Effect of Propofol versus Sevoflurane Anesthesia on Acute Kidney Injury after Lung Transplantation Surgery: A Prospective Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Patient Enrollment
2.2. Study Protocol
2.3. Perioperative Care
2.4. Study Endpoints
2.5. Other Assessments
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Perioperative Data
3.3. Perioperative Renal Function and Post-Operative 30-Day Morbidity
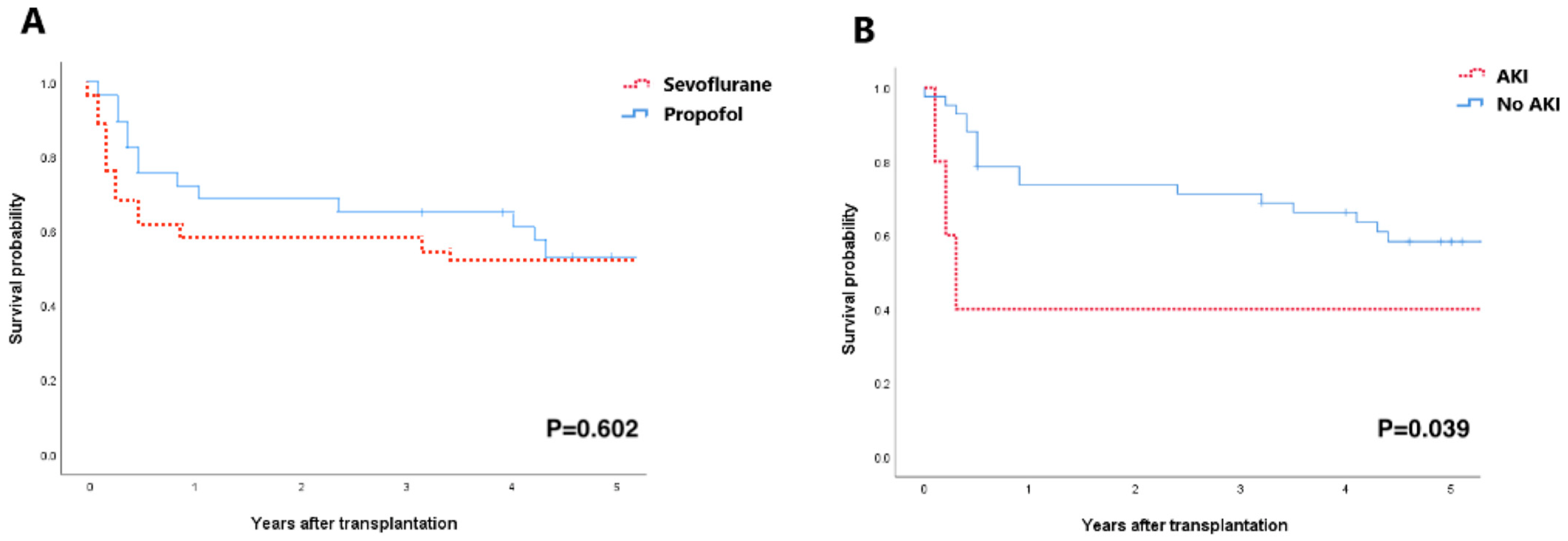
3.4. Long-Term Prognosis of AKI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, P.N.; Rocha, A.T.; Palmer, S.M.; Davis, R.D.; Smith, S.R. Acute renal failure after lung transplantation: Incidence, predictors and impact on perioperative morbidity and mortality. Am. J. Transplant. 2005, 5, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutakis, G.J.; George, T.J.; Robinson, C.W.; Gibbs, K.W.; Orens, J.B.; Merlo, C.A.; Shah, A.S. Severe acute kidney injury according to the RIFLE (risk, injury, failure, loss, end stage) criteria affects mortality in lung transplantation. J. Heart Lung Transplant. 2011, 30, 1161–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaoutakis, G.J.; George, T.J.; Robinson, C.W.; Gibbs, K.W.; Orens, J.B.; Merlo, C.A.; Shah, A.S. Incidence and outcomes of acute kidney injury following orthotopic lung transplantation: A population-based cohort study. Nephrol. Dial. Transplant. 2014, 29, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Meersch, M.; Schmidt, C.; Zarbock, A. Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth. Analg. 2017, 125, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Jacques, F.; El-Hamamsy, I.; Fortier, A.; Maltais, S.; Perrault, L.P.; Liberman, M.; Noiseux, N.; Ferraro, P. Acute renal failure following lung transplantation: Risk factors, mortality, and long-term consequences. Eur. J. Cardiothorac. Surg. 2012, 41, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, L.; Chen, W.; Guo, L.; Zhao, L.; Liang, C.; Chen, J.; Wang, C. Acute kidney injury after lung transplantation: A narrative review. Ann. Transl. Med. 2021, 9, 717. [Google Scholar] [CrossRef]
- Bae, H.B. Volatile anesthetics and ischemia-reperfusion injury. Korean J. Anesthesiol. 2015, 68, 211–212. [Google Scholar] [CrossRef] [Green Version]
- Motayagheni, N.; Phan, S.; Eshraghi, C.; Nozari, A.; Atala, A. A Review of Anesthetic Effects on Renal Function: Potential Organ Protection. Am. J. Nephrol. 2017, 46, 380–389. [Google Scholar] [CrossRef]
- Lee, Y.M.; Song, B.C.; Yeum, K.J. Impact of Volatile Anesthetics on Oxidative Stress and Inflammation. BioMed Res. Int. 2015, 2015, 242709. [Google Scholar] [CrossRef]
- Wagner, J.; Strosing, K.M.; Spassov, S.G.; Lin, Z.; Engelstaedter, H.; Tacke, S.; Hoetzel, A.; Faller, S. Sevoflurane posttreatment prevents oxidative and inflammatory injury in ventilator-induced lung injury. PLoS ONE 2018, 13, e0192896. [Google Scholar] [CrossRef]
- Hsu, H.T.; Tseng, Y.T.; Hsu, Y.Y.; Cheng, K.I.; Chou, S.H.; Lo, Y.C. Propofol attenuates lipopolysaccharide-induced reactive oxygen species production through activation of Nrf2/GSH and suppression of NADPH oxidase in human alveolar epithelial cells. Inflammation 2015, 38, 415–423. [Google Scholar] [CrossRef]
- Durand, F.; Francoz, C.; Asrani, S.K.; Khemichian, S.; Pham, T.A.; Sung, R.S.; Genyk, Y.S.; Nadim, M.K. Acute Kidney Injury After Liver Transplantation. Transplantation 2018, 102, 1636–1649. [Google Scholar] [CrossRef]
- Gumbert, S.D.; Kork, F.; Jackson, M.L.; Vanga, N.; Ghebremichael, S.J.; Wang, C.Y.; Eltzschig, H.K. Perioperative Acute Kidney Injury. Anesthesiology 2020, 132, 180–204. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.C.; Shim, J.K.; Song, Y.; Yang, S.Y.; Kwak, Y.L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014, 86, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.Y.; Lee, J.; Oh, J.; Song, J.G.; Hwang, G.S. The Influence of Propofol and Sevoflurane on Acute Kidney Injury after Colorectal Surgery: A Retrospective Cohort Study. Anesth. Analg. 2016, 123, 363–370. [Google Scholar] [CrossRef]
- Li, H.; Weng, Y.; Yuan, S.; Liu, W.; Yu, H.; Yu, W. Effect of sevoflurane and propofol on acute kidney injury in pediatric living donor liver transplantation. Ann. Transl. Med. 2019, 7, 340. [Google Scholar] [CrossRef]
- Shin, S.; Joo, D.J.; Kim, M.S.; Bae, M.I.; Heo, E.; Lee, J.S.; Kim, D.W.; Yoo, Y.C. Propofol intravenous anesthesia with desflurane compared with desflurane alone on postoperative liver function after living-donor liver transplantation: A randomized controlled trial. Eur. J. Anesthesiol. 2019, 36, 656–666. [Google Scholar] [CrossRef]
- Yu, W.S.; Paik, H.C.; Haam, S.J.; Lee, C.Y.; Nam, K.S.; Jung, H.S.; Do, Y.W.; Shu, J.W.; Lee, J.G. Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes. J. Thorac. Dis. 2016, 8, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.S.; Shim, J.K.; Soh, S.; Kim, M.K.; Kwak, Y.L. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016, 89, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Ricci, Z.; Cruz, D.N.; Ronco, C. Classification and staging of acute kidney injury: Beyond the RIFLE and AKIN criteria. Nat. Rev. Nephrol. 2011, 7, 201–208. [Google Scholar] [CrossRef]
- Ius, F.; Kuehn, C.; Tudorache, I.; Sommer, W.; Avsar, M.; Boethig, D.; Fuehner, T.; Gottlieb, J.; Hoeper, M.; Haverich, A.; et al. Lung transplantation on cardiopulmonary support: Venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2012, 144, 1510–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Cantu, E.; Christie, J.D. Primary graft dysfunction. Semin. Respir. Crit. Care Med. 2013, 34, 305–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertjitbanjong, P.; Thongprayoon, C.; Cheungpasitporn, W.; O’Corragain, O.A.; Srivali, N.; Bathini, T.; Watthanasuntorn, K.; Aeddula, N.R.; Salim, S.A.; Ungprasert, P.; et al. Acute Kidney Injury after Lung Transplantation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Craft, M.L.; Wang, P.; Wyburn, K.R.; Chen, G.; Ma, J.; Hambly, B.; Chadban, S.J. IL-18 contributes to renal damage after ischemia-reperfusion. J. Am. Soc. Nephrol. 2008, 19, 2331–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, H.; Matsunami, E.; Blazon-Brown, N.; Koutsogiannaki, S.; Hou, L.; Bu, W.; Babazada, H.; Odegard, K.C.; Liu, R.; Eckenhoff, R.G.; et al. Volatile anesthetics affect macrophage phagocytosis. PLoS ONE 2019, 14, e0216163. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Yang, Z.; Luo, F.; Zheng, S. Gut microbial balance and liver transplantation: Alteration, management, and prediction. Front. Med. 2018, 12, 123–129. [Google Scholar] [CrossRef]
- Han, C.; Zhang, Z.; Guo, N.; Li, X.; Yang, M.; Peng, Y.; Ma, X.; Yu, K.; Wang, C. Effects of Sevoflurane Inhalation Anesthesia on the Intestinal Microbiome in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 633527. [Google Scholar] [CrossRef]
- Toews, G.B. Cytokines and the lung. Eur. Respir. J. Suppl. 2001, 34, 3s–17s. [Google Scholar] [CrossRef] [Green Version]
- Takada, M.; Nadeau, K.C.; Hancock, W.W.; MacKenzie, H.S.; Shaw, G.D.; Waaga, A.M.; Chandraker, A.; Sayegh, M.H.; Tilney, N.L. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation 1998, 65, 1533–1542. [Google Scholar] [CrossRef]
- Nijboer, W.; Schuurs, T.; van der Hoeven, J.; Leuvenink, H.; van der Heide, J.H.; van Goor, H.; Ploeg, R. Effects of brain death on stress and inflammatory response in the human donor kidney. Transplant. Proc. 2005, 37, 367–369. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Svokos, A.A.; Svokos, K.A.; Zacharoulis, D. Extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation: A meta-analysis. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 38–47. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Lertjitbanjong, P.; Aeddula, N.R.; Bathini, T.; Watthanasuntorn, K.; Srivali, N.; Mao, M.A.; Kashani, K. Incidence and Impact of Acute Kidney Injury in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. J. Clin. Med. 2019, 8, 981. [Google Scholar] [CrossRef] [Green Version]
- Sbrana, S.; Nunziata, A.; Storti, S.; Haxhiademi, D.; Mazzone, A.; Leone, M.; Solinas, M.; Del Sarto, P. Differential modulatory effects of Propofol and Sevoflurane anesthesia on blood monocyte HLA-DR and CD163 expression during and after cardiac surgery with cardiopulmonary bypass: A preliminary randomized flow cytometry study. Perfusion 2020, 35, 48–56. [Google Scholar] [CrossRef]
- Ihn, C.; Joo, J.; Choi, J.; Kim, D.; Jeon, Y.; Kim, Y.; Jung, H.; Kwon, S. Comparison of stress hormone response, interleukin-6 and anesthetic characteristics of two anesthetic techniques: Volatile induction and maintenance of anesthesia using sevoflurane versus total intravenous anesthesia using propofol and remifentanil. J. Int. Med. Res. 2009, 37, 1760–1771. [Google Scholar] [CrossRef]
- Kostopanagiotou, G.; Kalimeris, K.; Christodoulaki, K.; Nastos, C.; Papoutsidakis, N.; Dima, C.; Chrelias, C.; Pandazi, A.; Mourouzis, I.; Pantos, C. The differential impact of volatile and intravenous anesthetics on stress response in the swine. Hormones 2010, 9, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Erturk, E. Ischemia-reperfusion injury and volatile anesthetics. BioMed Res. Int. 2014, 2014, 526301. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Yang, N.; Zhang, X.; Xu, X. The significance and mechanism of propofol on treatment of ischemia reperfusion induced lung injury in rats. Cell Biochem. Biophys. 2014, 70, 1527–1532. [Google Scholar] [CrossRef]
- Vasileiou, I.; Xanthos, T.; Koudouna, E.; Perrea, D.; Klonaris, C.; Katsargyris, A.; Papadimitriou, L. Propofol: A review of its non-anesthetic effects. Eur. J. Pharmacol. 2009, 605, 1–8. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Oshima, Y.; Sano, M.; Kajiwara, I.; Ichimaru, Y.; Itaya, T.; Kuramochi, T.; Hayashi, E.; Kim, J.; Kitajima, O.; Masugi, Y.; et al. Midazolam exhibits antitumour and anti-inflammatory effects in a mouse model of pancreatic ductal adenocarcinoma. Br. J. Anaesth. 2022, 128, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Liao, K.S.; Yeh, L.R.; Wang, Y.K. Drug Repurposing: The Mechanisms and Signaling Pathways of Anti-Cancer Effects of Anesthetics. Biomedicines 2022, 10, 1589. [Google Scholar] [CrossRef] [PubMed]

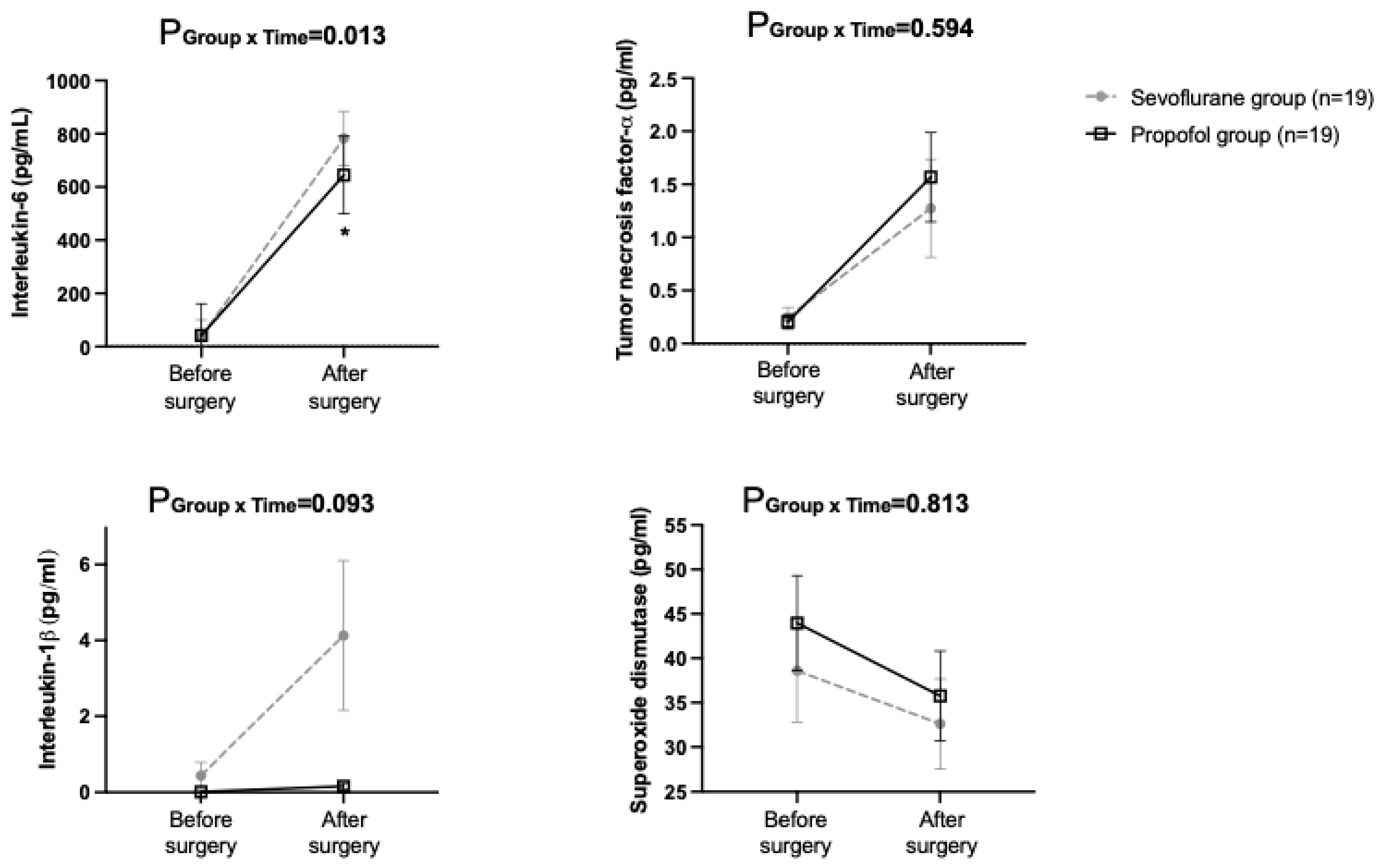
| Variables | Sevoflurane Group (n = 29) | Propofol Group (n = 30) | p Value | |
|---|---|---|---|---|
| Age, y | 51 ± 14 | 54 ± 11 | 0.381 | |
| Male sex | 17 (59) | 16 (53) | 0.908 | |
| Body surface area, m2 | 1.7 ± 0.2 | 1.6 ± 0.1 | 0.095 | |
| Hypertension | 5 (17) | 4 (13) | 0.731 | |
| Diabetes | 5 (17) | 5 (17) | 1.000 | |
| Left ventricular ejection fraction, % | 61 ± 13 | 62 ± 14 | 0.761 | |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 111.2 ± 22.9 | 110.3 ± 15.7 | 0.854 | |
| Primary diagnosis leading to transplantation | ||||
| Idiopathic pulmonary fibrosis | 12 (41) | 16 (53) | 0.358 | |
| Bronchiectasis | 5 (17) | 2 (7) | 0.254 | |
| Interstitial lung disease | 5 (17) | 7 (23) | 0.561 | |
| GVHD after HSCT | 5 (17) | 1 (3) | 0.103 | |
| Chronic obstructive pulmonary disease | 0 | 2 (7) | 0.492 | |
| Primary pulmonary hypertension | 1 (3) | 0 | 0.492 | |
| Others | 1 (3) | 2 (7) | 1.000 | |
| Waitlist time, days | 62 [6–141] | 97 [34–146] | 0.318 | |
| FVC (% predicted) | 43 [33–47] | 38 [32–51] | 0.335 | |
| FEV1 (% predicted) | 50 ± 23 | 43 ± 20 | 0.234 | |
| Mean PA pressure, mmHg | 27 ± 7 | 25 ± 8 | 0.446 | |
| Ventilatory support | 9 (31) | 4 (13) | 0.101 | |
| ECMO support | 6 (21) | 3 (10) | 0.299 | |
| Variables | Sevoflurane Group (n = 29) | Propofol Group (n = 30) | p Value |
|---|---|---|---|
| Donor age | 42 ± 12 | 41 ± 13 | 0.805 |
| Sex matching, % | 23 (79) | 18 (60) | 0.107 |
| Ischemic time, h | 244 ± 84 | 237 ± 84 | 0.750 |
| Intraoperative data | |||
| Surgery time, min | 397 ± 54 | 372 ± 72 | 0.165 |
| Fluid input, mL | 10263 ± 3592 | 10402 ± 5408 | 0.549 |
| pRBC transfusion, mL | 1820 [1000–2700] | 1500 [1200–2400] | 0.853 |
| FFP transfusion, mL | 260 [0–390] | 130 [0–390] | 0.475 |
| Platelet transfusion, mL | 240 [0–240] | 0 [0–240] | 0.500 |
| Urine output, mL | 1225 [720–2130] | 953 [800–2440] | 0.848 |
| Blood loss, mL | 2050 [3500–14,000] | 1925 [1100–2500] | 0.473 |
| Norepinephrine administered, (μg/kg/min)/n | 0.03 [0.02–0.07]/27 | 0.04 [0.02–0.06]/30 | 0.788 |
| Vasopressin administered, (IU)/n | 3.0 [0.4–7.0]/14 | 4.0 [2.0–7.5]/15 | 0.664 |
| Milrinone administered, (mg)/n | 5.0 [0–9.5]/20 | 4.7 [2.4–7.2]/26 | 0.994 |
| SpO2 below 90% > consecutive 15 min | 3 (10) | 2 (7) | >0.999 |
| ECMO wean-off at the OR | 13 (45) | 20 (67) | 0.091 |
| Postoperative data for 48 h | |||
| Fluid input, mL | 7586 ± 1274 | 7125 ± 984 | 0.124 |
| pRBC transfusion, mL | 250 [0–500] | 250 [0–250] | 0.664 |
| FFP transfusion, mL | 0 [0–390] | 260 [0–390] | 0.857 |
| Platelet transfusion, mL | 240 [0–240] | 240 [0–240] | 1.000 |
| Urine output, mL | 5468 [4280–6822] | 5987 [4922–6892] | 0.647 |
| Blood loss, mL | 1690 [1560–3640] | 1834 [1275–2575] | 0.336 |
| Norepinephrine administered, (μg/kg/min)/n | 0.08 [0.03–0.10] | 0.04 [0.02–0.09] | 0.104 |
| Vasopressin administered, (IU)/n | 0 [0–20.0] | 0 [0–0] | 0.122 |
| Milrinone administered, (mg)/n | 47.4 [0–67.2] | 48.0 [0–76.8] | 0.401 |
| Variables | Sevoflurane Group (n = 29) | Propofol Group (n = 30) | p Value |
|---|---|---|---|
| Baseline serum creatinine, mg/dL | 0.64 ± 0.26 | 0.60 ± 0.16 | 0.497 |
| AKI stratified by AKIN criteria | 11 (38) | 4 (13) | 0.030 |
| Stage I | 7 (24) | 4 (13) | 0.287 |
| Stage II | 3 (10) | 0 | 0.112 |
| Stage III | 1 (3) | 0 | 0.492 |
| Serum NGAL, ng/mL | 0.039 a | ||
| Baseline | 115.3 ± 88.0 | 118.5 ± 94.2 | 0.938 |
| Immediately after surgery | 184.4 ± 141.6 | 103.3 ± 74.6 | 0.012 |
| After 24 h | 193.2 ± 168.1 | 108.7 ± 98.7 | 0.037 |
| After 48 h | 98.8 ± 0.5 | 84.5 ± 65.4 | 0.047 |
| Serum Cystatin C, mg/L | 0.223 a | ||
| Baseline | 0.93 ± 0.48 | 0.74 ± 0.41 | 0.110 |
| Immediately after surgery | 0.77 ± 0.43 | 0.67 ± 0.32 | 0.284 |
| After 24 h | 1.07 ± 0.58 | 0.88 ± 0.36 | 0.134 |
| After 48 h | 1.34 ± 0.72 | 1.00 ± 0.35 | 0.084 |
| Furosemide usage within 48 h, mg | 120 [60–185] | 100 [40–160] | 0.132 |
| RRT within 48 h | 3 (10) | 0 | 0.237 |
| RRT during hospitalization | 6 (21) | 1 (3) | 0.052 |
| In-hospital morbidity | |||
| Reoperation for bleeding | 3 (10) | 2 (7) | 0.671 |
| Rejection | 2 (7) | 2 (7) | 1.000 |
| Stroke or transient ischemic attack | 2 (7) | 1 (3) | 0.612 |
| Primary graft dysfunction grade 3 | 14 (48) | 8 (27) | 0.086 |
| Duration of prolonged ECMO, h | 55 [35–98] | 36 [31–85] | 0.329 |
| Duration of mechanical ventilation, h | 156 [96–372] | 132 [94–288] | 0.533 |
| ECMO re-insertion | 4 (14) | 1 (3) | 0.195 |
| Length of ICU stay, day | 8 [7–14] | 10 [7–17] | 0.490 |
| Length of hospital stay, day | 36 [22–64] | 29 [23–46] | 0.255 |
| Mortality | 2 (7) | 0 | 0.492 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Paik, H.-C.; Kim, N.; Jung, H.; Lee, J.-G.; Yoo, Y.-c. Effect of Propofol versus Sevoflurane Anesthesia on Acute Kidney Injury after Lung Transplantation Surgery: A Prospective Randomized Controlled Trial. J. Clin. Med. 2022, 11, 6862. https://doi.org/10.3390/jcm11226862
Song Y, Paik H-C, Kim N, Jung H, Lee J-G, Yoo Y-c. Effect of Propofol versus Sevoflurane Anesthesia on Acute Kidney Injury after Lung Transplantation Surgery: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(22):6862. https://doi.org/10.3390/jcm11226862
Chicago/Turabian StyleSong, Young, Hyo-Chae Paik, Namo Kim, Heejae Jung, Jin-Gu Lee, and Young-chul Yoo. 2022. "Effect of Propofol versus Sevoflurane Anesthesia on Acute Kidney Injury after Lung Transplantation Surgery: A Prospective Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 22: 6862. https://doi.org/10.3390/jcm11226862





