Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View
Abstract
1. Introduction
2. CRS Types and Epidemiological Data
3. Pathophysiology
4. Diagnosis
4.1. Traditional Diagnostic Methods
4.2. Renal Biomarkers
4.3. Cardiac Biomarkers
4.4. Imaging of the Heart and the Kidneys
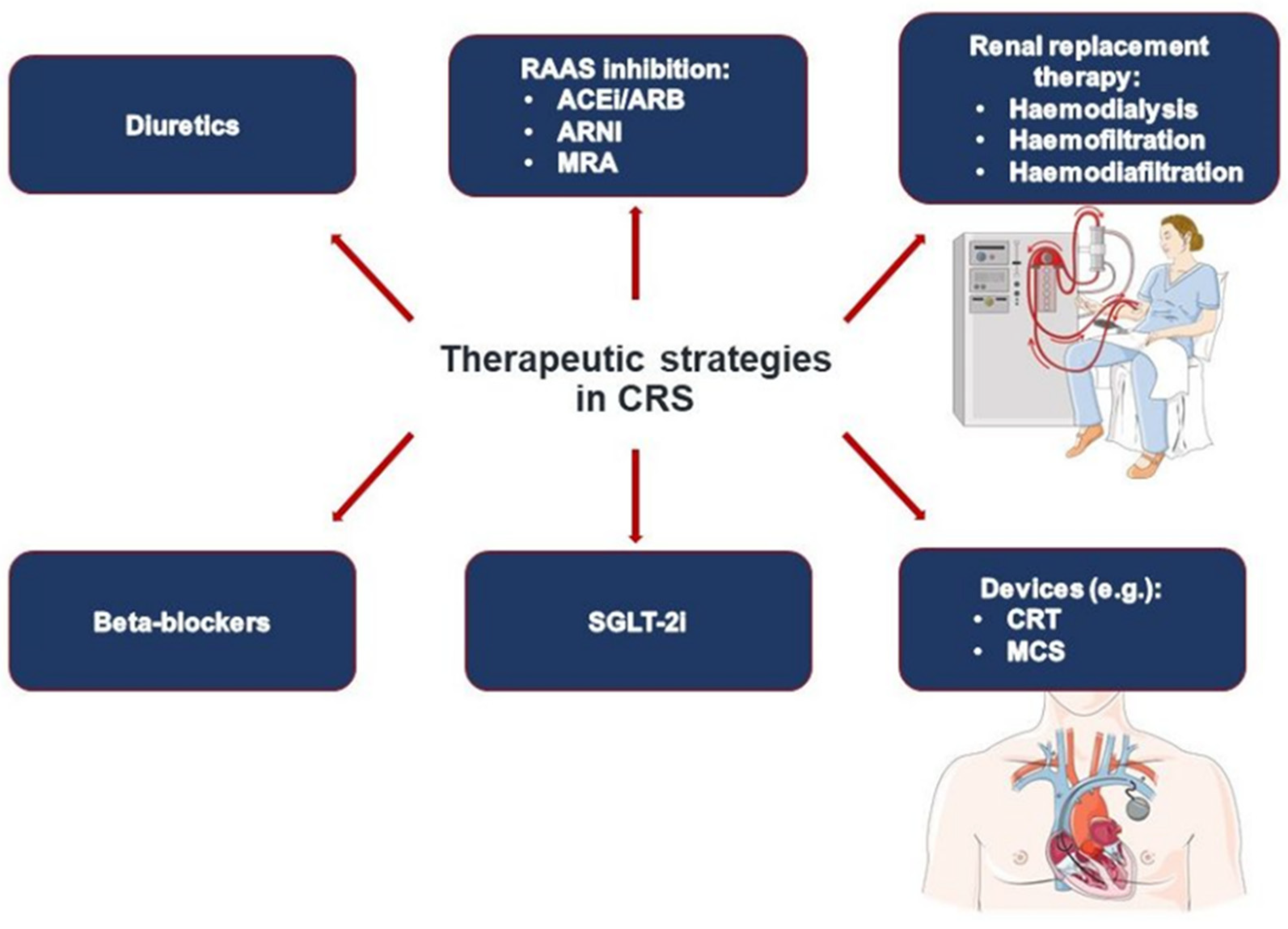
5. Treatment Strategies
5.1. Regulation of Volume Status
5.2. Heart Failure Medication
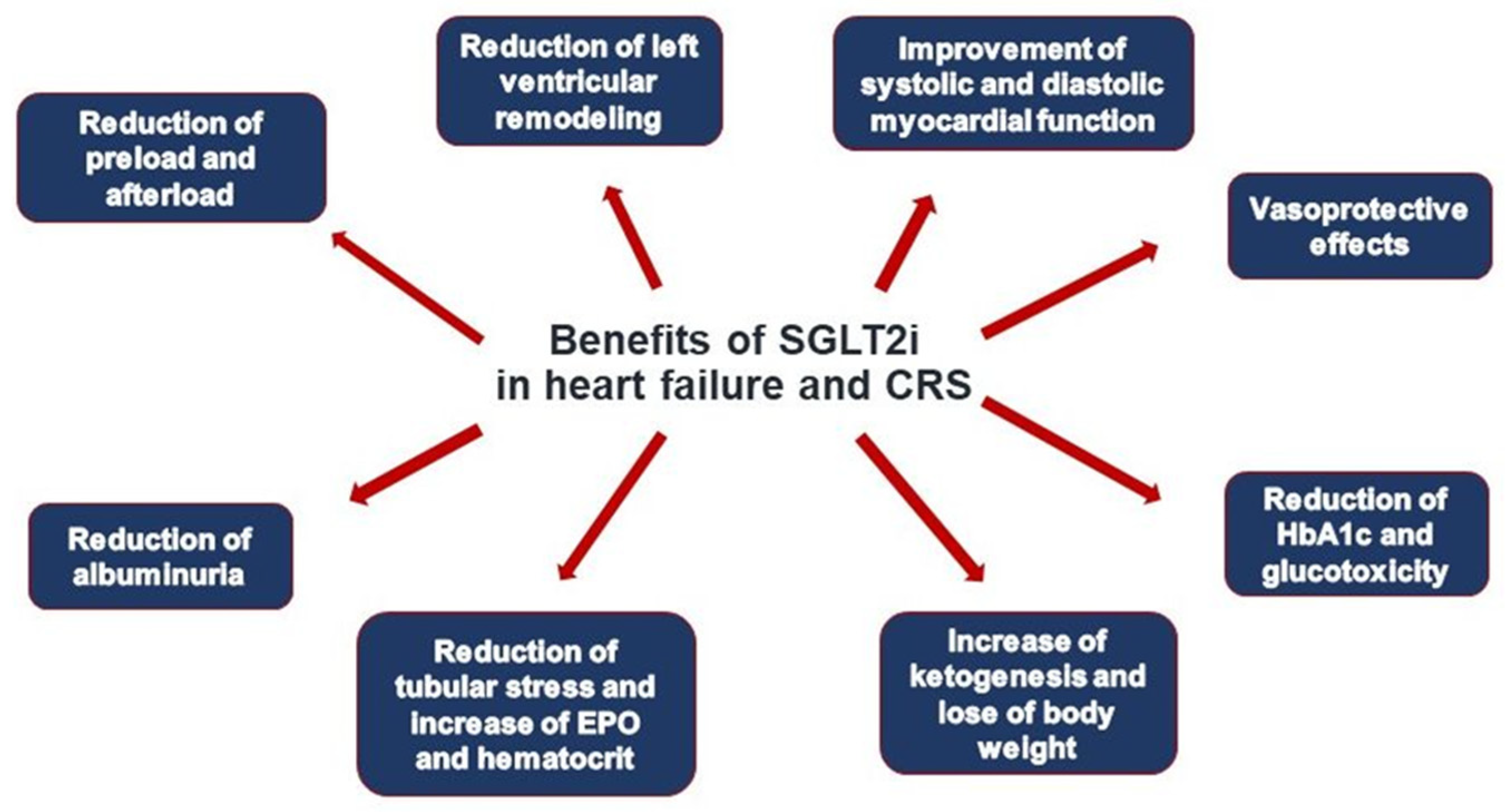
5.3. Novel Treatment Concepts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting-enzyme-inhibitors |
| ACS | acute coronary syndrome |
| ADH | antidiuretic hormone |
| ADHF | acute decompensated heart failure |
| AHF | acute heart failure |
| AKI | acute kidney injury |
| ARB | angiotensin-receptor-blockers |
| ARNI | angiotensin-renin-neprilysin-inhibitors |
| BB | beta-adrenergic blockers |
| BOLD MR | Blood oxygen level-dependent (BOLD) MR imaging |
| CHF | chronic heart failure |
| CKD | chronic kidney disease |
| CNS | central nervous system |
| CRS | cardiorenal syndrome |
| CysC | Cystatin C |
| GFR | glomerular filtration rate |
| HF | heart failure |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| KIM-1 | kidney injury molecule-1 |
| LVEF | left ventricular ejection fraction |
| MRA | mineraloreceptor antagonists |
| MRI | magnetic resonance imaging |
| RAAS | renin-angiotensin-aldosterone-system |
| SGLT2i | sodium glucose linked transporter 2 inhibitors |
| SIRS | systemic inflammatory response syndrome |
| SNS | sympathetic nervous system |
References
- Vaidya, V.S.; Ramirez, V.; Ichimura, T.; Bobadilla, N.A.; Bonventre, J.V. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal. Physiol. 2006, 290, F517–F529. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Abraham, E.; Ancukiewicz, M.; Edelstein, C.L. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J. Am. Soc. Nephrol. 2005, 16, 3046–3052. [Google Scholar] [CrossRef]
- Liangos, O.; Perianayagam, M.C.; Vaidya, V.S.; Han, W.K.; Wald, R.; Tighiouart, H.; MacKinnon, R.W.; Li, L.; Balakrishnan, V.S.; Pereira, B.J.; et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J. Am. Soc. Nephrol. 2007, 18, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; NGAL Meta-Analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef]
- Maisel, A.S.; Mueller, C.; Fitzgerald, R.; Brikhan, R.; Hiestand, B.C.; Iqbal, N.; Clopton, P.; van Veldhuisen, D.J. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur. J. Heart Fail. 2011, 13, 846–851. [Google Scholar] [CrossRef]
- Noiri, E.; Doi, K.; Negishi, K.; Tanaka, T.; Hamasaki, Y.; Fujita, T.; Portilla, D.; Sugaya, T. Urinary fatty acid-binding protein 1: An early predictive biomarker of kidney injury. Am. J. Physiol. Renal. Physiol. 2009, 296, F669–F679. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Nozaki, N.; Hirono, O.; Watanabe, T.; Nitobe, J.; Miyashita, T.; Miyamoto, T.; Koyama, Y.; et al. Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ. J. 2008, 72, 109–114. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Zarbock, A.; Kullmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Ronco, C.; Di Lullo, L. Cardiorenal syndrome. Heart Fail. Clin. 2014, 10, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Katsurada, K.; Zheng, H. Cardiorenal Syndrome: The Role of Neural Connections Between the Heart and the Kidneys. Circ. Res. 2022, 130, 1601–1617. [Google Scholar] [CrossRef]
- Bright, R. Cases and Observations Illustrative of Renal Disease, Accompanied with the Secretion of Albuminous Urine. Med. Chir. Rev. 1836, 25, 23–35. [Google Scholar]
- U.S. Department of Health & Human Services. Cardio-Renal Connections in Heart Failure and Cardiovascular Disease. 2004. Available online: https://www.nhlbi.nih.gov/events/2004/cardio-renal-connections-heart-failure-and-cardiovascular-disease (accessed on 25 March 2022).
- Ronco, C.; McCullough, P.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Acute Dialysis Quality Initiative consensus g. Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 2010, 31, 703–711. [Google Scholar] [CrossRef]
- George, S.M.; Kalantarinia, K. The role of imaging in the management of cardiorenal syndrome. Int. J. Nephrol. 2011, 2011, 245241. [Google Scholar] [CrossRef]
- Padeletti, L.; Innocenti, L.; Paoletti Perini, A.; Gronda, E. Arrhythmic complication in cardiorenal syndrome. Heart Fail. Rev. 2011, 16, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Y.; Han, D.; Yang, A.; Wang, A.Y.; Qi, W. Cardiorenal Syndrome in COVID-19 Patients: A Systematic Review. Front. Cardiovasc. Med. 2022, 9, 915533. [Google Scholar] [CrossRef]
- Uduman, J. Epidemiology of Cardiorenal Syndrome. Adv. Chronic Kidney Dis. 2018, 25, 391–399. [Google Scholar] [CrossRef]
- Ronco, C.; Di Lullo, L. Cardiorenal Syndrome in Western Countries: Epidemiology, Diagnosis and Management Approaches. Kidney Dis 2017, 2, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C. Cardiorenal and renocardiac syndromes: Clinical disorders in search of a systematic definition. Int. J. Artif. Organs 2008, 31, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kaze, A.D.; Zhuo, M.; Kim, S.C.; Patorno, E.; Paik, J.M. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: A meta-analysis. Cardiovasc. Diabetol. 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- de la Espriella, R.; Bayes-Genis, A.; Morillas, H.; Bravo, R.; Vidal, V.; Nunez, E.; Santas, E.; Minana, G.; Sanchis, J.; Facila, L.; et al. Renal function dynamics following co-administration of sacubitril/valsartan and empagliflozin in patients with heart failure and type 2 diabetes. ESC Heart Fail. 2020, 7, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Hebert, K.; Dias, A.; Delgado, M.C.; Franco, E.; Tamariz, L.; Steen, D.; Trahan, P.; Major, B.; Arcement, L.M. Epidemiology and survival of the five stages of chronic kidney disease in a systolic heart failure population. Eur. J. Heart Fail. 2010, 12, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Zannad, F.; Sopko, G.; Klein, L.; Pina, I.L.; Konstam, M.A.; Massie, B.M.; Roland, E.; Targum, S.; Collins, S.P.; et al. International Working Group on Acute Heart Failure S. Acute heart failure syndromes: Current state and framework for future research. Circulation 2005, 112, 3958–3968. [Google Scholar] [CrossRef]
- Ronco, F.; Mazzone, P.; Hosseinian, L.; Genovesi, S. Recent Advances in Stroke Prevention in Patients with Atrial Fibrillation and End-Stage Renal Disease. Cardiorenal Med. 2017, 7, 207–217. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Bansal, N.; Keane, M.; Delafontaine, P.; Dries, D.; Foster, E.; Gadegbeku, C.A.; Go, A.S.; Hamm, L.L.; Kusek, J.W.; Ojo, A.O.; et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2013, 8, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Hasselblad, V.; Stebbins, A.; Pauly, D.F.; Fonarow, G.C.; Shah, M.; Yancy, C.W.; Califf, R.M.; Stevenson, L.W.; Hill, J.A. Cardiorenal interactions: Insights from the ESCAPE trial. J. Am. Coll. Cardiol. 2008, 51, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Fonarow, G.C.; Greene, S.J.; Zhang, S.; Alhanti, B.; DeVore, A.D.; Butler, J.; Heidenreich, P.A.; Huang, J.C.; Kittleson, M.M.; et al. Kidney Function and Outcomes in Patients Hospitalized With Heart Failure. J. Am. Coll. Cardiol. 2021, 78, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kataja, A.; Tarvasmaki, T.; Lassus, J.; Kober, L.; Sionis, A.; Spinar, J.; Parissis, J.; Carubelli, V.; Cardoso, J.; Banaszewski, M.; et al. Altered mental status predicts mortality in cardiogenic shock—Results from the CardShock study. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.H.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients with Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Ahmad, T.; Brisco-Bacik, M.A.; Bonventre, J.V.; Wilson, F.P.; Siew, E.D.; Felker, G.M.; Anstrom, K.K.; Mahoney, D.D.; Bart, B.A.; et al. Renal Effects of Intensive Volume Removal in Heart Failure Patients With Preexisting Worsening Renal Function. Circ. Heart Fail. 2019, 12, e005552. [Google Scholar] [CrossRef]
- Damman, K.; Gori, M.; Claggett, B.; Jhund, P.S.; Senni, M.; Lefkowitz, M.P.; Prescott, M.F.; Shi, V.C.; Rouleau, J.L.; Swedberg, K.; et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Heart Fail. 2018, 6, 489–498. [Google Scholar] [CrossRef]
- Bradley, S.E.; Bradley, G.P. The Effect of Increased Intra-Abdominal Pressure on Renal Function in Man. J. Clin. Investig. 1947, 26, 1010–1022. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef]
- Tarvasmaki, T.; Haapio, M.; Mebazaa, A.; Sionis, A.; Silva-Cardoso, J.; Tolppanen, H.; Lindholm, M.G.; Pulkki, K.; Parissis, J.; Harjola, V.P.; et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Heart Fail. 2018, 20, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, D.; Phillips, M.I.; Mehta, P.; Mehta, J.L. Myocardial angiotensin II receptor expression and ischemia-reperfusion injury. Vasc. Med. 1998, 3, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.O.; Long, C.S.; Kalinyak, J.E.; Li, H.T.; Karliner, J.S. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc. Res. 1998, 40, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Senchenkova, E.Y.; Russell, J.; Esmon, C.T.; Granger, D.N. Roles of Coagulation and fibrinolysis in angiotensin II-enhanced microvascular thrombosis. Microcirculation 2014, 21, 401–407. [Google Scholar] [CrossRef]
- Hitomi, H.; Kiyomoto, H.; Nishiyama, A. Angiotensin II and oxidative stress. Curr. Opin. Cardiol. 2007, 22, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bidani, A.K.; Griffin, K.A. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr. Opin. Nephrol. Hypertens. 2002, 11, 73–80. [Google Scholar] [CrossRef]
- Kontogiannis, J.; Burns, K.D. Role of AT1 angiotensin II receptors in renal ischemic injury. Am. J. Physiol. 1998, 274, F79–F90. [Google Scholar]
- Cravedi, P.; Remuzzi, G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br. J. Clin. Pharmacol. 2013, 76, 516–523. [Google Scholar]
- Bohrer, M.P.; Deen, W.M.; Robertson, C.R.; Brenner, B.M. Mechanism of angiotensin II-induced proteinuria in the rat. Am. J. Physiol. 1977, 233, F13–F21. [Google Scholar] [CrossRef]
- Lins, B.B.; Casare, F.A.M.; Fontenele, F.F.; Goncalves, G.L.; Oliveira-Souza, M. Long-Term Angiotensin II Infusion Induces Oxidative and Endoplasmic Reticulum Stress and Modulates Na(+) Transporters through the Nephron. Front. Physiol. 2021, 12, 642752. [Google Scholar] [CrossRef]
- Mezzano, S.A.; Ruiz-Ortega, M.; Egido, J. Angiotensin II and renal fibrosis. Hypertension 2001, 38 Pt 2, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Arenare, F.; Spaziani, D.; Mancia, G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension 2009, 53, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Aghel, A.; Shrestha, K.; Mullens, W.; Borowski, A.; Tang, W.H. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J. Card. Fail. 2010, 16, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, Y.; Garty, M.; Schwartz, R.; Sandach, A.; Behar, S.; Caspi, A.; Gottlieb, S.; Ezra, D.; Lewis, B.S.; Leor, J. Prevalence and significance of unrecognized renal insufficiency in patients with heart failure. Eur. Heart J. 2008, 29, 1029–1036. [Google Scholar] [CrossRef]
- Waikar, S.S.; Bonventre, J.V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 672–679. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; van Essen, B.J.; Zannad, F.; van Veldhuisen, D.J.; Samani, N.J.; Dickstein, K.; Metra, M.; Filippatos, G.; et al. Albuminuria as a marker of systemic congestion in patients with heart failure. Eur. Heart J. 2022, ehac528. [Google Scholar] [CrossRef]
- Roos, J.F.; Doust, J.; Tett, S.E.; Kirkpatrick, C.M. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—A meta-analysis. Clin. Biochem. 2007, 40, 383–391. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Schmid, C.H.; Feldman, H.I.; Froissart, M.; Kusek, J.; Rossert, J.; Van Lente, F.; Bruce, R.D., 3rd; Zhang, Y.L.; et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am. J. Kidney Dis. 2008, 51, 395–406. [Google Scholar] [CrossRef]
- Macdonald, J.; Marcora, S.; Jibani, M.; Roberts, G.; Kumwenda, M.; Glover, R.; Barron, J.; Lemmey, A. GFR estimation using cystatin C is not independent of body composition. Am. J. Kidney Dis. 2006, 48, 712–719. [Google Scholar] [CrossRef]
- Newman, D.J.; Thakkar, H.; Edwards, R.G.; Wilkie, M.; White, T.; Grubb, A.O.; Price, C.P. Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995, 47, 312–318. [Google Scholar] [CrossRef]
- Maisel, A.S.; Wettersten, N.; van Veldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Muller, G.A.; et al. Neutrophil Gelatinase-Associated Lipocalin for Acute Kidney Injury during Acute Heart Failure Hospitalizations: The AKINESIS Study. J. Am. Coll. Cardiol. 2016, 68, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.T.; Wettersten, N.; van Veldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Mueller, G.A.; et al. Utility of Urine Neutrophil Gelatinase-Associated Lipocalin for Worsening Renal Function during Hospitalization for Acute Heart Failure: Primary Findings of the Urine N-gal Acute Kidney Injury N-gal Evaluation of Symptomatic Heart Failure Study (AKINESIS). J. Card. Fail. 2019, 25, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Valente, M.A.E.; van Veldhuisen, D.J.; Cleland, J.G.F.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Cotter, G.; Davison, B.; Givertz, M.M.; et al. Plasma Neutrophil Gelatinase-Associated Lipocalin and Predicting Clinically Relevant Worsening Renal Function in Acute Heart Failure. Int. J. Mol. Sci. 2017, 18, 1470. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Mueller, C.; Adams, K., Jr.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef]
- Dieplinger, B.; Mueller, T. Soluble ST2 in heart failure. Clin. Chim. Acta 2015, 443, 57–70. [Google Scholar] [CrossRef]
- Colbert, G.; Jain, N.; de Lemos, J.A.; Hedayati, S.S. Utility of traditional circulating and imaging-based cardiac biomarkers in patients with predialysis CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 515–529. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, X.; Dekkers, I.A.; Lamb, H.J. Cardiorenal Syndrome: Emerging Role of Medical Imaging for Clinical Diagnosis and Management. J. Pers. Med. 2021, 11, 734. [Google Scholar] [CrossRef]
- Di Lullo, L.; Floccari, F.; Granata, A.; D’Amelio, A.; Rivera, R.; Fiorini, F.; Malaguti, M.; Timio, M. Ultrasonography: Ariadne’s Thread in the Diagnosis of the Cardiorenal Syndrome. Cardiorenal Med. 2012, 2, 11–17. [Google Scholar] [CrossRef]
- Grenier, N.; Merville, P.; Combe, C. Radiologic imaging of the renal parenchyma structure and function. Nat. Rev. Nephrol. 2016, 12, 348–359. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A.; Hoyt, K. New Ultrasound Techniques Promise Further Advances in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 3452–3460. [Google Scholar] [CrossRef]
- Mahoney, M.; Sorace, A.; Warram, J.; Samuel, S.; Hoyt, K. Volumetric contrast-enhanced ultrasound imaging of renal perfusion. J. Ultrasound. Med. 2014, 33, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Osugi, N.; Suzuki, S.; Shibata, Y.; Tatami, Y.; Harata, S.; Ota, T.; Hayashi, M.; Yasuda, Y.; Ishii, H.; Shimizu, A.; et al. Coronary artery calcification scores improve contrast-induced nephropathy risk assessment in chronic kidney disease patients. Clin. Exp. Nephrol. 2017, 21, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.W.; et al. Post-contrast acute kidney injury—Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- American College of Cardiology Foundation Task Force on Expert Consensus Documents; Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010, 121, 2462–2508. [Google Scholar]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Gadolinium-contrast toxicity in patients with kidney disease: Nephrotoxicity and nephrogenic systemic fibrosis. Curr. Drug Saf. 2008, 3, 67–75. [Google Scholar] [CrossRef]
- Pursnani, A.; Prasad, P.V. Science to Practice: Can Functional MR Imaging Be Useful in the Evaluation of Cardiorenal Syndrome? Radiology 2018, 286, 1–3. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Noutsias, M.; Rigopoulos, A.G.; Giannakopoulou, A.; Gatzonis, S.; Pons, R.M.; Papavasiliou, A.; Vartela, V.; Bonou, M.; Kolovou, G.; et al. The emerging role of combined brain/heart magnetic resonance imaging for the evaluation of brain/heart interaction in heart failure. J. Clin. Med. 2022, 11, 4009. [Google Scholar] [CrossRef]
- Breidthardt, T.; Cox, E.F.; Squire, I.; Odudu, A.; Omar, N.F.; Eldehni, M.T.; Francis, S.T.; McIntyre, C.W. The pathophysiology of the chronic cardiorenal syndrome: A magnetic resonance imaging study. Eur. Radiol. 2015, 25, 1684–1691. [Google Scholar] [CrossRef]
- Noutsias, M.; Hauptmann, M.; Voller, H. Pointing a FINGER at the contribution of lifestyle to cardiovascular events and dementia. Eur. Heart J. 2022, 43, 2062–2064. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Piaditis, G.; Bacopoulou, F.; Chrousos, G.P. Cardiac Remodeling in Hypertension: Clinical Impact on Brain, Heart, and Kidney Function. Horm. Metab. Res. 2022, 54, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Wang, Y.C.; Xu, T.T.; Peng, X.G.; Cai, Y.; Wang, L.; Bai, Y.Y.; Ju, S. Noninvasive Identification of Renal Hypoxia in Experimental Myocardial Infarctions of Different Sizes by Using BOLD MR Imaging in a Mouse Model. Radiology 2018, 286, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bane, O.; Mendichovszky, I.A.; Milani, B.; Dekkers, I.A.; Deux, J.F.; Eckerbom, P.; Grenier, N.; Hall, M.E.; Inoue, T.; Laustsen, C.; et al. Consensus-based technical recommendations for clinical translation of renal BOLD MRI. MAGMA 2020, 33, 199–215. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- He, T.; Zhang, Z.; Staessen, J.A.; Mischak, H.; Latosinska, A.; Beige, J. Proteomic Biomarkers in the Cardiorenal Syndrome: Toward Deciphering Molecular Pathophysiology. Am. J. Hypertens 2021, 34, 669–679. [Google Scholar] [CrossRef]
- Rangaswami, J.; Mathew, R.O. Pathophysiological Mechanisms in Cardiorenal Syndrome. Adv. Chronic. Kidney Dis. 2018, 25, 400–407. [Google Scholar] [CrossRef]
- Prosek, J.; Agarwal, A.; Parikh, S.V. Cardiorenal syndrome and the role of ultrafiltration in heart failure. Curr. Heart Fail. Rep. 2013, 10, 81–88. [Google Scholar] [CrossRef]
- Testani, J.M.; Chen, J.; McCauley, B.D.; Kimmel, S.E.; Shannon, R.P. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010, 122, 265–272. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Ronco, C.; McCullough, P.A.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardiorenal syndromes: An executive summary from the consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2010, 165, 54–67. [Google Scholar] [PubMed]
- Ellison, D.H.; Felker, G.M. Diuretic Treatment in Heart Failure. N. Engl. J. Med. 2017, 377, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Marton, A.; Kaneko, T.; Kovalik, J.P.; Yasui, A.; Nishiyama, A.; Kitada, K.; Titze, J. Organ protection by SGLT2 inhibitors: Role of metabolic energy and water conservation. Nat. Rev. Nephrol. 2021, 17, 65–77. [Google Scholar] [CrossRef]
- Chou, Y.H.; Huang, T.M.; Wu, V.C.; Wang, C.Y.; Shiao, C.C.; Lai, C.F.; Tsai, H.B.; Chao, C.T.; Young, G.H.; Wang, W.J.; et al. Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit. Care 2011, 15, R134. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Badve, S.V.; Roberts, M.A.; Hawley, C.M.; Cass, A.; Garg, A.X.; Krum, H.; Tonkin, A.; Perkovic, V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 1152–1161. [Google Scholar] [CrossRef]
- McCallum, W.; Tighiouart, H.; Ku, E.; Salem, D.; Sarnak, M.J. Trends in Kidney Function Outcomes Following RAAS Inhibition in Patients With Heart Failure With Reduced Ejection Fraction. Am. J. Kidney Dis. 2020, 75, 21–29. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Investigators P-H, Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef]
- Haynes, R.; Judge, P.K.; Staplin, N.; Herrington, W.G.; Storey, B.C.; Bethel, A.; Bowman, L.; Brunskill, N.; Cockwell, P.; Hill, M.; et al. Effects of Sacubitril/Valsartan Versus Irbesartan in Patients With Chronic Kidney Disease. Circulation 2018, 138, 1505–1514. [Google Scholar] [CrossRef]
- Spannella, F.; Giulietti, F.; Filipponi, A.; Sarzani, R. Effect of sacubitril/valsartan on renal function: A systematic review and meta-analysis of randomized controlled trials. ESC Heart Fail. 2020, 7, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Oestreicher, N.; Knispel, J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am. J. Manag. Care 2015, 21 (Suppl. S11), S212–S220. [Google Scholar] [PubMed]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggiioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan, I. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Shin, J.I.; Chen, T.K.; Inker, L.A.; Coresh, J.; Alexander, G.C.; Jackson, J.W.; Chang, A.R.; Grams, M.E. Association between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality among Persons with Low Estimated Glomerular Filtration Rate. JAMA Intern. Med. 2020, 180, 718–726. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Lam, C.S.P.; Testani, J.M.; Voors, A.A.; Van Spall, H.G.C.; Ter Maaten, J.M.; Damman, K. Evidence-Based Medical Therapy in Patients with Heart Failure with Reduced Ejection Fraction and Chronic Kidney Disease. Circulation 2022, 145, 693–712. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Investigators F-D. Effect of Finerenone on Chronic Kidney Disea.as.se Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Filippatos, G.; Pitt, B.; Agarwal, R.; Farmakis, D.; Ruilope, L.M.; Rossing, P.; Bauersachs, J.; Mentz, R.J.; Kolkhof, P.; Scott, C.; et al. Investigators F-D. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: A prespecified subgroup analysis of the FIDELIO-DKD trial. Eur. J. Heart Fail. 2022, 24, 996–1005. [Google Scholar] [CrossRef]
- Chiu, N.; Aggarwal, R.; Bakris, G.L.; Pitt, B.; Bhatt, D.L. Generalizability of FIGARO-DKD and FIDELIO-DKD Trial Criteria to the US Population Eligible for Finerenone. J. Am. Heart Assoc. 2022, 11, e025079. [Google Scholar] [CrossRef]
- Agarwal, R.; Rossignol, P.; Romero, A.; Garza, D.; Mayo, M.R.; Warren, S.; Ma, J.; White, W.B.; Williams, B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 1540–1550. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Berg, D.D.; Jhund, P.S.; Docherty, K.F.; Murphy, S.A.; Verma, S.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients with Heart Failure with Reduced Ejection Fraction. JAMA Cardiol. 2021, 6, 499–507. [Google Scholar] [CrossRef]
- Vukadinovic, D.; Abdin, A.; Anker, S.D.; Rosano, G.M.C.; Mahfoud, F.; Packer, M.; Butler, J.; Bohm, M. Side effects and treatment initiation barriers of sodium-glucose cotransporter 2 inhibitors in heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2022, 24, 1625–1632. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Mobius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients with Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefansson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Nangaku, M. More reasons to use SGLT2 inhibitors: EMPEROR-reduced and DAPA-CKD. Kidney Int. 2020, 98, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, B.V.; Heerspink, H.J.L.; Wheeler, D.C.; Sjostrom, C.D.; Greasley, P.J.; Sartipy, P.; Cain, V.; Correa-Rotter, R. Correction of anemia by dapagliflozin in patients with type 2 diabetes. J. Diabetes Complications 2020, 34, 107729. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Braunwald, E. Cardiac and Renal Effects of Sodium-Glucose Co-Transporter 2 Inhibitors in Diabetes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1845–1855. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Vickneson, K.; Singh, J.S. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail. Rev. 2021, 26, 623–642. [Google Scholar] [CrossRef]
- Davis, M.K.; Virani, S.A. Cardiac resynchronization therapy in the cardiorenal syndrome. Int. J. Nephrol. 2011, 2011, 168461. [Google Scholar] [CrossRef][Green Version]
- Grassi, G.; Vincenti, A.; Brambilla, R.; Trevano, F.Q.; Dell’Oro, R.; Ciro, A.; Trocino, G.; Vincenzi, A.; Mancia, G. Sustained sympathoinhibitory effects of cardiac resynchronization therapy in severe heart failure. Hypertension 2004, 44, 727–731. [Google Scholar] [CrossRef]
- Napp, L.C.; Mariani, S.; Ruhparwar, A.; Schmack, B.; Keeble, T.R.; Reitan, O.; Hanke, J.S.; Dogan, G.; Hiss, M.; Bauersachs, J.; et al. First-in-Man Use of the Percutaneous 10F Reitan Catheter Pump for Cardiorenal Syndrome. ASAIO J. 2022, 68, e99–e101. [Google Scholar] [CrossRef]
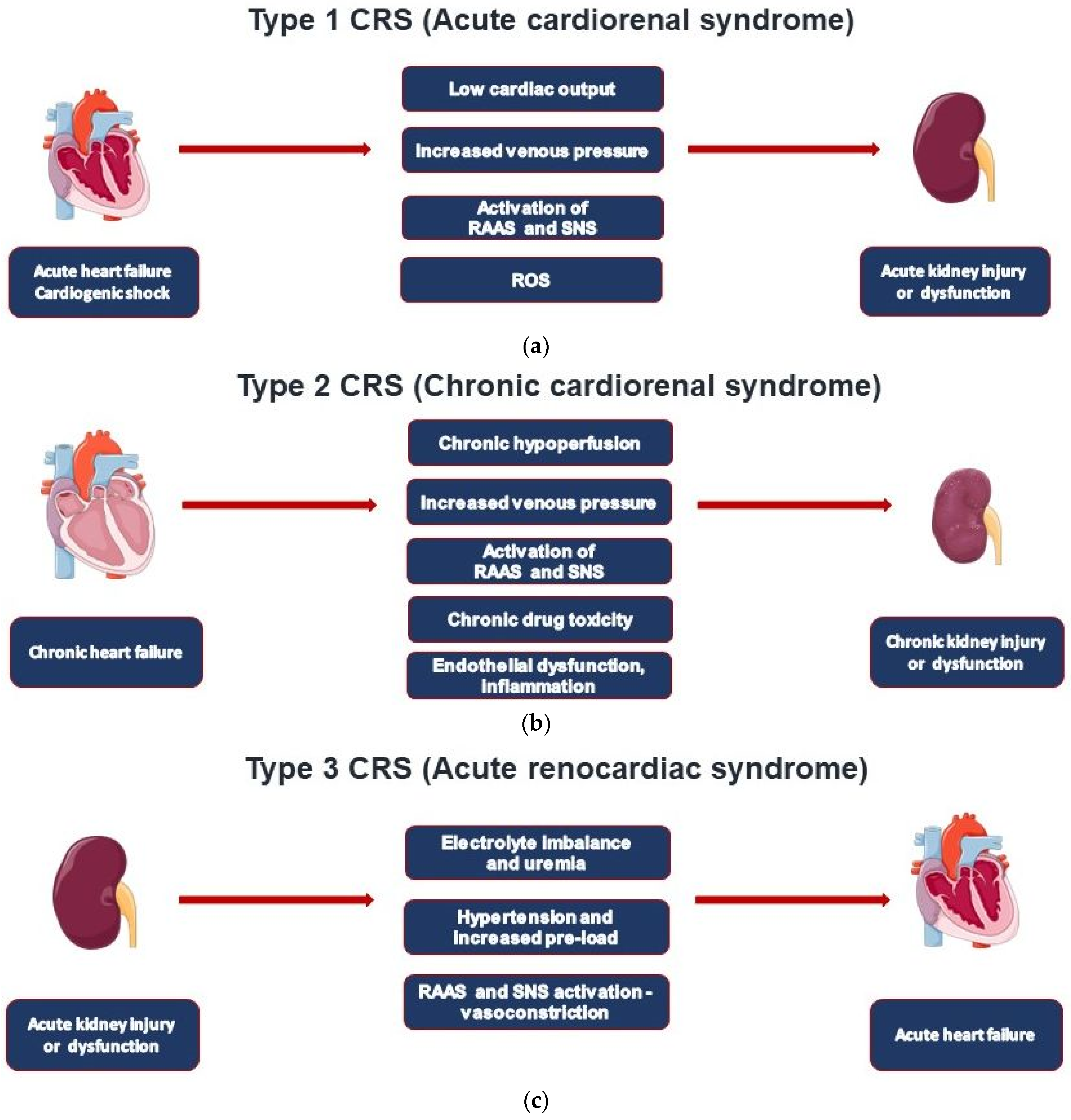
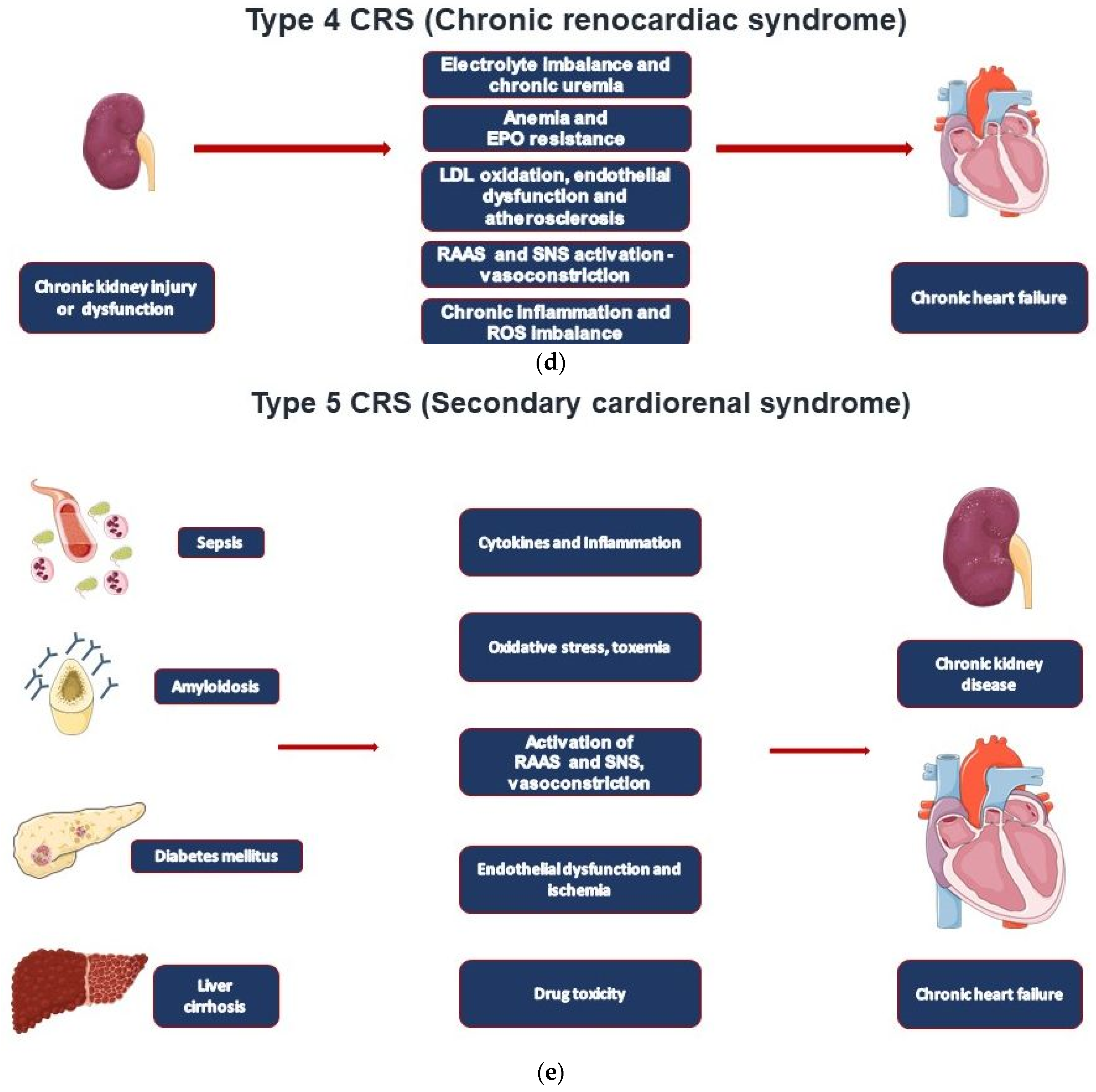
| CRS Types | Mechanisms | Clinical Conditions |
|---|---|---|
| Type 1—Acute cardiorenal syndrome | AHF leading to AKI | AHF, ACS, cardiogenic shock |
| Type 2—Chronic cardiorenal syndrome | CHF leading to CKD | CHF regardless of cause |
| Type 3—Acute renocardiac syndrome | AKI leading to AHF | Volume overload, uremic metabolic disturbances, and inflammatory eruption |
| Type 4—Chronic renocardiac syndrome | CKD leading to CHF | CKD-induced cardiomyopathy resulting in cardiac remodeling and heart failure |
| Type 5—Secondary cardiorenal syndrome | Systemic disorder leading to cardiorenal dysfunction | Sepsis, diabetes, liver cirrhosis, amyloidosis, M. Fabry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsas, A.C.; Elzawawi, M.; Mavrogeni, S.; Boekels, M.; Khan, A.; Eldawy, M.; Stamatakis, I.; Kouris, D.; Daboul, B.; Gunkel, O.; et al. Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View. J. Clin. Med. 2022, 11, 7041. https://doi.org/10.3390/jcm11237041
Mitsas AC, Elzawawi M, Mavrogeni S, Boekels M, Khan A, Eldawy M, Stamatakis I, Kouris D, Daboul B, Gunkel O, et al. Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View. Journal of Clinical Medicine. 2022; 11(23):7041. https://doi.org/10.3390/jcm11237041
Chicago/Turabian StyleMitsas, Angelos C., Mohamed Elzawawi, Sophie Mavrogeni, Michael Boekels, Asim Khan, Mahmoud Eldawy, Ioannis Stamatakis, Dimitrios Kouris, Baraa Daboul, Oliver Gunkel, and et al. 2022. "Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View" Journal of Clinical Medicine 11, no. 23: 7041. https://doi.org/10.3390/jcm11237041
APA StyleMitsas, A. C., Elzawawi, M., Mavrogeni, S., Boekels, M., Khan, A., Eldawy, M., Stamatakis, I., Kouris, D., Daboul, B., Gunkel, O., Bigalke, B., van Gisteren, L., Almaghrabi, S., & Noutsias, M. (2022). Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View. Journal of Clinical Medicine, 11(23), 7041. https://doi.org/10.3390/jcm11237041










