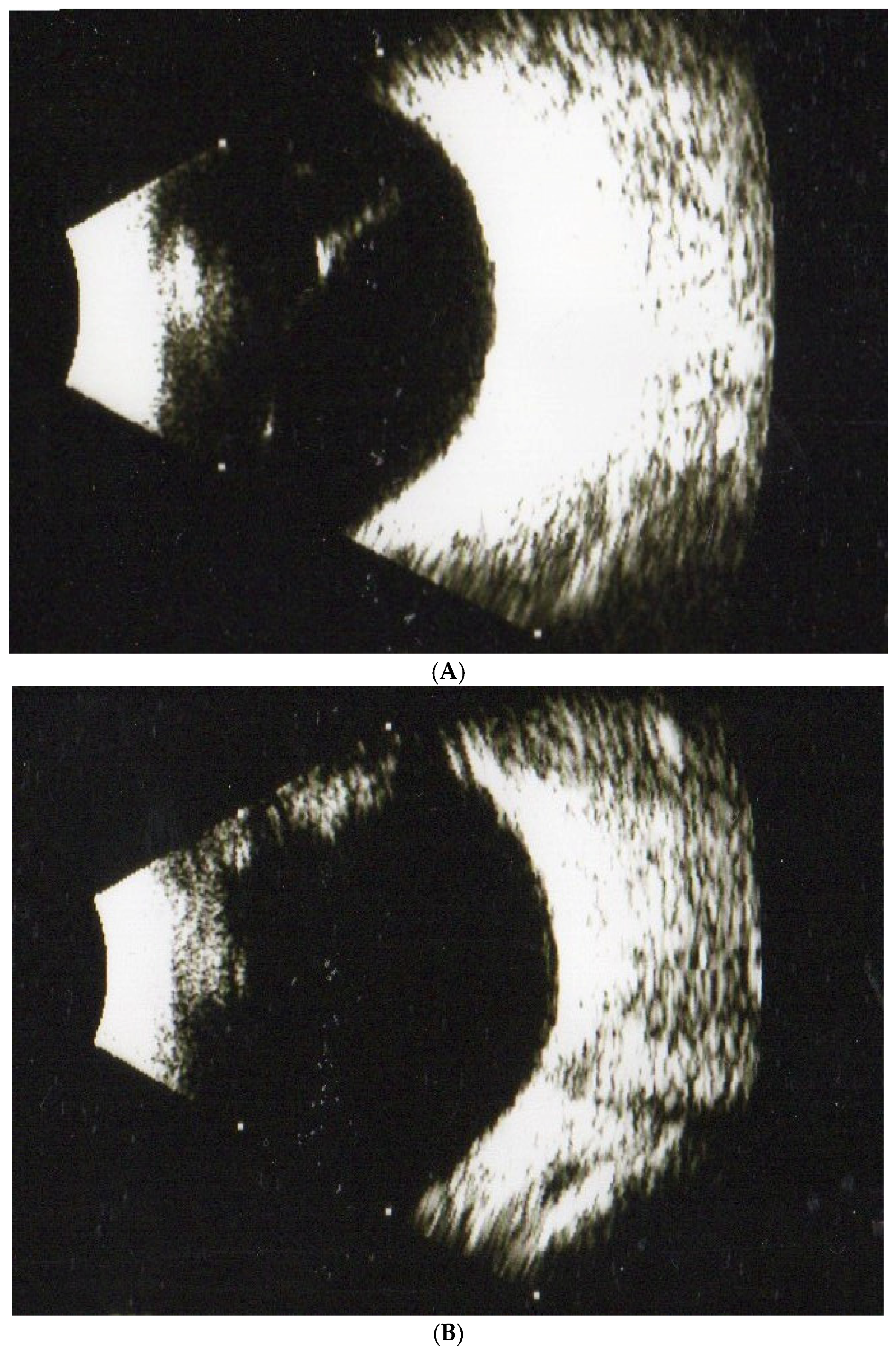
2.4. Procedure
2.5. Statistical Analysis
2.6. 1,1 Diphenyl-2 Picrylhydrazyl (DPPH) Test
2.7. Questionnaire on Satisfaction and Subjective Sensation
3. Results
3.1. Human Study
3.2. The Evaluation of Antioxidant Abilities (DDPH Test)
3.3. Questionnaire on Satisfaction and Subjective Sensation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Aguirre, G.; Henaine-Berra, A.; Scalcedo-Villanueva, G. Visualization and grading of vitreous floaters using dy-namic ultra-wildfield infrared confocal scanning laser optholomology: A pilot study. J. Clin. Med. 2020, 11, 5502. [Google Scholar] [CrossRef] [PubMed]
- Lamande, S.R.; Bateman, J.F. Collagen VI disorders: Insights on forms and functions in the extracellular matrix and be-yond. Matrix Biol. 2018, 71–72, 348–367. [Google Scholar] [CrossRef] [PubMed]
- Gishti, O.; van den Nieuwenhol, R.; Verhoekx, J.; van Overdam, K. Symptoms related to posterior vitreous detachment and the risk of developing retinal tears: A systematic review. Acta Ophthalmol. 2019, 97, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Vitreous and vision degrading myodesopsia. Prog. Retin. Eye. Res. 2020, 79, 100847. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.; Jalil, A.; Antoniou, Y.; Bishop, P.N.; Vallejo-Garcia, J.L.; Patton, N. Vitrectomy for primary symptomatic vitreous opacities: An evidence-based review. Eye 2016, 30, 645–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond-Taylor, M.; Jakosson, G.; Zetterberg, M. Posterior vitreous detachment–prevalence of and risk factors for retinal tears. Clin. Ophthalmol. 2017, 11, 1689–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Moon, S.Y.; Yim, K.M.; Seong, S.J.; Hwang, J.Y.; Park, S.P. Psychologic distress in patients with symptomatic vitreous floaters. J. Ophthalmol. 2017, 2017, 3191576. [Google Scholar]
- Chen, J.T.; Wu, H.J. Blue light fromelectronic devicesmay bean important factorforvitreous floaters. Med. Hypotheses. 2020, 139, 109698. [Google Scholar] [CrossRef]
- Webb, B.F.; Webb, J.R.; Schroeder, M.C.; North, M.C. Prevalence of vitreous floaters in a community sample of smartphone users. Int. J. Ophthalmol. 2013, 6, 402–405. [Google Scholar]
- de Nie, K.F.; Crama, N.; Tilanus, A.D.; Klevering, B.J.; Boon, J.F. Pars plana vitrectomy for disturbing primary vitreous floaters: Clinical outcome and patient satisfaction. Grafes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1373–1382. [Google Scholar] [CrossRef]
- Nian, S.; Lo, C.Y.; Mi, Y.; Ren, D.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Mudvari, S.S.; Shott, S.S. Clinical characteristics affecting the outcome of pneumatic retinopexy. Arch Ophthalmol. 2011, 129, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous antioxidants, degeneration, and vitreo-retinopathy. Antioxidants 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollands, H.; Johnson, D.; Brox, A.C.; Almedia, D.; Simel, D.A.; Sharma, S. Acute-onset floaters and flashes. Is this patient at risk for retinal detachment? JAMA 2009, 302, 2243–2249. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.E. Vitreous opacity vitrectomy (VOV): Safest possible removal of “Floaters”. Clin. Ophthalmol. 2022, 16, 1653–1663. [Google Scholar] [CrossRef]
- Wu, R.H.; Jiang, J.H.; Gu, Y.F.; Moonasar, N.; Lin, Z. Pars plana vitrectomy relieves the depression in patients with symptomatic vitreous floaters. Int. J. Ophthalmol. 2020, 13, 412–416. [Google Scholar] [CrossRef]
- Lumi, X.; Hawlina, M.; Glavač, D.; Facskó, A.; Moe, M.C.; Kaarniranta, K.; Petrovski, G. Ageing of the vitreous: Floaters from acute onset and flashes to retinal detachment. Ageing Res. Rev. 2015, 21, 71–77. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cheang, W.M.; Hwang, D.K.; Lin, C.H. Vitreous haemorrhage: A population-based study of the incidence and risk factors in Taiwan. Int. J. Ophthalmol. 2017, 10, 461–466. [Google Scholar]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy-ocular complications of diabetes mellitus. World. J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef]
- Wagle, A.M.; Lim, W.Y.; Yap, T.P.; Neelman, K.; Au Eong, K.G. Utility values associated with vitreous floater. Am. J. Ophthalmol. 2011, 152, 60–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.P. Management of Floaters. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 388–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, H.D.; Liu, H.T.; Xu, X.; Zhang, X. The impact of persistent visually disabling vitreous floaters on health status utility values. Qual. Life Res. 2013, 22, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.E.; Lima, L.H.; Nasscimento, H.; Zett, C.; Belfort, R., Jr. Objective assessment of YAG laser vitreolysis in patients with symptomatic viotreous floaters. Int. J. Retin. Vitr. 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.P.; Heier, J.S. YAG laser vitreolysis vs. sham YAG vitreolysis for symptomatic vitreous floaters: A randomized clinical trial. JAMA Ophthlamol. 2017, 135, 918–923. [Google Scholar] [CrossRef]
- Cordero, I. Understanding and satefy using ophthalmic lasers. Community Eye Health 2015, 28, 76–77. [Google Scholar]
- GEvans, J.R.; Michelessi, M.; Virgili, G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2014, 2014, 11234. [Google Scholar]
- Takeuichi, M.; Shieh, P.C.; Horng, C.T. Treatment of symptomatic vitreous opacities with pharmacologic vitrelysis using a mixture of bromelain, papain and ficin supplement. Appl. Sci. 2020, 10, 5901. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Hong, T.; Chang, A.A. To treat or not to treat: Management options for symptomatic vitreous floaters. Asia. Pac. J. Ophthalmol. 2020, 9, 96–103. [Google Scholar] [CrossRef]
- Horng, C.T.; Chen, F.A.; Kuo, D.H.; Chen, L.C.; Yen, S.S.; Shieh, P.C. Pharmacologic vitreolysis of vitreous floaters by 3-month pineapple in Taiwan: A pilot Study. J. Am. Sci. 2019, 15, 17–30. [Google Scholar]
- Dupps, W.J., Jr.; Kohnen, T.; Mamalis, N.; Rosen, E.S.; Koch, D.D.; Obstaum, S.A.; Waring, G.O., 3rd; Reinstein, D.Z.; Stulting, R.D. Standardized graphs and terms for refractive surgery results. J. Cataract Refract. Surg. 2011, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food. Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.Z.; Li, T.T.; Zhang, X.M.; Hui, Y.N.; Moutari, S.; Pazo, E.E.; Dai, G.Z.; Shen, L.J. The efficacy and safety of YAG Laser vitreolysis for symptomatic vitreous floaters of vomplete PVD or Non-PVD. Ophthalmol. Ther. 2022, 11, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Goa, Q.Y.; Hui, Y.N. Vitreous function and intyervention of it with vitrectomy and other modalities. Int. J. Ophthalmol. 2022, 15, 857–867. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.D.; Gad Elkareem, A.M.; Zwinderman, A.H. The vitreous, the retinal interface in ocular health and disease. Ophthalmologica 2013, 230, 165–178. [Google Scholar] [CrossRef] [PubMed]
- El-Sanhouri, A.A.; Foster, R.E.; Petersen, M.R.; Hutchins, R.K.; Miller, D.M.; Evans, T.M.; Trichopoulos, N.; Riemann, C.D. Retinal tears after posterior vitreous detachment and vitreous hemorrhage in patients on systemic anticoagulants. Eye 2011, 25, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Bergandi, L.; Skorokhod, O.A.; La Grotta, R.; Schwarzer, E.; Nuzzi, R. Oxidative stress, lipid peroxidation, and loss of hyaluronic acid in the human vitreous affected by synchysis scintillans. J. Ophthalmol. 2019, 2019, 7231015. [Google Scholar] [CrossRef]
- Schulz-Key, S.; Carlsson, J.O.; Crafoord, S. Long-term follow-up of pars plana vitrectomy for anterior vitreous floaters: Complication, outcome, and patient satisfaction. Acta Ophthalmol. 2011, 89, 159–165. [Google Scholar] [CrossRef]
- Manandhar, L.D.; Thapa, R.; Poudyal, G. Clinical profile and management of vitreous hemorrhage in tertiary eye care centre in Nepal. Nepal. J. Ophthalmol. 2020, 12, 99–105. [Google Scholar] [CrossRef]
- Alfayyadh, M.A.; Alabdulhadi, H.A.; Almubarak, M.H. Advanced Eales’ disease with neovascular glaucoma at first presentation. Cureus 2021, 13, e18302. [Google Scholar] [CrossRef]
- Hahn, P.; Schneider, E.W.; Tabandeh, H.; Wong, R.W. Reported complications following laser vitreolysis. JAMA Ophthalmol. 2017, 135, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Adelman, R.A. Recent developments in laser treatment of diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2015, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Heny, C.R.; Smiddy, W.E.; Flynn, H.W., Jr. Pars plana vitrectomy for vitreous floaters: Is there such a thing as minimally-invasive vitreoretinal surgery? Retina 2014, 34, 1043–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, G.L.; Silva, P.S.; Arring, P.G.; Sun, J.K. Postoperative complications of pars plana vitrectomy for diabetic retinal disease. Semin. Ophthalmol. 2018, 33, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Kampik, A.; Kuppermann, B.D.; Girach, A.; Rizzo, S.; Sergott, R.C. Safety profile of ocriplasmin for the pharmacologic treatment of symptomatic vitreomacular adhesion/traction. Retina 2015, 35, 1111–1127. [Google Scholar] [CrossRef]
- Stalmans, P.; Duker, J.S.; Kaiser, P.K.; Heier, J.S.; Dugel, P.U.; Gandorfer, A.; Sebag, J.; Haller, J.A. Oct-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina 2013, 33, 2003–2011. [Google Scholar] [CrossRef]
- Ha, M.; Bekhitb, A.E.; Carnea, A.; Hopkinsc, D.L. Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food. Chem. 2012, 134, 95–105. [Google Scholar] [CrossRef]
- Pavan, R.; Shraddha, S.J.; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.H.; Huang, X.Q.; Liu, L.J.; Tang, M.S.; Zhan, C.G. Reaction pathway and free energy profile for papain-catalyzed hydrolysis of N-acetyl-Phe-Gly 4-nitroanilide. Biochemistry 2013, 52, 5145–5154. [Google Scholar] [CrossRef] [Green Version]
- Wihastyoko, Y.L.; Soeharto, S.; Widjajanto, E.; Handono, K.; Pardjianto, B. Does papain enzyme improve collagen degradation? Rev. Pharm. 2021, 12, 676–684. [Google Scholar]
- Nishimura, K.; Higashiya, K.; Uesshima, N.; Abe, T.; Yasukawa, K. Characterization of proteases activities in Ficus carica cultivars. J. Food. Sci. 2020, 85, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Chakaborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compond: A comprehesive overview from a pharmacologic perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Ataide, J.A.; de carvalho, N.M. Bacterial nancellulose loaded with bromelain: Assement of antimicrobial, antioxidant and physical-chemical properities. Sci. Rep. 2017, 7, 18031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J.R. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food. Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Pattamapun, K.; Manosroi, W.; Manosroi, J. Antioxidant and gelatinolytic activities of papain from papaya Latex and bromelain from pineapple fruits. Chiang Mai J. Sci. 2014, 41, 635–648. [Google Scholar]
- Cho, U.M.; Choi, D.H.; Yoo, D.S.; Park, S.J.; Hwang, H.S. Inhibitory effect of ficin derived from Fig latex on inflammation and melanin production in skin cells. Biotechnol. Bioprocess Eng. 2019, 24, 288–297. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Balakireva, A.V.; Kuzetsova, N.V.; Vukolova, M.N.; Litvitsky, P.F.; Zamyatnin, A.A., Jr. Collagenolytic enzymes and their application in biomedicine. Curr. Med. Chem. 2019, 26, 487–505. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging and diseases. Clin. Interv. Agin. 2018, 13, 753–772. [Google Scholar] [CrossRef]
- Singh, M.; Tyagi, S.C. Metalloproteinases as mediators of inflammation and the eye: Molecular genetic underprinnings governing ocular pathology. Int. J. Ophthalmol. 2017, 10, 1308–1318. [Google Scholar]
- Yang, T.; Guo, L.; Fang, Y.; Liang, M.; Zheng, Y.Z.; Pan, M.D.; Meng, C.; Liu, G.G. Pericytes of indirect contact coculture decrease intergrity of inner blood-retina barrier model in vitro by upgrading MMP-2/9 activity. Dis. Markers 2021, 2021, 7124835. [Google Scholar] [CrossRef]
- Sebag, J. Floaters and the quality of life. Am. J. Ophthalmol. 2011, 152, 3–4. [Google Scholar] [CrossRef] [PubMed]




| Numbers of Capsules Each Day | Before | 1st Month | 2nd Month | 3rd Month |
| None | 40 | 40 | 43 | 38 (95%) |
| 1 capsule | 40 | 40 | 36 | 18 * (45%) # |
| 2 capsules | 40 | 39 | 34 | 15 * (37.5) # |
| 3 capsules | 40 | 38 | 32 | 12 * (30%) # |
| Numbers of Capsules Each Day | Before | 1st Month | 2nd Month | 3rd Month |
|---|---|---|---|---|
| None | 16 | 15 | 15 | 13 (82%) |
| 1 capsule | 16 | 14 | 13 | 13 (82%) |
| 2 capsules | 16 | 13 | 12 | 12 (75%) |
| 3 capsules | 16 | 13 | 12 | 7 * (44%) # |
| Numbers of Capsules Each Day | Before | 1st Month | 2nd Month | 3rd Month |
|---|---|---|---|---|
| None | 0.61 ± 0.11 | 0.62 ± 0.12 | 0.58 ± 0.10 | 0.59 ± 0.15 |
| 1 capsule | 0.59 ± 0.08 | 0.54 ± 0.15 | 0.48 ± 0.19 | 0.40 ± 0.12 |
| 2 capsules | 0.62 ± 0.15 | 0.50 ± 0.20 | 0.45 ± 0.20 | 0.34 ± 0.15 |
| 3 capsules | 0.63 ± 0.19 | 0.49 ± 0.15 | 0.40 ± 0.25 | * 0.19 ± 0.09 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.-W.; Hung, J.-L.; Takeuchi, M.; Shieh, P.-C.; Horng, C.-T. A New Pharmacological Vitreolysis through the Supplement of Mixed Fruit Enzymes for Patients with Ocular Floaters or Vitreous Hemorrhage-Induced Floaters. J. Clin. Med. 2022, 11, 6710. https://doi.org/10.3390/jcm11226710
Ma J-W, Hung J-L, Takeuchi M, Shieh P-C, Horng C-T. A New Pharmacological Vitreolysis through the Supplement of Mixed Fruit Enzymes for Patients with Ocular Floaters or Vitreous Hemorrhage-Induced Floaters. Journal of Clinical Medicine. 2022; 11(22):6710. https://doi.org/10.3390/jcm11226710
Chicago/Turabian StyleMa, Jui-Wen, Jen-Lin Hung, Masaru Takeuchi, Po-Chuen Shieh, and Chi-Ting Horng. 2022. "A New Pharmacological Vitreolysis through the Supplement of Mixed Fruit Enzymes for Patients with Ocular Floaters or Vitreous Hemorrhage-Induced Floaters" Journal of Clinical Medicine 11, no. 22: 6710. https://doi.org/10.3390/jcm11226710
APA StyleMa, J. -W., Hung, J. -L., Takeuchi, M., Shieh, P. -C., & Horng, C. -T. (2022). A New Pharmacological Vitreolysis through the Supplement of Mixed Fruit Enzymes for Patients with Ocular Floaters or Vitreous Hemorrhage-Induced Floaters. Journal of Clinical Medicine, 11(22), 6710. https://doi.org/10.3390/jcm11226710








