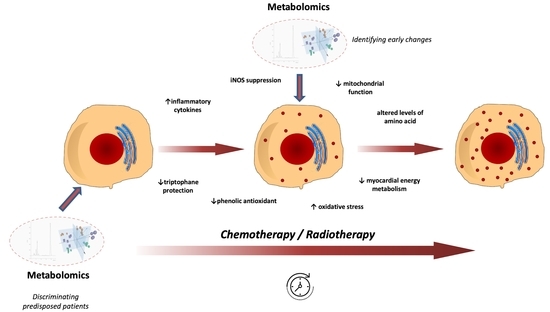
Metabolomic Profiles on Antiblastic Cardiotoxicity: New Perspectives for Early Diagnosis and Cardioprotection
Abstract
:1. Introduction
2. What’s Metabolomics?
- Sample collection and storage;
- Sample analysis, using nuclear magnetic resonance spectroscopy and mass spectrometry techniques;
- Data analysis using multivariate statistical tools.
3. Until 2019: Early Detection of Drug-Induced Cardiotoxicity in Animal Studies
4. Step Ahead: The Human Model
4.1. In Vitro
4.2. In Vivo
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CTX | Cardiotoxicity |
| ATP | Adenosine TriPhosphate |
| MRI | Magnetic Resonance Imaging |
| TCA | Tricarboxylic acid |
| PCr | Phosphocreatine |
| YWPC | Yellow Wine Polyphenolic Compound |
| NEFA | Non Esterified Fatty Acids |
| HF | Heart Failure |
| GY | Grey |
| ADP | Adenosine DiPhosphate |
| AMP | Adenosine MonoPhosphate |
| AMPK | Adenosine-MonoPhosphate Kinase |
| NMR | Nuclear Magnetic Resonance |
| MS | Mass Spectrometry |
| GC | Gas Chromatography |
| LC | Liquid Chromatograph |
| DOX | Doxorubicine |
| iNOS | inducible Nitric Oxide Synthases |
| eNOS | endothelial Nitric Oxide Synthases |
| THP | pirarubicin |
| L-THP | Liposome pirarubicin |
| F-THP | Free pirarubicin |
| UPLC–Q-TOF-MS | Ultra-Performance Liquid Chromatography–Quadrupole Time-of-Flight MS |
| H-NMR | Hydrogen NMR |
| hiPSC-CMs | Human induced Pluripotent Stem Cell-Derived CardioMyocytes |
| DZR | Dexrazoxane |
| TKIs | Tyrosine Kinase Inhibitors |
References
- Mele, D.; Nardozza, M.; Spallarossa, P.; Frassoldati, A.; Tocchetti, C.G.; Cadeddu, C.; Madonna, R.; Malagù, M.; Ferrari, R.; Mercuro, G. Current views on anthracycline cardiotoxicity. Heart Fail. Rev. 2016, 21, 621–634. [Google Scholar] [CrossRef]
- Madeddu, C.; Deidda, M.; Piras, A.; Cadeddu, C.; Demurtas, L.; Puzzoni, M.; Piscopo, G.; Scartozzi, M.; Mercuro, G. Pathophysiology of cardiotoxicity induced by non-anthracycline chemotherapy. J. Cardiovasc. Med. 2016, 17 (Suppl. S1), S12–S18. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.M.; Anzevino, S.; Rao, R. Cardiovascular disease in cancer survivors. Postgrad. Med. J. 2017, 93, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Vo, J.B.; Ramin, C.; Barac, A.; de Gonzalez, A.B.; Veiga, L. Trends in heart disease mortality among breast cancer survivors in the US, 1975–2017. Breast Cancer Res. Treat. 2022, 192, 611–622. [Google Scholar] [CrossRef]
- Mercuro, G.; Cadeddu, C.; Piras, A.; Dessì, M.; Madeddu, C.; Deidda, M.; Serpe, R.; Massa, E.; Mantovani, G. Early epirubicin-induced myocardial dysfunction revealed by serial tissue doppler echocardiography: Correlation with inflammatory and oxidative stress markers. Oncologist 2007, 12, 1124–1133. [Google Scholar] [CrossRef]
- Deidda, M.; Mercurio, V.; Cuomo, A.; Noto, A.; Mercuro, G.; Cadeddu Dessalvi, C. Metabolomic Perspectives in Antiblastic CardiotoxiCity and Cardioprotection. Int. J. Mol. Sci. 2019, 20, 4928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The human metabolome database in 2013. Nucl. Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucl. Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Andreadou, I.; Papaefthimiou, M.; Zira, A.; Constantinou, M.; Sigala, F.; Skaltsounis, A.L.; Tsantili-Kakoulidou, A.; Iliodromitis, E.K.; Kremastinos, D.T.; Mikros, E. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 2009, 22, 585–592. [Google Scholar] [CrossRef]
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.I.; et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Lou, Z.; Liao, W.; Zhu, Z.; Dong, X.; Zhang, W.; Li, W.; Chai, Y. Potential biomarkers in mouse myocardium of doxorubicin-induced cardiomyopathy: A metabolomic method and its application. PLoS ONE 2011, 6, e27683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, W.; Liang, Q.; Li, L.; Shi, J.; Liu, Q.; Feng, Y.; Wang, Y.; Luo, G. Metabonomic study on the cumulative cardiotoxicity of a pirarubicin liposome powder. Talanta 2012, 89, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ju, L.; Hou, Z.; Deng, H.; Zhang, Z.; Wang, L.; Yang, Z.; Yin, J.; Zhang, Y. Screening, verification, and optimization of biomarkers for early prediction of cardiotoxicity based on metabolomics. J. Proteome Res. 2015, 14, 2437–2445. [Google Scholar] [CrossRef]
- Schnackenberg, L.K.; Pence, L.; Vijay, V.; Moland, C.L.; George, N.; Cao, Z.; Yu, L.R.; Fuscoe, J.C.; Beger, R.D.; Desai, V.G. Early metabolomics changes in heart and plasma during chronic doxorubicin treatment in B6C3F1 mice. J. Appl. Toxicol. 2016, 36, 1486–1495. [Google Scholar] [CrossRef]
- Yin, J.; Xie, J.; Guo, X.; Ju, L.; Li, Y.; Zhang, Y. Plasma metabolic profiling analysis of cyclophosphamide-induced cardiotoxicity using metabolomics coupled with UPLC/Q-TOF-MS and ROC curve. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033, 428–435. [Google Scholar] [CrossRef]
- Chaudhari, U.; Ellis, J.K.; Wagh, V.; Nemade, H.; Hescheler, J.; Keun, H.C.; Sachinidis, A. Metabolite signatures of doxorubicin induced toxicity in human induced pluripotent stem cell-derived cardiomyocytes. Amino Acids 2017, 49, 1955–1963. [Google Scholar] [CrossRef] [Green Version]
- QuanJun, Y.; GenJin, Y.; LiLi, W.; YongLong, H.; Yan, H.; Jie, L.; JinLu, H.; Jin, L.; Run, G.; Cheng, G. Protective effects of dexrazoxane against doxorubicin-induced cardiotoxicity: A metabolomic study. PLoS ONE 2017, 12, e0169567. [Google Scholar] [CrossRef] [Green Version]
- Yun, W.; Qian, L.; Yuan, R.; Xu, H. Periplocymarin Alleviates Doxorubicin-Induced Heart Failure and Excessive Accumulation of Ceramides. Front. Cardiovasc. Med. 2021, 8, 732554. [Google Scholar] [CrossRef]
- Timm, K.N.; Perera, C.; Ball, V.; Henry, J.A.; Miller, J.J.; Kerr, M.; West, J.A.; Sharma, E.; Broxholme, J.; Logan, A.; et al. Early detection of doxorubicin-induced cardiotoxicity in rats by its cardiac metabolic signature assessed with hyperpolarized MRI. Commun. Biol. 2020, 3, 692. [Google Scholar] [CrossRef]
- Timm, K.N.; Ball, V.; Miller, J.J.; Savic, D.; West, J.A.; Griffin, J.L.; Tyler, D.J. Metabolic Effects of Doxorubicin on the Rat Liver Assessed With Hyperpolarized MRI and Metabolomics. Front. Physiol. 2022, 12, 782745. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Cui, C.; Wang, C.; Lu, S.; Zhang, M.; Chen, D.; Jiang, P. Systematic Evaluations of Doxorubicin-Induced Toxicity in Rats Based on Metabolomics. ACS Omega 2020, 6, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M.; Boguszewicz, Ł.; Ciszek, M.; Gabryś, D.; Kulik, R.; Sokół, M. Metabolic changes in mice cardiac tissue after low-dose irradiation revealed by 1H NMR spectroscopy. J. Radiat. Res. 2020, 61, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.M.; Wang, Y.K.; Yan, D.M.; Fang, J.H.; Xiao, X.R.; Zhang, T.; Cheng, Y.; Xu, K.P.; Li, F. Metabolic profiling of tyrosine kinase inhibitor nintedanib using metabolomics. J. Pharm. Biomed. Anal. 2020, 180, 113045. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Meng, L.; Sun, Z.; Sun, S.; Huang, X.; Lin, N.; Zhang, J.; Lu, W.; Yang, Q.; Chi, J.; et al. Yellow Wine Polyphenolic Compound Protects Against Doxorubicin-Induced Cardiotoxicity by Modulating the Composition and Metabolic Function of the Gut Microbiota. Circ. Heart Fail. 2021, 14, e008220. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Mohamed, O.Y.; Ahamad, S.R.; Al-Jenoobi, F.I. The protective effect of losartan against sorafenib induced cardiotoxicity: Ex-vivo isolated heart and metabolites profiling studies in rat. Eur. J. Pharmacol. 2020, 882, 173229. [Google Scholar] [CrossRef]
- Alhazzani, K.; Alotaibi, M.R.; Alotaibi, F.N.; Aljerian, K.; As Sobeai, H.M.; Alhoshani, A.R.; Alanazi, A.Z.; Alanazi, W.A.; Alswayyed, M. Protective effect of valsartan against doxorubicin-induced cardiotoxicity: Histopathology and metabolomics in vivo study. J. Biochem. Mol. Toxicol. 2021, 35, e22842. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Mar. Drugs 2018, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J.A.; Smith, A.M.; Gryshkova, V.; Donley, E.L.R.; Valentin, J.P.; Burrier, R.E. A Targeted Metabolomics-Based Assay Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Identifies Structural and Functional Cardiotoxicity Potential. Toxicol. Sci. 2020, 174, 218–240. [Google Scholar] [CrossRef]
- Draguet, A.; Tagliatti, V.; Colet, J.M. Targeting Metabolic Reprogramming to Improve Breast Cancer Treatment: An In Vitro Evaluation of Selected Metabolic Inhibitors Using a Metabolomic Approach. Metabolites 2021, 11, 556. [Google Scholar] [CrossRef]
- Dionísio, F.; Araújo, A.M.; Duarte-Araújo, M.; Bastos, M.L.; Guedes de Pinho, P.; Carvalho, F.; Costa, V.M. Cardiotoxicity of cyclophosphamide’s metabolites: An in vitro metabolomics approach in AC16 human cardiomyocytes. Arch. Toxicol. 2022, 96, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Unger, K.; Li, Y.; Yeh, C.; Barac, A.; Srichai, M.B.; Ballew, E.A.; Girgis, M.; Jayatilake, M.; Sridharan, V.; Boerma, M.; et al. Plasma metabolite biomarkers predictive of radiation induced cardiotoxicity. Radiother. Oncol. 2020, 152, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A.; Shi, X.; Farrell, L.; Lall, R.; Sebag, I.A.; Plana, J.C.; Gerszten, R.E.; Scherrer-Crosbie, M. Changes in Citric Acid Cycle and Nucleoside Metabolism Are Associated with Anthracycline Cardiotoxicity in Patients with Breast Cancer. J. Cardiovasc. Transl. Res. 2020, 13, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Cocco, D.; Ferro, E.G.; Ricci, S.; Deidda, M.; Noto, A.; Madeddu, C.; Atzori, F.; Scartozzi, M.; Mercuro, G.; Cadeddu Dessalvi, C. University of Cagliari, Italy, Defining the metabolomic profile associated with early cardiotoxicity in patients with breast cancer treated with anthracyclines. Eur. Heart J. 2020, 41, ehaa946.3289. [Google Scholar] [CrossRef]
- Madonna, R.; Cadeddu, C.; Deidda, M.; Giricz, Z.; Madeddu, C.; Mele, D.; Monte, I.; Novo, G.; Pagliaro, P.; Pepe, A.; et al. Cardioprotection by gene therapy: A review paper on behalf of the Working Group on Drug Cardiotoxicity and Cardioprotection of the Italian Society of Cardiology. Int. J. Cardiol. 2015, 191, 203–210. [Google Scholar] [CrossRef]
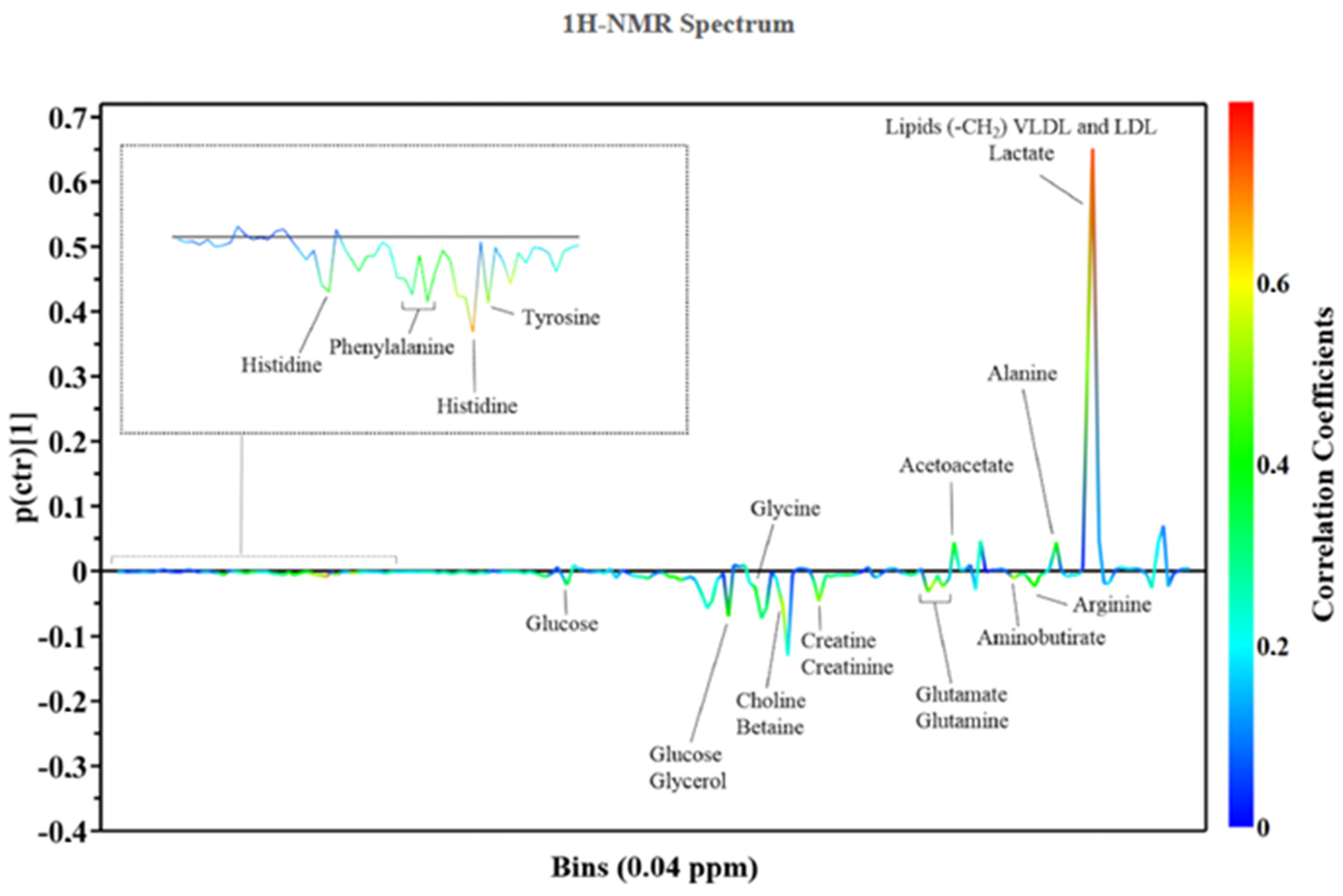
| Reference | Species | Biofluid/Tissue | Metabolites/Metabolism Discrimination |
|---|---|---|---|
| Andreadu et al., 2009 [4] | Wistar rats | Aqueous myocardial extracts | Increased levels of acetate and succinate, decreased levels of branched-chain amino acids |
| Andreadu et al., 2014 [5] | Wistar rats | Aqueous myocardial extracts | Perturbations of energy metabolism |
| Tan et al., 2011 [6] | ICR mice | Myocardial tissue | Increased levels of L-alanine, phosphate, glycine, succinate, malate, proline, threonic acid, glutamine, phenylalanine, dihydroxyacetonephosphate (DHAP), glycerol-3-phosphate (G-3-P), fructose, glucose, stearic acid, myo-inositol and cholesterol; decreased levels of lactate, β-hydroxybutyric acid, l-valine, isoleucine, threonine, citrate, linoleic acid, arachidonic acid |
| Cong et al., 2012 [7] | Sprague-Dawley rats | Urine | Metabolites involved in metabolic process related to myocardial energy metabolism: tricarboxylic acid cycle (citrate), glycolysis (lactate), pentose phosphate pathway (d-gluconate-1-phosphate) and amino acid metabolism (N-acetylglutamine and N-acetyl-dl-tryptophan) |
| Li et al., 2015 [8] | Wistar rats | Plasma | l-carnitine, 19-hydroxydioxycortic acid, LPC (14:0) and LPC (20:2) |
| Schnackenberg et al., 2016 [9] | B6C3F1 mice | Heart tissue, Plasma | Myocardial specimens: altered levels of 18 amino acids and acetylornithine, kynurenine, putrescine and serotonin, decreased levels of 5 acylcarnitines. Plasma samples: altered levels of 16 amino acids and acetylornithine and hydroxyproline, increased levels of 16 acylcarnitines |
| Yin et al., 2016 [10] | Wistar rats | Plasma | l-carnitine, proline, 19-hydroxydeoxycorticosterone, phuyoshingosine, cholic acid, LPC (14:0), LPC (18:3), LPC (16:1), LPE (18:2), LPC (22:5), LPC (22:6), linoleic acid, LPC (22:4), LPC (20:2), LPE (18:0), LPC (20:3) |
| Chaudhari et al., 2017 [11] | Human-induced pluripotent stem cell-derived cardiomyocytes | Culture medium | Reduction in the utilisation of pyruvate and acetate, and accumulation of formate |
| QuanJun et al., 2017 [12] | BALB/c mice | Serum | DOX administration: increase in 5-hydroxylisine, 2-hydroxybutyrate, 2-oxoglutarate, 3-hydroxybutyrate decrease in glucose, glutamate, cysteine, acetone, methionine, asparate, isoleucine and glycylproline. DZR treatment: increase in lactate, 3-hydroxybutyrate, glutamate, alanine; decrease in glucose, trimethylamine N-oxide and carnosine levels |
| Yun et al., 2021 [13] | C57BL/6 mice | Myocardial tissue | Periplocymarin reduced cardiomyocyte apoptosis protecting myocytes from DOX-induced CTX |
| Timm et al., 2020 [14] | Wistar rats | Myocardial tissue, Plasma | DOX administration: decrease of the tricarboxylic acid (TCA) cycle intermediate malate, TCA cycle-related glutamate, total carnitine, acetyl carnitine, NAD, AMP, ADP, ATP |
| Timm et al., 2022 [15] | Wistar rats | Liver tissue | DOX administration: increase in several acyl-carnitine species as well as increases in high energy phosphates, citrate and markers of oxidative stress |
| Geng et al., 2020 [16] | Sprague-Dawley rats | Serum, heart, liver, kidney, and brain tissue | DOX administration: the altered metabolites in the heart were 3-methyl-1-pentanol, cholesterol, d-glucose, d-lactic acid, glycerol, glycine, l-alanine, l-valine, palmitic acid, phenol, propanoic acid, and stearic acid |
| Gramatyka et al., 2020 [17] | C57Bl/6NCrl mice | Heart tissue | Ionizing radiation with 2 Gy: high levels of pantothenate and glutamate and decreased levels of alanine, malonate, acetylcarnitine, glycine and adenosine |
| Zhou et al., 2020 [18] | C57BL/6 J mice | Feces, urine, plasma | Nintedanib metabolic pathways majorly included were hydroxylation, demethylation, glucuronidation, and acetylation reactions |
| Lin et al., 2021 [19] | Sprague-Dawley rats | Serum | YWPC influences the levels of metabolites altered by DOX (decreased levels of arachidonic and linoleic acid, increased levels of tryptophan) |
| Abdelgail et al., 2020 [20] | Wistar rats | Serum, heart tissue | Some metabolites were associated with sorafenib-induced CTX, particularly glycin and lattic acid; the coadministration of Losartan reverted these changes |
| Alhazzani et al.,2021 [21] | Sprague-Dawley rats | Serum | DOX monotherapy reduced concentrations of several amino acids, in contrast the combination therapy reverses these metabolic pathways |
| Reference | Species | Biofluid/Tissue | Metabolites/Metabolism Discrimination |
|---|---|---|---|
| Chin Yoon et al., [22] | Human cardiomyocyte cell line (AC 16) and human breast cancer cell line (MCF-7) | Culture medium | Spinochrome D (SpD) influenced glutathione metabolism in AC16 cells and and increased ATP production and the oxygen consumption rate in D-galactose-treated AC16 cells. SpD protected these cells from DOX-induced CTX, reducing the mitochondrial damage of DOX |
| Palmer et al., 2020 [23] | Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) | Culture medium | Arachidonic acid, lactic acid, 2′-deoxycytidine and thymidine have important roles in modulating oxidative stress, mitochondrial function and replication resulted associated with CTX |
| Draguet et al., 2021 [24] | MDA-MB-231 (ATCC® HTB-26TM) and MCF-7 (ATCC® HTB-22TM) cell lines | Culture medium | The combination of CB-839 (glutaminase inhibitor) and Oxamate (lactate dehydrogenase inhibitor) and the combination of CB-839/Oxamate/D609 (a phosphatidylcholine-specific phospholipase C inhibitor) caused significant cell mortality in two breast cancer cell lines (MDA-MB-231 and MCF-7) and were able to improve DOX-efficacy on the same cell lines |
| Dionisio et al., 2022 [25] | Human cardiac proliferative and differentiated AC16 cells | Culture medium | 4-hydroxycyclophosphamide and acrolein induced mitochondrial and lysosomal dysfunction: increased in sugar levels within the cells and a perturbed levels of some metabolites of the Krebs cycle and altered levels of amino acid |
| Unger et et al., 2020 [26] | Sprague-Dawley rats; Patients receiving radiation therapy for esophageal cancer | Heart Tissue; Plasma | Radiation therapy CTX: SM(d18:1/16:0), PC(16:0/14:0), SM(d18:1/18:0), PE(16:0/20:4), 1-(1,2-Dihexanoylphosphatidyl) inositol-4,5-bisphosphate and Gly-Arg-Gly-Asp-Asn-Pro were upregulated |
| Asnani et al., 2020 [27] | Women with breast cancer treated with anthracyclines and trastuzumab | Plasma | Changes in citric acid and aconitic acid that differentiated patients who developed CTX |
| Cocco et al., 2020 [28] | Human population of breast cancer patient | Plasma | In patients with CTX were identified a higher prevalence of Krebs cycle intermediates (fumarate and succinate) and fatty acid (e.g., linoleic acid). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazzini, L.; Caggiari, L.; Deidda, M.; Onnis, C.; Saba, L.; Mercuro, G.; Cadeddu Dessalvi, C. Metabolomic Profiles on Antiblastic Cardiotoxicity: New Perspectives for Early Diagnosis and Cardioprotection. J. Clin. Med. 2022, 11, 6745. https://doi.org/10.3390/jcm11226745
Fazzini L, Caggiari L, Deidda M, Onnis C, Saba L, Mercuro G, Cadeddu Dessalvi C. Metabolomic Profiles on Antiblastic Cardiotoxicity: New Perspectives for Early Diagnosis and Cardioprotection. Journal of Clinical Medicine. 2022; 11(22):6745. https://doi.org/10.3390/jcm11226745
Chicago/Turabian StyleFazzini, Luca, Ludovica Caggiari, Martino Deidda, Carlotta Onnis, Luca Saba, Giuseppe Mercuro, and Christian Cadeddu Dessalvi. 2022. "Metabolomic Profiles on Antiblastic Cardiotoxicity: New Perspectives for Early Diagnosis and Cardioprotection" Journal of Clinical Medicine 11, no. 22: 6745. https://doi.org/10.3390/jcm11226745