Destroying the Shield of Cancer Stem Cells: Natural Compounds as Promising Players in Cancer Therapy
Abstract
1. Introduction
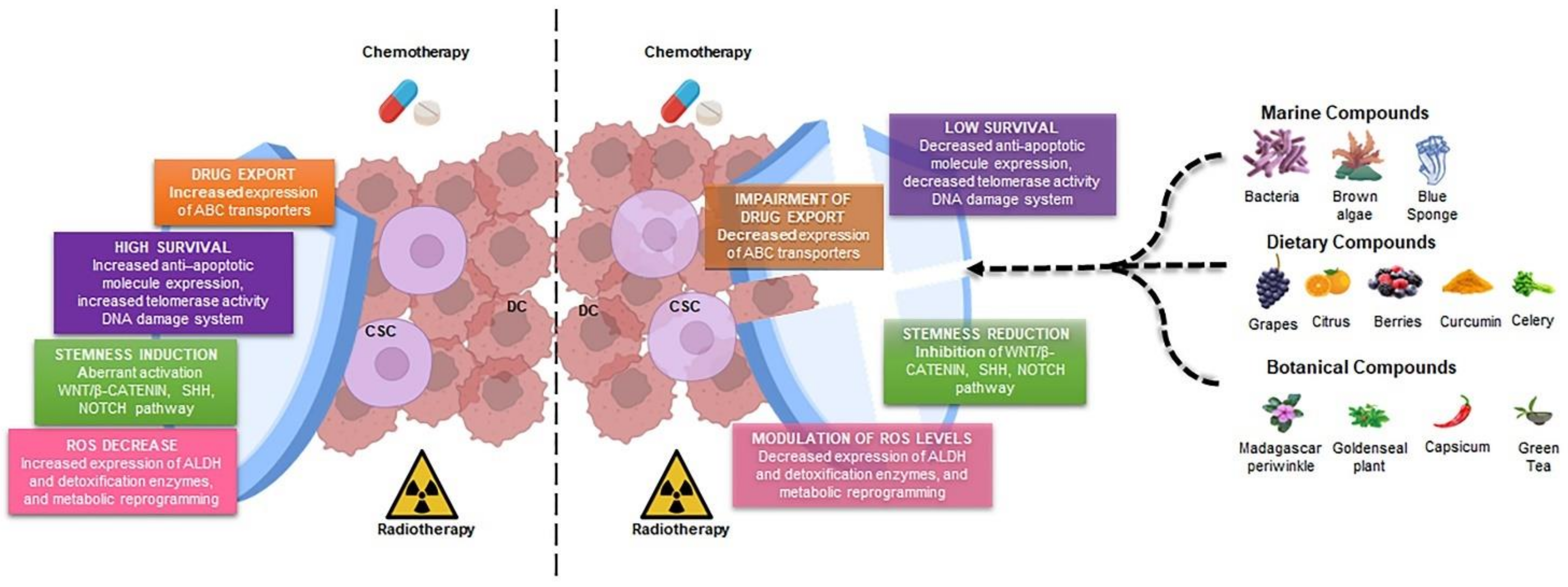
2. Cancer Stem Cells: The Main Players in Drug Resistance
2.1. Drug Export in CSCs
2.2. Enhanced Survival Ability in CSCs
2.3. Stemness Induction in CSCs by Different Signaling Pathways
3. Natural Products as Adjuvant against Cancer Stem Cells
3.1. NPs Derived from Dietary Sources
3.2. NPs Derived from Botanical Sources
3.3. NPs Derived from Marine Sources
3.4. Other Natural Compounds
4. Natural Products in Clinical Trial for Cancer Treatment
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turdo, A.; Veschi, V.; Gaggianesi, M.; Chinnici, A.; Bianca, P.; Todaro, M.; Stassi, G. Meeting the Challenge of Targeting Cancer Stem Cells. Front. Cell Dev. Biol. 2019, 7, 16. [Google Scholar] [CrossRef]
- Moselhy, J.; Srinivasan, S.; Ankem, M.K.; Damodaran, C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015, 35, 5773–5788. [Google Scholar]
- Schmidt, F.; Efferth, T. Tumor Heterogeneity, Single-Cell Sequencing, and Drug Resistance. Pharmaceuticals 2016, 9, 33. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem. Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Hausser, J.; Alon, U. Tumour heterogeneity and the evolutionary trade-offs of cancer. Nat. Rev. Cancer 2020, 20, 247–257. [Google Scholar] [CrossRef]
- Gaggianesi, M.; Di Franco, S.; Pantina, V.D.; Porcelli, G.; D’Accardo, C.; Verona, F.; Veschi, V.; Colarossi, L.; Faldetta, N.; Pistone, G.; et al. Messing Up the Cancer Stem Cell Chemoresistance Mechanisms Supported by Tumor Microenvironment. Front. Oncol. 2021, 11, 702642. [Google Scholar] [CrossRef]
- Veschi, V.; Verona, F.; Lo Iacono, M.; D’Accardo, C.; Porcelli, G.; Turdo, A.; Gaggianesi, M.; Forte, S.; Giuffrida, D.; Memeo, L.; et al. Cancer Stem Cells in Thyroid Tumors: From the Origin to Metastasis. Front. Endocrinol. 2020, 11, 566. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef]
- Loening-Baucke, V. Lichen sclerosus et atrophicus in children. Am. J. Dis. Child. 1991, 145, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Zhang, J.G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target Ther. 2021, 6, 62. [Google Scholar] [CrossRef]
- Begicevic, R.R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef]
- Rezayatmand, H.; Razmkhah, M.; Razeghian-Jahromi, I. Drug resistance in cancer therapy: The Pandora’s Box of cancer stem cells. Stem Cell Res.Ther. 2022, 13, 181. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Ho, M.M.; Ng, A.V.; Lam, S.; Hung, J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007, 67, 4827–4833. [Google Scholar] [CrossRef]
- Yin, W.; Xiang, D.; Wang, T.; Zhang, Y.; Pham, C.V.; Zhou, S.; Jiang, G.; Hou, Y.; Zhu, Y.; Han, Y.; et al. The inhibition of ABCB1/MDR1 or ABCG2/BCRP enables doxorubicin to eliminate liver cancer stem cells. Sci. Rep. 2021, 11, 10791. [Google Scholar] [CrossRef]
- Safa, A.R. Resistance to drugs and cell death in cancer stem cells (CSCs). J. Transl. Sci. 2020, 6, 341. [Google Scholar] [CrossRef]
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab. Rev. 2015, 47, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.C.; Cloos, J.; Lemos, C.; Stam, R.W.; Kaspers, G.J.L.; Jansen, G.; Peters, G.J. Ex vivo resistance in childhood acute lymphoblastic leukemia: Correlations between BCRP, MRP1, MRP4 and MRP5 ABC transporter expression and intracellular methotrexate polyglutamate accumulation. Leuk. Res. 2019, 79, 45–51. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Sheehan, N.T.; Monico, J.; Drummond, H.A.; Haikel, Y.; Brodell, R.T.; Megahed, M.; Hassan, M. CD133(+) melanoma subpopulation acquired resistance to caffeic acid phenethyl ester-induced apoptosis is attributed to the elevated expression of ABCB5: Significance for melanoma treatment. Cancer Lett. 2015, 357, 83–104. [Google Scholar] [CrossRef]
- Sugano, T.; Seike, M.; Noro, R.; Soeno, C.; Chiba, M.; Zou, F.; Nakamichi, S.; Nishijima, N.; Matsumoto, M.; Miyanaga, A.; et al. Inhibition of ABCB1 Overcomes Cancer Stem Cell-like Properties and Acquired Resistance to MET Inhibitors in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2015, 14, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Maugeri-Sacca, M.; Bartucci, M.; De Maria, R. DNA damage repair pathways in cancer stem cells. Mol. Cancer Ther. 2012, 11, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Manic, G.; Musella, M.; Corradi, F.; Sistigu, A.; Vitale, S.; Soliman Abdel Rehim, S.; Mattiello, L.; Malacaria, E.; Galassi, C.; Signore, M.; et al. Control of replication stress and mitosis in colorectal cancer stem cells through the interplay of PARP1, MRE11 and RAD51. Cell Death Differ. 2021, 28, 2060–2082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burness, M.L.; Martin-Trevino, R.; Guy, J.; Bai, S.; Harouaka, R.; Brooks, M.D.; Shang, L.; Fox, A.; Luther, T.K.; et al. RAD51 Mediates Resistance of Cancer Stem Cells to PARP Inhibition in Triple-Negative Breast Cancer. Clin. Cancer Res. 2017, 23, 514–522. [Google Scholar] [CrossRef]
- Turdo, A.; Gaggianesi, M.; Di Franco, S.; Veschi, V.; D’Accardo, C.; Porcelli, G.; Lo Iacono, M.; Pillitteri, I.; Verona, F.; Militello, G.; et al. Effective targeting of breast cancer stem cells by combined inhibition of Sam68 and Rad51. Oncogene 2022, 41, 2196–2209. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Grzybowska-Izydorczyk, O.; Cebula, B.; Robak, T.; Smolewski, P. Expression and prognostic significance of the inhibitor of apoptosis protein (IAP) family and its antagonists in chronic lymphocytic leukaemia. Eur. J. Cancer 2010, 46, 800–810. [Google Scholar] [CrossRef]
- Eckelman, B.P.; Salvesen, G.S.; Scott, F.L. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006, 7, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Ji, S.Y.; Mia-Jan, K.; Cho, M.Y. Chemoresistance of CD133(+) colon cancer may be related with increased survivin expression. Biochem. Biophys. Res. Commun. 2015, 463, 229–234. [Google Scholar] [CrossRef] [PubMed]
- AlShamaileh, H.; Wang, T.; Xiang, D.; Yin, W.; Tran, P.H.; Barrero, R.A.; Zhang, P.Z.; Li, Y.; Kong, L.; Liu, K.; et al. Aptamer-mediated survivin RNAi enables 5-fluorouracil to eliminate colorectal cancer stem cells. Sci. Rep. 2017, 7, 5898. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yu, Y.; Li, Z.L.; Chen, M.Y.; Deng, R.; Huang, X.; Wang, G.F.; Zhang, M.X.; Yang, Q.; Ravichandran, S.; et al. XIAP Limits Autophagic Degradation of Sox2 and Is A Therapeutic Target in Nasopharyngeal Carcinoma Stem Cells. Theranostics 2018, 8, 1494–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Scadden, D.T. Harnessing the apoptotic programs in cancer stem-like cells. EMBO Rep. 2015, 16, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, Q.; Li, Y.; Lei, G.; Scott, A.; Huo, L.; Li, C.Y.; Estrella, J.S.; Correa, A.; Pizzi, M.P.; et al. Targeting cancer stem cells with a pan-BCL-2 inhibitor in preclinical and clinical settings in patients with gastroesophageal carcinoma. Gut 2021, 70, 2238–2248. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Zanoni, M.; Bravaccini, S.; Fabbri, F.; Arienti, C. Emerging Roles of Aldehyde Dehydrogenase Isoforms in Anti-cancer Therapy Resistance. Front. Med. 2022, 9, 795762. [Google Scholar] [CrossRef] [PubMed]
- Emmink, B.L.; Verheem, A.; Van Houdt, W.J.; Steller, E.J.; Govaert, K.M.; Pham, T.V.; Piersma, S.R.; Borel Rinkes, I.H.; Jimenez, C.R.; Kranenburg, O. The secretome of colon cancer stem cells contains drug-metabolizing enzymes. J. Proteomics 2013, 91, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Pierscianek, D.; El Hindy, N.; Ahmadipour, Y.; Keyvani, K.; Sure, U.; Zhu, Y. The predominant expression of cancer stem cell marker ALDH1A3 in tumor infiltrative area is associated with shorter overall survival of human glioblastoma. BMC Cancer 2020, 20, 672. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.M.F.; Vermeulen, L. Wnt Signaling in Cancer Stem Cell Biology. Cancers 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Vermeulen, L.; De Sousa, E.M.F.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, J.; Wang, L.; Xiao, S.; Li, L.; Yan, X.; Yao, W.; Wu, L.; Zhang, S.; Zhang, Y.; et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/beta-catenin signaling. Cancer Lett. 2019, 450, 132–143. [Google Scholar] [CrossRef]
- Lu, H.; Ju, D.D.; Yang, G.D.; Zhu, L.Y.; Yang, X.M.; Li, J.; Song, W.W.; Wang, J.H.; Zhang, C.C.; Zhang, Z.G.; et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine 2019, 40, 276–289. [Google Scholar] [CrossRef]
- Yoon, C.; Park, D.J.; Schmidt, B.; Thomas, N.J.; Lee, H.J.; Kim, T.S.; Janjigian, Y.Y.; Cohen, D.J.; Yoon, S.S. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014, 20, 3974–3988. [Google Scholar] [CrossRef]
- Zhu, R.; Gires, O.; Zhu, L.; Liu, J.; Li, J.; Yang, H.; Ju, G.; Huang, J.; Ge, W.; Chen, Y.; et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 2019, 10, 2863. [Google Scholar] [CrossRef]
- Bai, X.Y.; Zhang, X.C.; Yang, S.Q.; An, S.J.; Chen, Z.H.; Su, J.; Xie, Z.; Gou, L.Y.; Wu, Y.L. Blockade of Hedgehog Signaling Synergistically Increases Sensitivity to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small-Cell Lung Cancer Cell Lines. PLoS ONE 2016, 11, e0149370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mathur, A.; Zhang, Y.; Xi, S.; Atay, S.; Hong, J.A.; Datrice, N.; Upham, T.; Kemp, C.D.; Ripley, R.T.; et al. Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res. 2012, 72, 4178–4192. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.A.; Chen, Y.F.; Bao, Y.; Mahara, S.; Yatim, S.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Asiedu, M.K.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011, 71, 4707–4719. [Google Scholar] [CrossRef]
- Naka, K.; Hoshii, T.; Muraguchi, T.; Tadokoro, Y.; Ooshio, T.; Kondo, Y.; Nakao, S.; Motoyama, N.; Hirao, A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 2010, 463, 676–680. [Google Scholar] [CrossRef]
- Verona, F.; Pantina, V.D.; Modica, C.; Lo Iacono, M.; D’Accardo, C.; Porcelli, G.; Cricchio, D.; Turdo, A.; Gaggianesi, M.; Di Franco, S.; et al. Targeting epigenetic alterations in cancer stem cells. Front. Mol. Med. 2022, 2, 882. [Google Scholar] [CrossRef]
- Wajapeyee, N.; Gupta, R. Epigenetic Alterations and Mechanisms That Drive Resistance to Targeted Cancer Therapies. Cancer Res. 2021, 81, 5589–5595. [Google Scholar] [CrossRef]
- Di Franco, S.; Bianca, P.; Sardina, D.S.; Turdo, A.; Gaggianesi, M.; Veschi, V.; Nicotra, A.; Mangiapane, L.R.; Lo Iacono, M.; Pillitteri, I.; et al. Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nat. Commun. 2021, 12, 5006. [Google Scholar] [CrossRef]
- Taylor, W.F.; Jabbarzadeh, E. The use of natural products to target cancer stem cells. Am. J. Cancer Res. 2017, 7, 1588–1605. [Google Scholar] [PubMed]
- Dyshlovoy, S.A. Recent Updates on Marine Cancer-Preventive Compounds. Mar. Drugs 2021, 19, 558. [Google Scholar] [CrossRef] [PubMed]
- Deldar Abad Paskeh, M.; Asadi, S.; Zabolian, A.; Saleki, H.; Khoshbakht, M.A.; Sabet, S.; Naghdi, M.J.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; et al. Targeting Cancer Stem Cells by Dietary Agents: An Important Therapeutic Strategy against Human Malignancies. Int. J. Mol. Sci. 2021, 22, 1669. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. Biomed. Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.M.F.; Cardoso, D.S.P.; Ferreira, M.-J.U. Overcoming Multidrug Resistance: Flavonoid and Terpenoid Nitrogen-Containing Derivatives as ABC Transporter Modulators. Molecules 2020, 25, 3364. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Zinger, H.; Kazanov, D.; Yona, D.; Ben-Yosef, R.; Starr, A.; Figer, A.; Arber, N. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed. Pharmacother. 2005, 59 (Suppl. S2), S276–S280. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, S.H.; Price, J.E.; Kim, L.S. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB in breast cancer cells and potentiates the growth inhibitory effect of paclitaxel in a breast cancer nude mice model. Breast J. 2009, 15, 223–229. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Veena, M.S.; Basak, S.K.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: A pilot study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Gomez-Quiroz, L.; Arreguin Camacho, L.O.; Pinna, F.; Lee, Y.H.; Kitade, M.; Dominguez, M.P.; Castven, D.; Breuhahn, K.; Conner, E.A.; et al. Curcumin effectively inhibits oncogenic NF-kappaB signaling and restrains stemness features in liver cancer. J. Hepatol. 2015, 63, 661–669. [Google Scholar] [CrossRef]
- Wu, L.; Guo, L.; Liang, Y.; Liu, X.; Jiang, L.; Wang, L. Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol. Rep. 2015, 34, 3311–3317. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Yang, X.; Chen, Y.; Jiang, Y.; Wang, S.J.; Li, Y.; Wang, X.Q.; Meng, Y.; Zhu, M.M.; Ma, X.; et al. Curcumin Suppresses Lung Cancer Stem Cells via Inhibiting Wnt/beta-catenin and Sonic Hedgehog Pathways. Phytother. Res. 2017, 31, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, X.; Zheng, X.; Chen, Y.; Xuan, Z.; Huang, P. Curcumin suppresses LGR5(+) colorectal cancer stem cells by inducing autophagy and via repressing TFAP2A-mediated ECM pathway. J. Nat. Med. 2021, 75, 590–601. [Google Scholar] [CrossRef]
- Baharuddin, P.; Satar, N.; Fakiruddin, K.S.; Zakaria, N.; Lim, M.N.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B.H. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol. Rep. 2016, 35, 13–25. [Google Scholar] [CrossRef]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.; Fathima, H.; Prabhu, K.S.; Siveen, K.S.; Kulinski, M.; Azizi, F.; Dermime, S.; Ahmad, A.; et al. Curcumin-Mediated Apoptotic Cell Death in Papillary Thyroid Cancer and Cancer Stem-Like Cells through Targeting of the JAK/STAT3 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 438. [Google Scholar] [CrossRef]
- Shaikh, S.; Shaikh, J.; Naba, Y.S.; Doke, K.; Ahmed, K.; Yusufi, M. Curcumin: Reclaiming the lost ground against cancer resistance. Cancer Drug Resist. 2021, 4, 298–320. [Google Scholar] [CrossRef]
- Gusman, J.; Malonne, H.; Atassi, G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 2001, 22, 1111–1117. [Google Scholar] [CrossRef]
- Gescher, A.J. Resveratrol from red grapes-pedestrian polyphenol or useful anticancer agent? Planta Med. 2008, 74, 1651–1655. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS ONE 2018, 13, e0205918. [Google Scholar] [CrossRef]
- Ferraresi, A.; Esposito, A.; Girone, C.; Vallino, L.; Salwa, A.; Ghezzi, I.; Thongchot, S.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells 2021, 10, 3213. [Google Scholar] [CrossRef]
- Qin, T.; Cheng, L.; Xiao, Y.; Qian, W.; Li, J.; Wu, Z.; Wang, Z.; Xu, Q.; Duan, W.; Wong, L.; et al. NAF-1 Inhibition by Resveratrol Suppresses Cancer Stem Cell-Like Properties and the Invasion of Pancreatic Cancer. Front. Oncol. 2020, 10, 1038. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, S.; Zhou, J.; Li, X. Effect of resveratrol on drug resistance in colon cancer chemotherapy. RSC Adv. 2019, 9, 2572–2580. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef]
- Blanquer-Rossello, M.D.; Hernandez-Lopez, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef]
- Juan, M.E.; Alfaras, I.; Planas, J.M. Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol.Res. 2012, 65, 584–591. [Google Scholar] [CrossRef]
- Suh, J.; Kim, D.H.; Surh, Y.J. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch. Biochem. Biophys. 2018, 643, 62–71. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Huo, C.; Chen, D.; Milacic, V.; Shi, G.; Chan, T.H.; Dou, Q.P. A novel prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007, 67, 4303–4310. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Human cancer stem cells are a target for cancer prevention using (-)-epigallocatechin gallate. J. Cancer Res. Clin. Oncol. 2017, 143, 2401–2412. [Google Scholar] [CrossRef]
- Luo, K.W.; Xia, J.; Cheng, B.H.; Gao, H.C.; Fu, L.W.; Luo, X.L. Tea polyphenol EGCG inhibited colorectal-cancer-cell proliferation and migration via downregulation of STAT3. Gastroenterol. Rep. 2021, 9, 59–70. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y.; et al. (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/beta-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, C.; Zhang, P.; Ren, J.; Mageed, F.; Wu, X.; Chen, L.; Zeb, F.; Feng, Q.; Li, S. Epigallocatechin3gallate inhibits selfrenewal ability of lung cancer stemlike cells through inhibition of CLOCK. Int. J. Mol. Med. 2020, 46, 2216–2224. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/beta-catenin pathway mediates (-)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Vaya, J.; Khatib, S. The effects and mechanism of flavonoid-rePON1 interactions. Structure-activity relationship study. Bioorg. Med. Chem. 2013, 21, 3348–3355. [Google Scholar] [CrossRef]
- Montales, M.T.; Rahal, O.M.; Kang, J.; Rogers, T.J.; Prior, R.L.; Wu, X.; Simmen, R.C. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis 2012, 33, 652–660. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Meiyanto, E.; Hermawan, A.; Anindyajati. Natural products for cancer-targeted therapy: Citrus flavonoids as potent chemopreventive agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar]
- Nabekura, T.; Yamaki, T.; Kitagawa, S. Effects of chemopreventive citrus phytochemicals on human P-glycoprotein and multidrug resistance protein 1. Eur. J. Pharmacol. 2008, 600, 45–49. [Google Scholar] [CrossRef]
- Ma, W.; Feng, S.; Yao, X.; Yuan, Z.; Liu, L.; Xie, Y. Nobiletin enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cancer cells. Sci. Rep. 2015, 5, 18789. [Google Scholar] [CrossRef]
- Han, S.H.; Han, J.H.; Chun, W.J.; Lee, S.S.; Kim, H.S.; Lee, J.W. Nobiletin Inhibits Non-Small-Cell Lung Cancer by Inactivating WNT/beta-Catenin Signaling through Downregulating miR-15-5p. Evid.-Based Complement. Alternat. Med. 2021, 2021, 7782963. [Google Scholar] [CrossRef]
- Turdo, A.; Glaviano, A.; Pepe, G.; Calapa, F.; Raimondo, S.; Fiori, M.E.; Carbone, D.; Basilicata, M.G.; Di Sarno, V.; Ostacolo, C.; et al. Nobiletin and Xanthohumol Sensitize Colorectal Cancer Stem Cells to Standard Chemotherapy. Cancers 2021, 13, 3927. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Huang, K.M. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol. 2013, 53, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Turkekul, K.; Serttas, R.; Erdogan, Z. The natural flavonoid apigenin sensitizes human CD44(+) prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017, 88, 210–217. [Google Scholar] [CrossRef]
- Li, Y.W.; Xu, J.; Zhu, G.Y.; Huang, Z.J.; Lu, Y.; Li, X.Q.; Wang, N.; Zhang, F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; He, W.; Xia, S.; Jiang, X.; Li, X.; Bai, J.; Li, N.; Chen, L.; Yang, B. Apigenin Enhanced Antitumor Effect of Cisplatin in Lung Cancer via Inhibition of Cancer Stem Cells. Nutr. Cancer 2021, 73, 1489–1497. [Google Scholar] [CrossRef]
- Cao, L.; Yang, Y.; Ye, Z.; Lin, B.; Zeng, J.; Li, C.; Liang, T.; Zhou, K.; Li, J. Quercetin3methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int. J. Mol. Med. 2018, 42, 1625–1636. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(-)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412–420. [Google Scholar] [CrossRef]
- Cao, C.; Sun, L.; Mo, W.; Sun, L.; Luo, J.; Yang, Z.; Ran, Y. Quercetin Mediates beta-Catenin in Pancreatic Cancer Stem-Like Cells. Pancreas 2015, 44, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S.; et al. Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. Int. 2022, 29, 31025–31041. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, A.; Ikawati, M.; Jenie, R.I.; Khumaira, A.; Putri, H.; Nurhayati, I.P.; Angraini, S.M.; Muflikhasari, H.A. Identification of potential therapeutic target of naringenin in breast cancer stem cells inhibition by bioinformatics and in vitro studies. Saudi Pharm. J. 2021, 29, 12–26. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, O.P.; Gonzalez-Torres, A.; Alvarez-Salas, L.M.; Hernandez-Sanchez, H.; Garcia-Perez, B.E.; Thompson-Bonilla, M.D.R.; Jaramillo-Flores, M.E. Effect of naringenin and its combination with cisplatin in cell death, proliferation and invasion of cervical cancer spheroids. RSC Adv. 2020, 11, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.P.; Lim, G.; Li, Y.; Shah, R.B.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.; Lee, E.D.; Grandis, J.R.; Bauman, J.E.; Johnson, D.E. Gene targets of sulforaphane in head and neck squamous cell carcinoma. Mol. Med. Rep. 2019, 20, 5335–5344. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xie, Y.K.; Wu, Y.; Li, L.L.; Liu, Y.; Miao, X.B.; Liu, Q.Z.; Yao, K.T.; Xiao, G.H. Sulforaphane inhibits cancer stem-like cell properties and cisplatin resistance through miR-214-mediated downregulation of c-MYC in non-small cell lung cancer. Oncotarget 2017, 8, 12067–12080. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane Suppresses the Growth of Triple-negative Breast Cancer Stem-like Cells In vitro and In vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Khusro, F.H.; Mustafa Adhami, V.; Suh, Y.; Mukhtar, H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer 2012, 130, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Tabasum, S.; Singh, R.P. Fisetin suppresses migration, invasion and stem-cell-like phenotype of human non-small cell lung carcinoma cells via attenuation of epithelial to mesenchymal transition. Chem. Biol. Interact. 2019, 303, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, A.B.; Lopes, R.M.; Schwartsmann, G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001, 1, 364–369. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, G.Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.J.; Peng, S.X.; Chen, L.W.; Li, Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Tsai, K.J.; Tsai, H.Y.; Tsai, C.C.; Chen, T.Y.; Hsieh, T.H.; Chen, C.L.; Mbuyisa, L.; Huang, Y.B.; Lin, M.W. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules 2021, 26, 6452. [Google Scholar] [CrossRef]
- Tsai, P.H.; Cheng, C.H.; Lin, C.Y.; Huang, Y.T.; Lee, L.T.; Kandaswami, C.C.; Lin, Y.C.; Lee, K.P.; Hung, C.C.; Hwang, J.J.; et al. Dietary Flavonoids Luteolin and Quercetin Suppressed Cancer Stem Cell Properties and Metastatic Potential of Isolated Prostate Cancer Cells. Anticancer. Res. 2016, 36, 6367–6380. [Google Scholar] [CrossRef]
- Tu, D.G.; Lin, W.T.; Yu, C.C.; Lee, S.S.; Peng, C.Y.; Lin, T.; Yu, C.H. Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J. Formos Med. Assoc. 2016, 115, 1032–1038. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.; Katiyar, S.K. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 2008, 229, 33–43. [Google Scholar] [CrossRef]
- Iizuka, N.; Miyamoto, K.; Okita, K.; Tangoku, A.; Hayashi, H.; Yosino, S.; Abe, T.; Morioka, T.; Hazama, S.; Oka, M. Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Lett. 2000, 148, 19–25. [Google Scholar] [CrossRef]
- Qing, Y.; Hu, H.; Liu, Y.; Feng, T.; Meng, W.; Jiang, L.; Sun, Y.; Yao, Y. Berberine induces apoptosis in human multiple myeloma cell line U266 through hypomethylation of p53 promoter. Cell. Biol. Int. 2014, 38, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Xue, Y.; Hu, H.; Ye, J.; Li, X.; Lu, Z.; Meng, F.; Liang, S. Berberine acts as a putative epigenetic modulator by affecting the histone code. Toxicol. In Vitro 2016, 36, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zeng, J.; Guo, Q.; Pu, K.; Yang, Y.; Chen, N.; Zhang, G.; Zhao, M.; Zheng, Q.; Tang, J.; et al. Berberine Suppresses Stemness and Tumorigenicity of Colorectal Cancer Stem-Like Cells by Inhibiting m(6)A Methylation. Front. Oncol. 2021, 11, 775418. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sung, J.H.; Chung, N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol. Cell. Biochem. 2014, 394, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.; Zhao, J.; Gao, M.; Zhang, M.; Shi, T.; Zhang, F.; Zheng, X.; Pan, Y.; Shao, D.; et al. Berberine inhibits chemotherapy-exacerbated ovarian cancer stem cell-like characteristics and metastasis through GLI1. Eur. J. Pharmacol. 2021, 895, 173887. [Google Scholar] [CrossRef]
- Naveen, C.R.; Gaikwad, S.; Agrawal-Rajput, R. Berberine induces neuronal differentiation through inhibition of cancer stemness and epithelial-mesenchymal transition in neuroblastoma cells. Phytomedicine 2016, 23, 736–744. [Google Scholar] [CrossRef]
- Camplejohn, R.S. A critical review of the use of vincristine (VCR) as a tumour cell synchronizing agent in cancer therapy. Cell Tissue Kinet. 1980, 13, 327–335. [Google Scholar] [CrossRef]
- Tu, Y.; Cheng, S.; Zhang, S.; Sun, H.; Xu, Z. Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Med. 2013, 31, 113–119. [Google Scholar] [CrossRef]
- Gingrich, R.D.; Armitage, J.O.; Burns, C.P. Treatment of adult acute lymphoblastic leukemia with cytosine arabinoside, vincristine, and prednisone. Cancer Treat. Rep. 1978, 62, 1389–1391. [Google Scholar]
- Gandhi, L.; Harding, M.W.; Neubauer, M.; Langer, C.J.; Moore, M.; Ross, H.J.; Johnson, B.E.; Lynch, T.J. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer 2007, 109, 924–932. [Google Scholar] [CrossRef]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.J.; Tveit, K.M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Dalton, W.S.; Mosley, K.; Dorr, R.T.; Salmon, S.E. Combination chemotherapy with cyclophosphamide, vincristine, adriamycin, and dexamethasone (CVAD) plus oral quinine and verapamil in patients with advanced breast cancer. Breast Cancer Res. Treat. 1997, 42, 7–14. [Google Scholar] [CrossRef]
- Moon, J.W.; Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, N.; Kim, J.; Kim, H.S.; Park, S.H. Demethylation of RUNX3 by vincristine in colorectal adenocarcinoma cells. Anticancer Res. 2014, 34, 133–140. [Google Scholar] [PubMed]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Isaka, Y. Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 2013, 73, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Cufi, S.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Martin-Castillo, B.; Vellon, L.; Menendez, J.A. Autophagy positively regulates the CD44+ CD24-/low breast cancer stem-like phenotype. Cell Cycle 2011, 10, 3871–3885. [Google Scholar] [CrossRef]
- Choi, D.S.; Blanco, E.; Kim, Y.S.; Rodriguez, A.A.; Zhao, H.; Huang, T.H.; Chen, C.L.; Jin, G.; Landis, M.D.; Burey, L.A.; et al. Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells 2014, 32, 2309–2323. [Google Scholar] [CrossRef]
- Liang, D.H.; Choi, D.S.; Ensor, J.E.; Kaipparettu, B.A.; Bass, B.L.; Chang, J.C. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016, 376, 249–258. [Google Scholar] [CrossRef]
- Balic, A.; Sorensen, M.D.; Trabulo, S.M.; Sainz, B., Jr.; Cioffi, M.; Vieira, C.R.; Miranda-Lorenzo, I.; Hidalgo, M.; Kleeff, J.; Erkan, M.; et al. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol. Cancer Ther. 2014, 13, 1758–1771. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, D.; Shi, X.; Liu, S.; Li, A.; Wang, D.; Qin, G.; Ping, Y.; Qiao, Y.; Chen, X.; et al. Chloroquine Inhibits Stemness of Esophageal Squamous Cell Carcinoma Cells Through Targeting CXCR4-STAT3 Pathway. Front. Oncol. 2020, 10, 311. [Google Scholar] [CrossRef]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, X.; Zheng, Z.; Huang, J.; Yang, X.; Shi, H. Capsaicin suppressed activity of prostate cancer stem cells by inhibition of Wnt/beta-catenin pathway. Phytother. Res. 2020, 34, 817–824. [Google Scholar] [CrossRef]
- Shi, S.; Li, C.; Zhang, Y.; Deng, C.; Liu, W.; Du, J.; Li, Q.; Ji, Y.; Guo, L.; Liu, L.; et al. Dihydrocapsaicin Inhibits Cell Proliferation and Metastasis in Melanoma via Down-regulating beta-Catenin Pathway. Front. Oncol. 2021, 11, 648052. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, Y.S.; Lim, S.C.; Hou, Y.F.; Chang, I.Y.; You, H.J. Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner. Autophagy 2008, 4, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Bhutkar, S.; Dhulap, S.; Hirwani, R.R. Patent analysis as a tool for research planning: Study on natural based therapeutics against cancer stem cells. Recent Pat. Anticancer Drug Discov. 2015, 10, 72–86. [Google Scholar] [CrossRef]
- Schwartsmann, G. Marine organisms and other novel natural sources of new cancer drugs. Ann. Oncol. 2000, 11 (Suppl. S3), 235–243. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Parrino, B.; Barraja, P.; Spano, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.H. Synthesis and antiproliferative activity of 2,5-bis(3’-indolyl)pyrroles, analogues of the marine alkaloid nortopsentin. Mar. Drugs 2013, 11, 643–654. [Google Scholar] [CrossRef]
- Cascioferro, S.; Attanzio, A.; Di Sarno, V.; Musella, S.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Mar. Drugs 2019, 17, 35. [Google Scholar] [CrossRef]
- Di Franco, S.; Parrino, B.; Gaggianesi, M.; Pantina, V.D.; Bianca, P.; Nicotra, A.; Mangiapane, L.R.; Lo Iacono, M.; Ganduscio, G.; Veschi, V.; et al. CHK1 inhibitor sensitizes resistant colorectal cancer stem cells to nortopsentin. iScience 2021, 24, 102664. [Google Scholar] [CrossRef]
- Sirimangkalakitti, N.; Chamni, S.; Suwanborirux, K.; Chanvorachote, P. Renieramycin M Attenuates Cancer Stem Cell-like Phenotypes in H460 Lung Cancer Cells. Anticancer Res. 2017, 37, 615–621. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Tanaka, T.; Miyamoto, S.; Mutoh, M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J. Clin. Biochem. Nutr. 2017, 61, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Mima, M.; Kudoh, S.; Endo, T.; Maeda, H.; Hamada, J.; Osada, K.; Miyashita, K.; Mutoh, M. Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol. Rep. 2018, 40, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef]
- Prendiville, J.; Crowther, D.; Thatcher, N.; Woll, P.J.; Fox, B.W.; McGown, A.; Testa, N.; Stern, P.; McDermott, R.; Potter, M.; et al. A phase I study of intravenous bryostatin 1 in patients with advanced cancer. Br. J. Cancer 1993, 68, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Zonder, J.A. Pharmacology and clinical experience with bryostatin 1: A novel anticancer drug. Expert Opin. Investig. Drugs 1999, 8, 2189–2199. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Koprowska, K.; Majchrzak, K.; Hartman, M.; Czyz, M. Natural compounds’ activity against cancer stem-like or fast-cycling melanoma cells. PLoS ONE 2014, 9, e90783. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.G.; Allan, E.K.; Mountford, J.C.; Richmond, L.; Harrison, S.; Elliott, M.A.; Holyoake, T.L. Enhanced CML stem cell elimination in vitro by bryostatin priming with imatinib mesylate. Exp. Hematol. 2005, 33, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, E.J.; Maillard, I.; Aster, J.C.; Pear, W.S. Notch signaling in cancer. Cancer Biol. Ther. 2002, 1, 466–476. [Google Scholar] [CrossRef]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef]
- Ginestier, C.; Wicinski, J.; Cervera, N.; Monville, F.; Finetti, P.; Bertucci, F.; Wicha, M.S.; Birnbaum, D.; Charafe-Jauffret, E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle 2009, 8, 3297–3302. [Google Scholar] [CrossRef]
- Karsy, M.; Albert, L.; Tobias, M.E.; Murali, R.; Jhanwar-Uniyal, M. All-trans retinoic acid modulates cancer stem cells of glioblastoma multiforme in an MAPK-dependent manner. Anticancer Res. 2010, 30, 4915–4920. [Google Scholar] [PubMed]
- Lim, Y.C.; Kang, H.J.; Kim, Y.S.; Choi, E.C. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/beta-catenin pathway. Eur. J. Cancer 2012, 48, 3310–3318. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, L.; Huang, H.; Li, X.; Wang, P.; Mi, K.; Cheng, J.; Liu, H.; Gu, C.; Huang, L.; et al. All-trans retinoic acid reduces cancer stem cell-like cell-mediated resistance to gefitinib in NSCLC adenocarcinoma cells. BMC Cancer 2020, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Kwak, J.; Jang, K.L. All-trans retinoic acid induces p53-depenent apoptosis in human hepatocytes by activating p14 expression via promoter hypomethylation. Cancer Lett. 2015, 362, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kaufman-Szymczyk, A.; Majda, K.; Szulawska-Mroczek, A.; Fabianowska-Majewska, K.; Lubecka, K. Clofarabinephytochemical combination exposures in CML cells inhibit DNA methylation machinery, upregulate tumor suppressor genes and promote caspasedependent apoptosis. Mol. Med. Rep. 2019, 20, 3597–3608. [Google Scholar] [CrossRef] [PubMed]
- Dave, B.; Mittal, V.; Tan, N.M.; Chang, J.C. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012, 14, 202. [Google Scholar] [CrossRef]
- Gullett, N.P.; Ruhul Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Park, W.; Amin, A.R.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. 2013, 6, 387–400. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Diamond, T.H.; Alexander, W.; Manoharan, A.; Golombick, T. Use of Curcumin in Multiple Myeloma patients intolerant of steroid therapy. Clin. Case Rep. 2020, 8, 739–744. [Google Scholar] [CrossRef]
- Santosa, D.; Suharti, C.; Riwanto, I.; Dharmana, E.; Pangarsa, E.A.; Setiawan, B.; Suyono, S.; Tobing, M.L.; Suhartono, S.; Hadisapurto, S. Curcumin as adjuvant therapy to improve remission in myeloma patients: A pilot randomized clinical trial. Caspian J. Intern. Med. 2022, 13, 375–384. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, V.I.; Ashrafyan, L.A.; Muyzhnek, E.L.; Gerfanova, E.V.; Antonova, I.B.; Aleshikova, O.I.; Sarkar, F.H. A new promising way of maintenance therapy in advanced ovarian cancer: A comparative clinical study. BMC Cancer 2018, 18, 904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jia, L.; Chen, G.; Li, X.; Meng, X.; Zhao, X.; Xing, L.; Zhu, W. A prospective, three-arm, randomized trial of EGCG for preventing radiation-induced esophagitis in lung cancer patients receiving radiotherapy. Radiother. Oncol. 2019, 137, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, L.; Zhang, Y.; Xie, P.; Zhu, W.; Meng, X.; Wang, Y.; Kong, L.; Zhao, H.; Yu, J. Phase II Trial of Epigallocatechin-3-Gallate in Acute Radiation-Induced Esophagitis for Esophagus Cancer. J. Med. Food 2020, 23, 43–49. [Google Scholar] [CrossRef]
- Gee, J.R.; Saltzstein, D.R.; Kim, K.; Kolesar, J.; Huang, W.; Havighurst, T.C.; Wollmer, B.W.; Stublaski, J.; Downs, T.; Mukhtar, H.; et al. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev. Res. 2017, 10, 298–307. [Google Scholar] [CrossRef]
- Crew, K.D.; Ho, K.A.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Sneige, N.; Hudis, C.; McArthur, H.L.; Chang, J.; et al. Effects of a green tea extract, Polyphenon E, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. J. Hum. Nutr. Diet 2015, 28, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [PubMed]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: Updates 2020. Mar. Drugs 2020, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Bronstrup, M.; Sasse, F. Natural products targeting the elongation phase of eukaryotic protein biosynthesis. Nat. Prod. Rep. 2020, 37, 752–762. [Google Scholar] [CrossRef]
- Deeks, E.D. Polatuzumab Vedotin: First Global Approval. Drugs 2019, 79, 1467–1475. [Google Scholar] [CrossRef]
- Markham, A. Lurbinectedin: First Approval. Drugs 2020, 80, 1345–1353. [Google Scholar] [CrossRef]
- Skubnik, J.; Pavlickova, V.S.; Ruml, T.; Rimpelova, S. Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef]
- Gnekow, A.K.; Walker, D.A.; Kandels, D.; Picton, S.; Giorgio, P.; Grill, J.; Stokland, T.; Sandstrom, P.E.; Warmuth-Metz, M.; Pietsch, T.; et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (</=16 years) low grade glioma-A final report. Eur. J. Cancer 2017, 81, 206–225. [Google Scholar] [CrossRef]
- Peyrade, F.; Bologna, S.; Delwail, V.; Emile, J.F.; Pascal, L.; Ferme, C.; Schiano, J.M.; Coiffier, B.; Corront, B.; Farhat, H.; et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: An open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol. 2017, 4, e46–e55. [Google Scholar] [CrossRef] [PubMed]
- Clamp, A.; Jayson, G.C. The clinical development of the bryostatins. Anticancer Drugs 2002, 13, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, L.; Daliani, D.; Amato, R.; Tu, S.M.; Jones, D.; Smith, T.; Logothetis, C.; Millikan, R. A Phase II trial of bryostatin-1 for patients with metastatic renal cell carcinoma. Cancer 2000, 89, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Varterasian, M.L.; Mohammad, R.M.; Shurafa, M.S.; Hulburd, K.; Pemberton, P.A.; Rodriguez, D.H.; Spadoni, V.; Eilender, D.S.; Murgo, A.; Wall, N.; et al. Phase II trial of bryostatin 1 in patients with relapsed low-grade non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Clin. Cancer Res. 2000, 6, 825–828. [Google Scholar] [PubMed]
- Zhang, Z.; Garzotto, M.; Davis, E.W., 2nd; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2020, 72, 74–87. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Farsad-Naeimi, A.; Alizadeh, M.; Esfahani, A.; Darvish Aminabad, E. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018, 9, 2025–2031. [Google Scholar] [CrossRef]
| Natural Products (Source) | Tumor Type | Effects on CSCs | Alone or in Combination with Other Compounds | References |
|---|---|---|---|---|
| Curcumin (Curcumalonga) | Liver cancer | In vitro reduction in SP subpopulation and sphere formation. | HDAC inhibitors | [73] |
| Lung cancer | In vitro reduction in tumorsphere formation and inhibition of JAK2/STAT3 pathway. In vivo impaired tumor growth. | [74] | ||
| Lung cancer | In vitro arrest of cell proliferation, induction of apoptosis, and reduction in the main stemness markers via Wnt/β-catenin and SHH pathways downregulation. | [75] | ||
| Breast cancer | In vitro reduction in tumorsphere formation | Piperidine | [76] | |
| Colorectal cancer | In vitro reduction in LGR5+ cell proliferation and induction of autophagy. | [77] | ||
| Lung cancer | In vitro induction of apoptosis, reduction in CD166+/EpCAM+ cell migration. | Cisplatin | [78] | |
| Thyroid cancer | In vitro impairment of sphere formation and reduction in stemness marker expression via JAK/STAT3 downregulation. | Cisplatin | [79] | |
| Resveratrol (skin of grapes and berries) | Osteosarcoma | In vitro inhibition of STAT3 pathway and reduction in CD133 expression. | [84] | |
| Ovarian cancer | In vitro reduction in migration and viability, inhibition of SHH pathway, and induction of autophagy. | Lysophosphatidic acid | [85] | |
| Pancreatic cancer | In vitro reduction in stem-like features. In vivo reduction in tumorigenic and invasive potential. | [86] | ||
| Colorectal cancer | In vitro reduction in CD133+ cell survival. | 5-FU | [87] | |
| Breast cancer | In vitro reduction in tumorsphere formation via autophagy induction and Wnt pathway reduction. In vivo reduction in tumor growth. | [88] | ||
| Colorectal cancer | In vitro reduction in stemness markers and sphere formation. | [89,90] | ||
| Breast cancer | In vitro reduction in CD44+/CD24− subpopulations and SOX2 and BMI1 expression levels. | [91] | ||
| EGCG (green tea) | Colorectal cancer | In vitro reduction in invasive capacity and induction of apoptosis and chemosensitivity via STAT3 and Wnt pathway downregulation. | [99] | |
| Lung cancer | In vitro reduction in self-renewal of CD133+ cells. In vivoreduction in tumorigenic potential. | [100] | ||
| Lung cancer | In vitro downregulation of Wnt pathway and reduction in proliferation and stemness marker expression. | [101] | ||
| Nobiletin (Citrus depressa and Citrus sinensis) | Lung cancer | In vitro reduction in Wnt pathway and CD133 and ALDH1 expression levels. | [110] | |
| Colorectal cancer | In vitro reduction in cell viability, CD44v6 expression, Wnt activation, and induction of apoptosis. | Xanthohumol (flavonoid) and FOX | [111] | |
| Apigenin (fruits, vegetables, and drinks) | Prostate cancer | In vitroinduction of apoptosis and inhibition of cell migration and NF-kB pathway. | Cisplatin | [114] |
| Breast cancer | In vivo reduction in CD44+/CD24− tumorigenic potential via downregulation of YAP/TAZ pathway. | [115] | ||
| Lung cancer | In vivoreduction in CD133+ tumorigenic potential. | Cisplatin | [116] | |
| Quercetin (fruits and vegetables) | Breast cancer | In vitro reduction in tumorsphere formation and decrease in stemness gene (SOX2, NANOG) expression and Notch and PI3K/AKT pathway activation. | [117] | |
| Breast cancer | In vitro reduction in ALDH1A1 and CXCR4 expression levels. In vivo reduction in CD44+/CD24− tumor growth and metastasis formation. | [118,119] | ||
| Pancreatic cancer | In vitro reduction in Wnt pathway activation and stemness markers. | [120] | ||
| Naringine and naringenin (Citrus fruits) | Breast cancer | In vitro reduction in mammosphere and colony formation and migration, decrease in stem-like markers (β-catenin, ALDH1). | [124] | |
| Cervical cancer | In vitro reduction in cell viability and invasive capacity. | Cisplatin | [125] | |
| Sulforaphane (Brassicaceae) | Lung cancer | In vitro decrease in CD133+ and ALDH+ cells. | Cisplatin | [128] |
| Breast cancer | In vitro reduction in mammosphere formation and decrease in ALDH expression and Wnt/β-catenin pathway activation. In vivo decrease in tumor growth and second engraftment. | [129] | ||
| Breast cancer | In vitro reduction in cell proliferation, tumorsphere formation, and cell viability. In vivo decrease in xenograft tumor growth. | [130] | ||
| Fisetin (vegetables and fruits) | Lung cancer | In vitro reduction in cell growth and colony formation. | [132] | |
| Lung cancer | In vitro reduction in proliferative and invasive capacity via the downregulation of CD44 and CD133 stem-like markers. | [133] |
| Natural Products | Tumor Type | Effects on CSCs | Alone or in Combination with Other Compounds | References |
|---|---|---|---|---|
| Luteolin (plants) | Breast cancer | In vitro reduction in tumorsphere formation, decrease in stemness marker (ABCG2 and CD44) expression and ALDH1 activity. In vitro reduction in cell viability. | Taxol | [136] |
| Prostate cancer | In vitro reduction in tumorsphere formation and of the expression levels of stem-like markers (NANOG, SOX2, and CD44). | Quercetin | [137] | |
| Oral cancer | In vitro arrest of cell proliferation and migration and decrease in ALDH activity, CD44 expression levels, and IL6/STAT3 axis. In vivo reduction in tumor growth through IL-6/STAT3 signaling inactivation. | [138] | ||
| Berberine (plants) | Colorectal cancer | In vitro reduction in tumorsphere formation, cell proliferation, and CD44 and CD133 expression levels. In vivo reduction in tumor growth. | 5-FU and irinotecan | [143] |
| Pancreatic cancer | In vitro decrease in SP percentage and the expression of the stemness genes (POU5F1, SOX2, and NANOG). | Gemcitabine | [144] | |
| Ovarian cancer | In vitro reduction in tumorsphere formation, expression of EMT stemness marker expression, and GLI1-BMI1 axis. | Carboplatin and VP-16 | [145] | |
| Neuroblastoma | In vitro downregulation of PI3K/Akt, TGF-β, and MAPK pathways. | [146] | ||
| Vincristine (Madagascar periwinkle) | Colorectal cancer | In vitro demethylation of RUNX3. | [153] | |
| Cloroquine (bark of the Cinchona officinalis tree) | Breast cancer | In vitro reduction in CD44+/CD24− stem-like population. | [155] | |
| Breast cancer | In vitro reduction in CD44+/CD24− and ALDH+ stem-like population, impairment of autophagy (increased levels of LCB3-II), and increase in apoptosis and cleaved caspase-3 levels. In vivo reduction in tumor growth and metastatic foci. | Paclitaxel Paclitaxel | [156] | |
| Breast cancer | In vitro induction of mitochondrial depolarization. In vitro reduction in autophagy and the expression levels of DNA repair machinery proteins. | Carboplatin | [157] | |
| Pancreatic cancer | In vitro inhibition of CXCL12/CXCR4 axis and SHH pathway. In vivo reduction in the tumorigenic potential of CD133+ subpopulation. | [158] | ||
| Esophageal carcinoma | In vitro reduction in the CXCR4/STAT3 axis. | [159] | ||
| Capsaicin (plants of the genus Capsicum) | Prostate | In vitro reduction in tumorsphere formation, cell viability, and the expression levels of stem-like markers (CD133, CD44, OCT-4, NANOG, and SOX2) and downregulation of GSK3β/β-catenin pathway. | [161] | |
| Dihydrocapsaicin (derivate of capsaicin) | Brain | In vitro decrease in CD133+ subpopulation and induction of cell death. | [164] |
| Natural Products (Source) | Tumor Type | Effects on CSCs | Alone or in Combination with Other Compounds | References |
|---|---|---|---|---|
| Alkaloids | ||||
| Nortopsentin (deep-sea sponges, Spongsoritesruetzleri) | Colorectal | In vitro arrest of cell proliferation (inhibition of CDK1 activity), induction of apoptosis (Caspase 3), and decrease in stem cell markers (CD44v6) and pathways (Wnt/β-catenin). | Rabusertib | [168] |
| Renieramycin M (blue sponge Xestospongia species) | Non-small-cell lung cancer | In vitro reduction in tumorsphere formation andstem-like markers (CD133, CD44, ALDH1A1). | [169] | |
| Carotenoids | ||||
| Fucoxanthinol (brown algae) | Colorectal cancer | In vitro reduction in tumorsphere formation by the inactivation of AKT signaling and the downregulation of PPARβ/δ and PPARγ protein expression, in vitro induction of apoptosis via the reduction in cellular adhesion molecule expression. | [171] | |
| Colorectal cancer | In vitro decrease in proliferation pathways (JAK/STAT, PI3K/Akt, MAPK, NF-κB). | [172] | ||
| Macrolides | ||||
| Bryostatin-1 (Bugula neritina) | Melanoma cells | In vitro reduction in proliferation and ABCB5+ subpopulation. | [176] | |
| Leukemia | In vitroinduction of apoptosis and reduction in CD34+ cell fraction | Gleevec | [177] |
| NPs (Source) | Tumor Type | Phase | Patient Number | Parameters | Results | References and CT Number |
|---|---|---|---|---|---|---|
| Curcumin (Curcuma longa) | Myeloma | I | 15 | Patients displaying intolerance to dexamethasone treated with curcumin (3.0–4.0 g/day oral administration) plus immunomodulatory drugs (IMD, lenalidomide) or proteasome inhibitors (PI, bortezomib) for about 6 years. | Curcumin in combination with IMD or PI lessened paraprotein (38%) and plasmacytosis (59%) levels; 12 out of 15 patients were stable. | [189] |
| Myeloma and prostate | II | 40 | Patients were treated with curcumin (4 g) plus piperidine (5 mg) by oral administration for 12 months. | No posted results. First results will be posted after May 2023. | NCT04731844 | |
| Myeloma | IIa | 33 | The treated patient group (17) was treated with curcumin (8 g/day) for 28 days plus melphalan (4 mg/m2) and prednisone (40 mg/m2) for 7 days. Control patient group (16) was treated with melphalan, prednisone, and a placebo. The two groups received 4 cycles of treatment. | The treated group displayed an increased overall remission with a reduction in IL-6, VEGF, and TNF-α levels compared with the control group. | [190] ISRCTN14131419 | |
| Metastatic breast cancer | II | 150 | Treated patient group was treated with intravenous curcumin (300 mg) and paclitaxel (80 mg/m2) weekly for 12 weeks. Control patient groups were treated with paclitaxel and a placebo. | Tumor reduction by 50.7% in curcumin treatment compared with 33.3% placebo. | [191] NCT03072992 | |
| Obese women characterized by high risk of developing breast cancer | I | 29 | The participants received curcumin (50 or 100 mg) by oral administration twice daily for 3 months. | No posted results. | NCT01975363 | |
| Breast cancer patients before surgery | Not applicable | 30 | Patients were treated with 8 g per day by oral administration for two to four weeks before surgery. | No posted results. | NCT03847623 | |
| Breast cancer patients treated with radiotherapy | II/III | 686 | Patients were treated with curcumin (6.0 g) by oral administration daily for the entire period of the radiation treatments plus another week. | Breast cancer patients treated with curcumin displayed a reduced dermatitis severity. | NCT01246973 | |
| Metastatic colorectal cancer | IIa | 41 | Treated patient group was treated with curcumin (2 g/day) orally administered plus standard chemotherapy (FOLFOX) every 2 weeks for 12 cycles. | The clinical trial assessed the safety and effect of curcumin in combination with FOLFOX-based chemotherapy in metastatic colorectal cancer patients. | [192] NCT01490996 | |
| EGCG | Ovarian cancer | II | 300 | Five treatment arms: (i) Combined treatment (TP: paclitaxel 175 mg/m2 plus cisplatin 75–100 mg/m2 by intravenous administration, or TC: paclitaxel 175 mg/m2 plus carboplatin AUC 5 by intravenous administration, plus surgery plus postoperative TP or TC regimen) in combination with I3C (200 mg/day) continuously; (ii) combined treatment plus I3C (200 mg/day) and EGCG (200 mg/day) continuously; (iii) combined treatment plus I3C and EGCG continuously and TP therapy; (iv) combined treatment without TP or TC postoperative regimen; (v) combined treatment. | Patients treated with I3C and EGCG plus chemotherapy showed an increased median overall survival (60 months) and median progression-free survival (48.5 months) compared with single treatment and chemotherapy alone. Moreover, I3C and EGCG treatment reduced cancer recurrence. | [193] ACTRN12616000394448 |
| Lung cancer | II | 83 | Prophylactic EGCG group: EGCG (440 umol/L) 0.9% saline solution 3 times/day at the beginning of radiotherapy treatment; therapeutic EGCG group: EGCG 0.9% saline solution 3 times/day in presence of grade 1 esophagitis radiotherapy side effects; conventional therapy group: mLDG (lidocaine 0.16 mg/mL, dexamethasone 0.02 mg/mL, and gentamycin 0.16 mg/mL) 3 times/day in presence of grade 1 esophagitis radiotherapy side effects. | Compared to standard therapies, EGCG preventive treatment and EGCG administration in radiation-treated patients reduced the severity of esophagitis and the levels of pro-inflammatory factors in the serum. | [194] NCT02577393 | |
| Esophageal Cancer | I | 15 | Six escalating doses (880 umol/L–4400 umol/L) of EGCG weredissolved in 0.9% saline solution and administered three times a day. EGCG solution was given continuously for 8 days before anti-tumor treatment. | No posted results (ongoing trial). | [195] NCT05039983 | |
| EGCG (Polyphenon E) | Bladder cancer | II | 31 | An amount of 800–1200 mg/day of orally administered EGCG for 14–28 days prior to surgery. | The dose-dependent downregulation of two tumor biomarkers, clusterin (an apoptosis marker) and PCNA (a proliferation marker), was seen in EGCG-treated patients, supporting the compound’s chemoprotective activity and use as a neoadjuvant therapy before transurethral resection of bladder tumor or cystectomy, despite the fact that there are no differences in EGCG tumor levels between EGCG-treated and placebo patient groups. | [196] |
| Breast cancer | II | 32 | Subjects are asked to take 4 polyphenol E (200 mg) capsules daily with a meal for the duration of the study. Biomarkers are measured at baseline and then again at presurgery, the end-point for the study within a time frame between 4 and 6 weeks. | Polyphenon E neoadjuvant daily administration induced a decreasein serum HGF levels, without altering those of VEGF. | NCT00676793 | |
| Breast cancer | Ib | 40 | After completing adjuvant therapy, women with stage I–III breast cancer were randomized to receive Poly E at dosages of 400, 600, or 800 mg twice daily for six months, or a placebo. Samples of blood and urine were collected at the beginning, 2, 4, and 6 months. | After completing adjuvant therapy, women with stage I–III breast cancer were randomized to receive Poly E at dosages of 400, 600, or 800 mg twice daily for six months, or a placebo. Samples of blood and urine were collected at the beginning, 2, 4, and 6 months. | [197] | |
| Resveratrol | Breast cancer | I | 39 | Resveratrol (5–50 mg) was orally administered for 3 months, twice a day. | After resveratrol treatment, no significant changes in p16, CCND2, APC, and RASSF-1α DNA methylation wereobserved, but only a decreased methylation profile of RASSF-1α and an increase inthe APC profile. | [199] |
| Prostate cancer | I | 14 | Patients were treated with 500 to 4000 mg of muscadine grape extract (MPX, containing 1.2 mg of ellagic acid, 9.2 g of quercetin, and 4.4 g of trans-resveratrol) per day, taken orally for 28 days, with a follow-up of >2 years. | No tolerability problems were observed in patients, and the treatment was deemed to be safe. Additionally, it demonstrated a delay in the recurrence process by extending the PSA doubling time (PSADT) by 5.3 months. | [200] | |
| Prostate cancer | I | 112 | Following stratification based on their initial PSADT and Gleason scores, the participants were randomly allocated 1:2:2 to receive a placebo, 500 mg of MPX (low), or 4000 mg of MPX (high), daily. | There was no discernible difference between the control and MPX-treated cohorts in the time it took for PSA to double. | [201] NCT00256334 | |
| Colorectal cancer | I | 8 | Resveratrol was administered at 20 and 80 mg/day. Resveratrol-containing freeze-dried grape powder (GP) was administered at 0.073 and 0.114 mg/day. Both treatments were taken orally. | Resveratrol/GP treatment significantly inhibited Wnt target gene expression (myc, jun, TCF7, cyclin D1, axin II) in healthy colonic mucosa without effects on tumor mucosa. | [202] | |
| Colorectal cancer | I | 9 | A 5.0 g daily dose for 14 days of SRT501 (micronized form of resveratrol), was administered to patients with colorectal cancer and hepatic metastases who were scheduled to undergo hepatectomy. | This treatment method increased drug availability and absorption. After 1–2 weeks of therapy with resveratrol or SRT501, the observed amounts of parent resveratrol and its primary metabolites in the colon tissue of patients were comparable to the efficacious doses of resveratrol utilized in pre-clinical investigations. In addition, cleaved caspase-3, an indication of death, dramatically increased in malignant hepatic tissue after SRT501 therapy by 39% in comparison to tissue from patients who received a placebo. | [203] | |
| Colorectal cancer | I | 20 | Colorectal cancer patients were treated daily with resveratrol (0.5 or 1.0 g) for 8 days before surgery. | Treatment reduced ki-67 levels. | [204] NCT00433576 | |
| Vincristine | Low-grade glioma | I | 497 | A total of 497 patients were randomized to receive vincristine carboplatin (VC, vincristine 1.5 mg/m2 × 10 weekly and carboplatin 550 mg/m2 q 3 weekly) (n = 249) or VC plus etoposide (VCE, etoposide 100 mg/m2, days 1, 2,and 3). | The high rates of non-progression after 24 weeks support the use of VC as a first-line treatment. | [210] European Union Clinical Trials Register No. 2005-005377-29 |
| Diffuse large B-cell lymphoma | II | 120 | Patients underwent a pre-treatment phase consisting of oral prednisone (60 mg total dosage commencing 1 week before cycle 1, for 4 days (day7 to day4)) and oral vincristine before the first cycle of the ofatumumab (1000 mg every 3 weeks) + miniCHOP regimen (400 mg/m2 of intravenous cyclophosphamide, 25 mg/m2 of intravenous doxorubicin, 1 mg/m2 of intravenous vincristine on day 1 of each cycle, and 40 mg/m2 of oral prednisone every day from days 1 to 5). | The pretreatment with ofatumumab and miniCHOP in 80-year-old patients improved overall survival in comparison with standard therapy. | [211] NCT001195714 | |
| Bryostatin-1 | Renal cell carcinoma | II | 30 | Patients were treated for 30 min with an intravenous infusion of bryostatin-1 (25 microg/m2) with formulation PET (polyethyleneglycol, ethanol, and Tween 80) on days 1, 8, and 15 of each 28-day cycle. | Patients responded to the treatment without severe adverse effects. | [213] |
| Low-grade non-Hodgkin lymphoma and chronic lymphocytic leukemia | II | 25 | Patients were treated for 72 h with a continuous infusion of bryostatin-1 (120 microg/m2) per course every 2 weeks immediately followed by vincristine (from 0.5 mg/m2 to 2 mg/m2) administration by bolus i.v. injection. | Treatment with bryostatin-1 resulted in one patient in complete remission and two in partial remission. Moreover, this treatment promoted a differentiative state of CLL cells, demonstrated bythe presence of CD11c/CD22/CD20 B-cell subpopulations. | [214] | |
| Sulforaphane | Prostate cancer | nd | 98 | Patients were treated with BSE (200 µmol daily) or a placebo for 4–8 weeks. | Forty differently expressed genes linked to BSE treatment, including the downregulation of two prostate cancer-related genes. Supplementing with BSE is associated with alterations in gene expression but not with changes in prostate tissue biomarkers. | [215] (NCT01265953) |
| Prostate cancer | II | 61 | Patients were given a weekly 300 mL serving of soup produced from either regular broccoli (the control) or one of two experimental broccoli genotypes with increased glucoraphanin concentrations that delivered 3 or 7 times the amount of the control, respectively. | Downregulation of genes linked to inflammation processes and epithelial–mesenchymal transition in a dose-dependent manner in glucoraphanin-rich broccoli-soup-consuming men. An inverse relationship was found between the consumption of cruciferous vegetables and a decreased risk of prostate cancer advancement in males under active monitoring. | [216] (NCT01950143) | |
| Fisetin | Colorectal cancer | I | 38 | CRC patients treated with chemotherapy were randomized to receive either 100 mg fisetin (n = 18) or placebo (n = 19) for 7 weeks. | After fisetin administration to CRC patients, plasma levels of IL-8 and hs-CRP dropped dramatically as a lower expression of MMP-7. | [217] (code: IRCT2015110511288N9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Iacono, M.; Gaggianesi, M.; Bianca, P.; Brancato, O.R.; Muratore, G.; Modica, C.; Roozafzay, N.; Shams, K.; Colarossi, L.; Colarossi, C.; et al. Destroying the Shield of Cancer Stem Cells: Natural Compounds as Promising Players in Cancer Therapy. J. Clin. Med. 2022, 11, 6996. https://doi.org/10.3390/jcm11236996
Lo Iacono M, Gaggianesi M, Bianca P, Brancato OR, Muratore G, Modica C, Roozafzay N, Shams K, Colarossi L, Colarossi C, et al. Destroying the Shield of Cancer Stem Cells: Natural Compounds as Promising Players in Cancer Therapy. Journal of Clinical Medicine. 2022; 11(23):6996. https://doi.org/10.3390/jcm11236996
Chicago/Turabian StyleLo Iacono, Melania, Miriam Gaggianesi, Paola Bianca, Ornella Roberta Brancato, Giampaolo Muratore, Chiara Modica, Narges Roozafzay, Kimiya Shams, Lorenzo Colarossi, Cristina Colarossi, and et al. 2022. "Destroying the Shield of Cancer Stem Cells: Natural Compounds as Promising Players in Cancer Therapy" Journal of Clinical Medicine 11, no. 23: 6996. https://doi.org/10.3390/jcm11236996
APA StyleLo Iacono, M., Gaggianesi, M., Bianca, P., Brancato, O. R., Muratore, G., Modica, C., Roozafzay, N., Shams, K., Colarossi, L., Colarossi, C., Memeo, L., Turdo, A., Veschi, V., Di Franco, S., Todaro, M., & Stassi, G. (2022). Destroying the Shield of Cancer Stem Cells: Natural Compounds as Promising Players in Cancer Therapy. Journal of Clinical Medicine, 11(23), 6996. https://doi.org/10.3390/jcm11236996









