A Machine-Learning Model for the Prognostic Role of C-Reactive Protein in Myocarditis
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
RF Algorithm Development
4. Results
4.1. Patients’ Characteristics in the Overall Cohort
4.2. Clinical and Diagnostic Correlates and CRP Levels
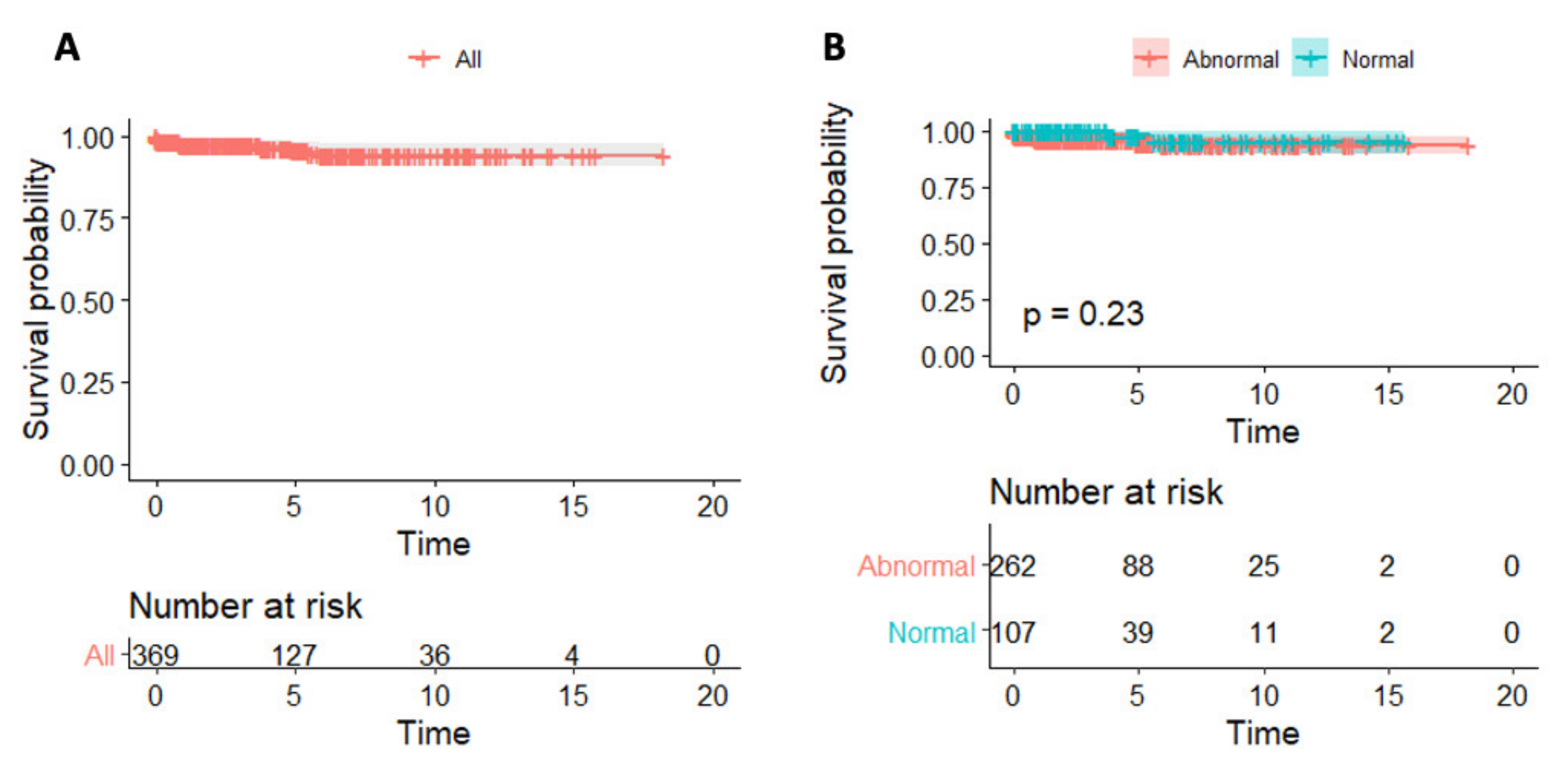
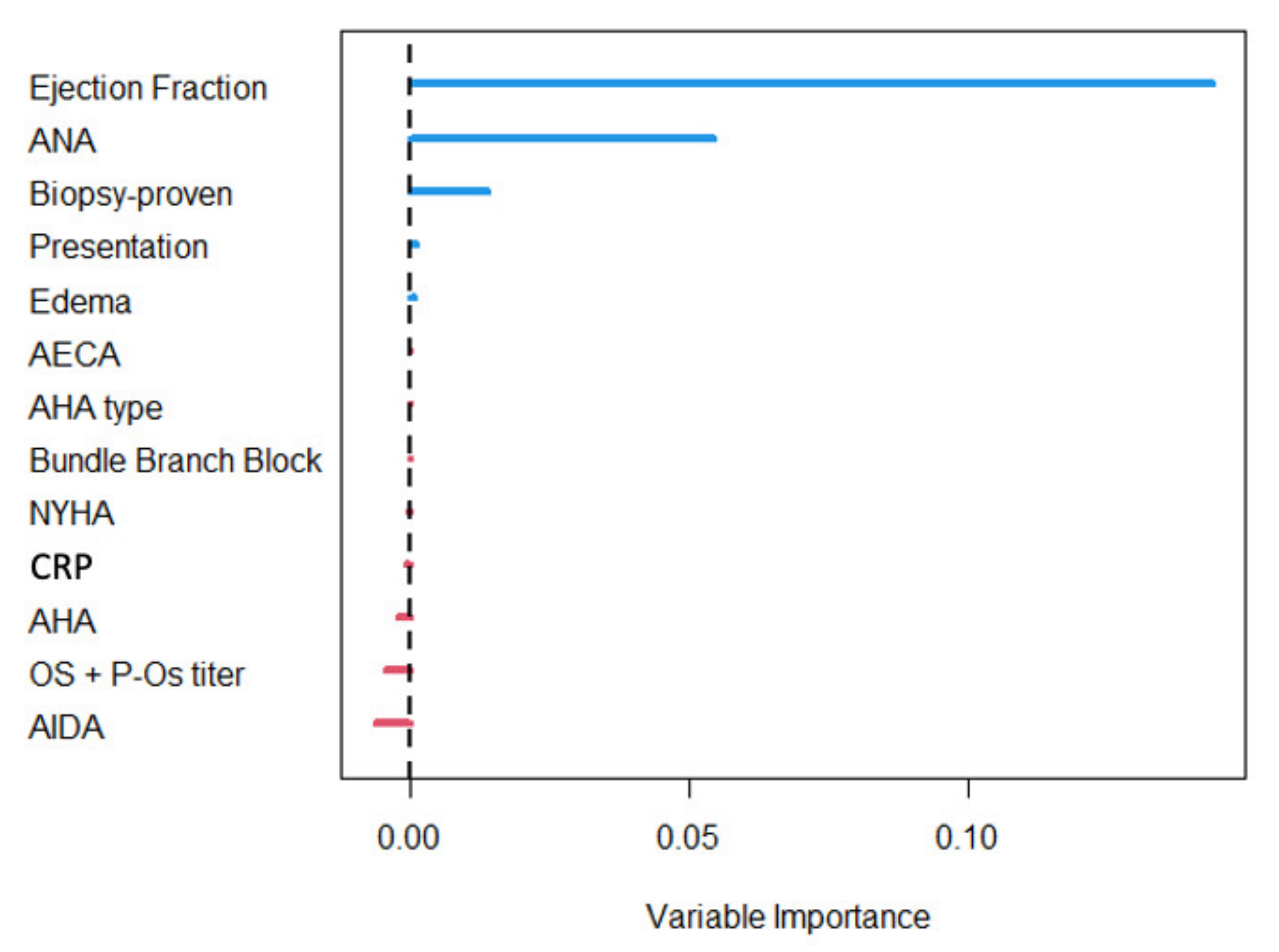
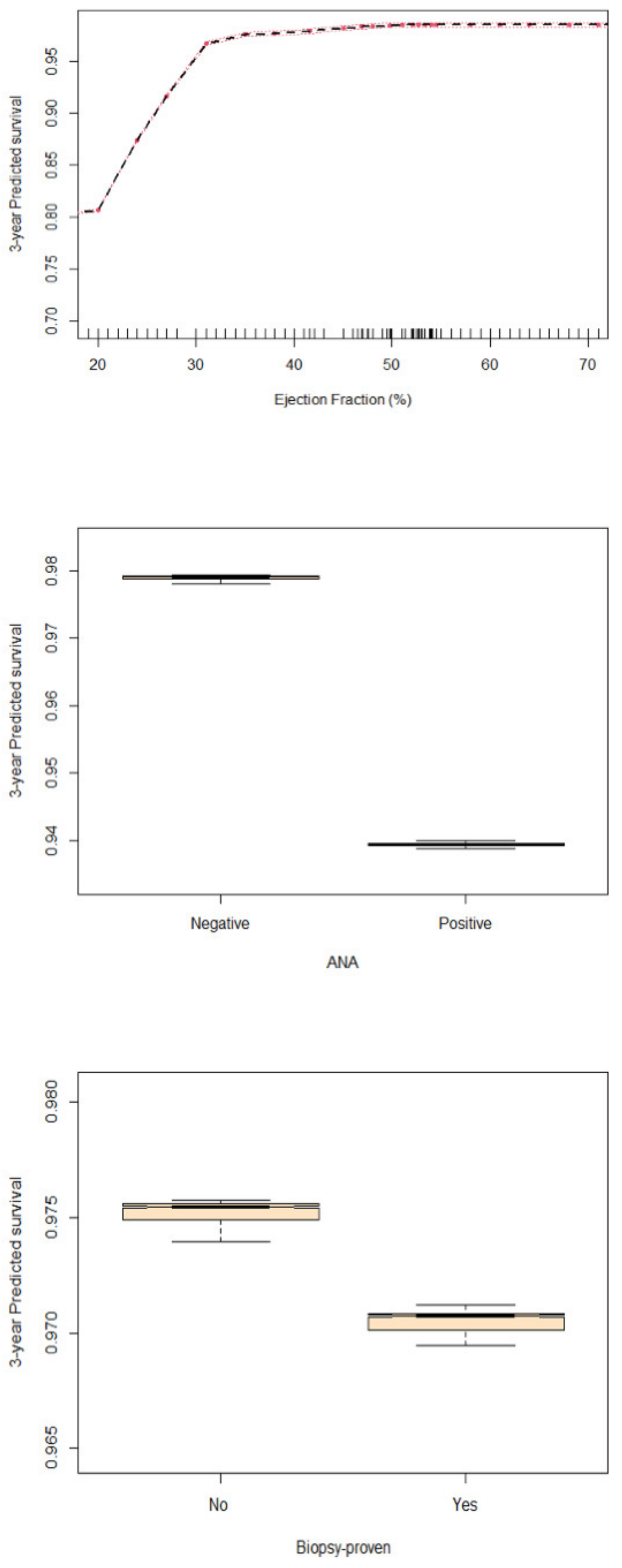
4.3. Predictors of Survival
5. Discussion
5.1. Diagnostic Role of CRP in Myocarditis
5.2. CRP and Myocarditis Prognosis
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, G.; Anzini, M.; Pereira, N.L.; Bussani, R.; Finocchiaro, G.; Bartunek, J.; Merlo, M. Myocarditis in Clinical Practice. Mayo Clin. Proc. 2016, 91, 1256–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tymińska, A.; Ozierański, K.; Caforio, A.L.P.; Marcolongo, R.; Marchel, M.; Kapłon-Cieślicka, A.; Baritussio, A.; Filipiak, K.J.; Opolski, G.; Grabowski, M. Myocarditis and inflammatory cardiomyopathy in 2021—An update. Pol. Arch. Intern. Med. 2021, 131, 594–606. [Google Scholar] [CrossRef]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell. Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef]
- Boncler, M.; Wu, Y.; Watala, C. The Multiple Faces of C-Reactive Protein—Physiological and Pathophysiological Implications in Cardiovascular Disease. Molecules 2019, 24, 2062. [Google Scholar] [CrossRef] [Green Version]
- Osman, R.; L’Allier, P.L.; Elgharib, N.; Tardif, J.-C. Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc. Heal. Risk Manag. 2006, 2, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Schwuchow-Thonke, S.; Göbel, S.; Emrich, T.; Schmitt, V.H.; Fueting, F.; Klank, C.; Escher, F.; Schultheiss, H.P.; Münzel, T.; Keller, K.; et al. Increased C reactive protein, cardiac troponin I and GLS are associated with myocardial inflammation in patients with non-ischemic heart failure. Sci. Rep. 2021, 11, 3008. [Google Scholar] [CrossRef]
- Kim, J.T.; Cho, S., III; Lee, S.Y.; Kim, D.; Lim, S.H.; Kang, T.S.; Lee, M.Y. The use of machine learning algorithms for the identification of stable obstructive coronary artery disease. J. Am. Coll. Cardiol. 2020, 75, 254. [Google Scholar] [CrossRef]
- Nakanishi, R.; Dey, D.; Commandeur, F.; Slomka, P.; Betancur, J.; Gransar, H.; Dailing, C.; Osawa, K.; Berman, D.; Budoff, M. Machine learning in predicting coronary heart disease and cardiovascular disease events: Results from the multi-ethnic study of atherosclerosis (mesa). J. Am. Coll. Cardiol. 2018, 71, A1483. [Google Scholar] [CrossRef]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular event prediction by machine learning: The multi-ethnic study of atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Sampedro-Gómez, J.; Dorado-Díaz, P.I.; Vicente-Palacios, V.; Sánchez-Puente, A.; Jiménez-Navarro, M.; Roman, J.A.S.; Galindo-Villardón, P.; Sanchez, P.L.; Fernández-Avilés, F. Machine Learning to Predict Stent Restenosis Based on Daily Demographic, Clinical, and Angiographic Characteristics. Can. J. Cardiol. 2020, 36, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Ye, G.; Kaya, M.; Gu, L. Simulation-Driven Machine Learning for Predicting Stent Expansion in Calcified Coronary Artery. Appl. Sci. 2020, 10, 5820. [Google Scholar] [CrossRef]
- Caforio, A.; Baritussio, A.; Marcolongo, R.; Cheng, C.-Y.; Pontara, E.; Bison, E.; Cattini, M.; Gallo, N.; Plebani, M.; Iliceto, S.; et al. Serum Anti-Heart and Anti-Intercalated Disk Autoantibodies: Novel Autoimmune Markers in Cardiac Sarcoidosis. J. Clin. Med. 2021, 10, 2476. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Tsutsui, H.; McNamara, D.M.; Ristić, A.D.; Basso, C.; Bozkurt, B.; Jr, L.T.C.; Filippatos, G.; Ide, T.; Inomata, T.; et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur. J. Heart Fail. 2021, 23, 854–871. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random survival forests. Ann. App. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Gorodeski, E.Z.; Minn, A.J.; Lauer, M.S. High-Dimensional Variable Selection for Survival Data. J. Am. Stat. Assoc. 2010, 105, 205–217. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Shaikhina, T.; Lowe, D.; Daga, S.; Briggs, D.; Higgins, R.; Khovanova, N. Decision tree and random forest models for outcome prediction in antibody incompatible kidney transplantation. Biomed. Signal Process. Control. 2019, 52, 456–462. [Google Scholar] [CrossRef]
- R Development Core Team. 3.0.1. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Volume 2, Available online: http://www.R-project.org (accessed on 1 June 2020).
- Harrell, F.E., Jr. Package ‘rms’: Regression Modeling Strategies; R Foundation for Statistical Computing: Vienna, Austria, 2019; pp. 1–246. Available online: https://cran.r-project.org/web/packages/rms/rms.pdf (accessed on 1 June 2020).
- Therneau, T.; Grambsch, P. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Alboukadel, K.; Marcin, K.; Przemyslaw, B.; Scheipl, F. Drawing Survival Curves Using ‘ggplot2’ [R package Survminer Version 0.4.3]; R Package Version 0.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Harrell, F.E., Jr.; Lee, K.L.; Matchar, D.B.; Reichert, T.A. Regression models for prognostic prediction: Advantages, problems, and suggested solutions. Cancer Treat. Rep. 1985, 69, 1071–1077. [Google Scholar] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis I. Background, goals, and general strategy. J. Clin. Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Salkind, N.J. Encyclopedia of Research Design; SAGE: Newbury Park, CA, USA, 2010; Volume 1. [Google Scholar]
- Steele, A.J.; Denaxas, S.C.; Shah, A.D.; Hemingway, H.; Luscombe, N.M. Machine learning models in electronic health records can outperform conventional survival models for predicting patient mortality in coronary artery disease. PLoS ONE 2018, 13, e0202344. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Merlo, M.; Dominguez, F.; Wang, P.; Henkens, M.T.H.M.; Adriaens, M.E.; Hazebroek, M.R.; Masè, M.; Escobar, L.E.; Cobas-Paz, R.; et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur. Heart J. 2021, 42, 162–174. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; De Luca, G.; Baritussio, A.; Seguso, M.; Gallo, N.; Bison, E.; Cattini, M.G.; Pontara, E.; Gargani, L.; Pepe, A.; et al. Serum Organ-Specific Anti-Heart and Anti-Intercalated Disk Autoantibodies as New Autoimmune Markers of Cardiac Involvement in Systemic Sclerosis: Frequency, Clinical and Prognostic Correlates. Diagnostics 2021, 11, 2165. [Google Scholar] [CrossRef]
- Baritussio, A.; Schiavo, A.; Basso, C.; Giordani, A.S.; Cheng, C.; Pontara, E.; Cattini, M.G.; Bison, E.; Gallo, N.; De Gaspari, M.; et al. Predictors of relapse, death or heart transplantation in myocarditis before the introduction of immunosuppression: Negative prognostic impact of female gender, fulminant onset, lower ejection fraction and serum autoantibodies. Eur. J. Heart Fail. 2022, 24, 1033–1044. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of Interleukin-1 Blockade with Anakinra on Adverse Cardiac Remodeling and Heart Failure After Acute Myocardial Infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) Pilot Study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, L.F.; Viscusi, M.M.; Van Tassell, B.W.; Abbate, A. Interleukin-1 blockade for the treatment of pericarditis. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brucato, A.; Imazio, M.; Gattorno, M.; Lazaros, G.; Maestroni, S.; Carraro, M.; Finetti, M.; Cumetti, D.; Carobbio, A.; Ruperto, N.; et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: The AIRTRIP Randomized Clinical Trial. JAMA 2016, 316, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating Noncoding Small RNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Anzulovic-Mirosevic, D.; Razzolini, R.; Zaninotto, M.; Plebani, M.; Mion, M.M.; Rozga, A.; Dalla-Volta, S. The C-Reactive Protein Levels in Left Ventricular Dysfunction of Different Etiology. Inflamm. Allergy Frug Targets 2009, 8, 247–251. [Google Scholar] [CrossRef]
- Lee, S.-S.; Singh, S.; Link, K.; Petri, M. High-Sensitivity C-Reactive Protein as an Associate of Clinical Subsets and Organ Damage in Systemic Lupus Erythematosus. Semin. Arthritis Rheum. 2008, 38, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Baritussio, A.; Vacirca, F.; Ocagli, H.; Tona, F.; Pergola, V.; Motta, R.; Marcolongo, R.; Lorenzoni, G.; Gregori, D.; Iliceto, S.; et al. Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation. J. Clin. Med. 2021, 10, 4200. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Herrmann, N.; Bittner, D.O.; Kilian, T.; Dey, D.; Raaz-Schrauder, D.; Marwan, M. Pericoronary adipose tissue CT attenuation and its association with serum levels of atherosclerosis-relevant inflammatory mediators, coronary calcification and major adverse cardiac events. J. Cardiovasc. Comput. Tomogr. 2021, 15, 449–454. [Google Scholar] [CrossRef]
- Baritussio, A.; Williams, M.C. Gaining evidence on coronary inflammation. J. Cardiovasc. Comput. Tomogr. 2021, 15, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Maestroni, S.; Cumetti, D.; Dominelli, A.; Natale, G.; Trinchero, R. Prevalence of C-Reactive Protein Elevation and Time Course of Normalization in Acute Pericarditis. Circulation 2011, 123, 1092–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, K.; Kanda, T.; Hasegawa, A.; Suzuki, T.; Kobayashi, I.; Nagai, R. C-reactive Protein as a Prognostic Marker in Lymphocytic Myocarditis. Jpn. Heart J. 2000, 41, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammirati, E.; Veronese, G.; Brambatti, M.; Merlo, M.; Cipriani, M.; Potena, L.; Sormani, P.; Aoki, T.; Sugimura, K.; Sawamura, A.; et al. Fulminant Versus Acute Nonfulminant Myocarditis in Patients with Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2019, 74, 299–311. [Google Scholar] [CrossRef]
- Anzini, M.; Merlo, M.; Sabbadini, G.; Barbati, G.; Finocchiaro, G.; Pinamonti, B.; Salvi, A.; Perkan, A.; Di Lenarda, A.; Bussani, R.; et al. Long-Term Evolution and Prognostic Stratification of Biopsy-Proven Active Myocarditis. Circulation 2013, 128, 2384–2394. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Cipriani, M.; Lilliu, M.; Sormani, P.; Varrenti, M.; Raineri, C.; Petrella, D.; Garascia, A.; Pedrotti, P.; Roghi, A.; et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation 2017, 136, 529–545. [Google Scholar] [CrossRef]
- Mason, J.W.; O’Connell, J.B.; Herskowitz, A.; Rose, N.R.; McManus, B.M.; Billingham, M.E.; Moon, T.E. A Clinical Trial of Immunosuppressive Therapy for Myocarditis. N. Engl. J. Med. 1995, 333, 269–275. [Google Scholar] [CrossRef]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef]
- Merken, J.; Hazebroek, M.; Van Paassen, P.; Verdonschot, J.; Van Empel, V.; Knackstedt, C.; Hamid, M.A.; Seiler, M.; Kolb, J.; Hoermann, P.; et al. Immunosuppressive Therapy Improves Both Short- and Long-Term Prognosis in Patients with Virus-Negative Nonfulminant Inflammatory Cardiomyopathy. Circ. Heart Fail. 2018, 11, e004228. [Google Scholar] [CrossRef]
- Chimenti, C.; Russo, M.A.; Frustaci, A. Immunosuppressive therapy in virus-negative inflammatory cardiomyopathy: 20-year follow-up of the TIMIC trial. Eur. Heart J. 2022, 43, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Kanda, T.; Yamauchi, Y.; Hasegawa, A.; Iwasaki, T.; Arai, M.; Suzuki, T.; Kobayashi, I.; Nagai, R. C-Reactive Protein in Dilated Cardiomyopathy. Cardiology 1999, 91, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Dernis, A.; Bresson, D.; Zouaghi, O.; Croisille, P.; Flocard, E.; Douek, P.; Bonnefoy-Cudraz, E. Myocardial biomarkers and delayed enhanced cardiac magnetic resonance relationship in clinically suspected myocarditis and insight on clinical outcome. J. Cardiovasc. Med. 2015, 16, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T., Jr.; Hare, J.M.; Tazelaar, H.D.; Edwards, W.D.; Starling, R.C.; Deng, M.C.; Menon, S.; Mullen, G.M.; Jaski, B.; Bailey, K.R.; et al. Giant Cell Myocarditis Treatment Trial Investigators. Usefulness of immunosuppression for giant cell myocarditis. Am. J. Cardiol. 2008, 102, 1535–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandolin, R.; Lehtonen, J.; Salmenkivi, K.; Räisänen-Sokolowski, A.; Lommi, J.; Kupari, M. Diagnosis, Treatment, and Outcome of Giant-Cell Myocarditis in the Era of Combined Immunosuppression. Circ. Heart Fail. 2013, 6, 15–22. [Google Scholar] [CrossRef]
| All n = 409 | Abnormal CRP Levels n = 288 | Normal CRP Levels n = 121 | Missing Data | p-Value | |
|---|---|---|---|---|---|
| Age, years | 37 ± 15 | 36 ± 15 | 39 ± 16 | - | 0.23 |
| Sex, male | 301 (74) | 224 (78) | 77 (64) | - | 0.003 |
| EMB-proven myocarditis | 129 (32) | 74 (26) | 55 (45) | - | <0.001 |
| Clinically suspected myocarditis | 280 (68) | 214 (76) | 66 (24) | - | <0.001 |
| Hypertension | 61 (15) | 39 (14) | 22 (18) | - | 0.23 |
| Diabetes | 11 (3) | 7 (2) | 4 (3) | - | 0.62 |
| Immune-mediated diseases | 56 (14) | 37 (13) | 19 (16) | 2 | 0.46 |
| Acute viral infection in the preceding 6 months | 191 (47) | 158 (55) | 33 (28) | 3 | <0.001 |
| Symptoms duration, months | 0.5 (0.06–3) | 0.2 (0.03–1) | 3 (0.3–18) | 257 | <0.001 |
| Chest pain | 223 (55) | 172 (60) | 51 (42) | - | <0.001 |
| Palpitations | 41 (10) | 20 (7) | 21 (17) | - | 0.002 |
| Syncope | 20 (5) | 8 (3) | 12 (10) | - | 0.002 |
| Symptoms variation in the 12 months preceding diagnosis | 66 (16) | 39 (14) | 27 (22) | - | 0.029 |
| Clinical presentation, infarct-like | 25 (6) | 19 (7) | 6 (5) | - | 0.53 |
| Clinical presentation, heart failure | 68 (17) | 46 (16) | 22 (18) | - | 0.58 |
| Clinical presentation, arrhythmias | 39 (10) | 12 (4) | 27 (22) | - | <0.001 |
| NYHA class II-IV | 61 (15) | 37 (12) | 24 (21) | 1 | 0.054 |
| LV heart failure at presentation | 69 (17) | 47 (16) | 22 (18) | - | 0.65 |
| RV heart failure at presentation | 26 (6) | 18 (6) | 8 (7) | - | 0.89 |
| Troponin I, ng/L | 4455 (438–13684) | 6140 (1148–15298) | 1150 (12–6030) | - | <0.001 |
| AHA positivity | 174 (52) | 112 (48) | 62 (61) | 75 | 0.025 |
| AIDA positivity | 116 (35) | 75 (32) | 41 (41) | 74 | 0.25 |
| AECA positivity | 3 (1) | 2 (1) | 1 (1) | 96 | 0.91 |
| ANA positivity | 37 (12) | 26 (11) | 11 (11) | 88 | 0.95 |
| LVEF Angio, % | 53 ± 17 | 54 ± 16 | 53 ± 18 | - | 0.87 |
| LVEDVi Echo, mL/m2 | 68 ± 24 | 67 ± 22 | 70 ± 28 | 60 | 0.69 |
| LVEF Echo, % | 51 ± 13 | 51 ± 13 | 50 ± 14 | 27 | 0.65 |
| RVEDA Echo, cm2 | 20.8 ± 4.7 | 20.7 ± 4.3 | 21.0 ± 5.7 | 205 | 0.96 |
| RVFAC Echo, % | 42.1 ± 9.8 | 42.2 ± 10.4 | 41.9 ± 8.5 | 219 | 0.73 |
| Mitral regurgitation Echo, moderate-severe | 34 (10) | 26 (10) | 8 (9) | 78 | 0.84 |
| LVEDVi CMR, mL/m2 | 88 (78–104) | 88 (79–102) | 90 (74–106) | - | 0.83 |
| RVEDVi CMR, mL/m2 | 82 (74–94) | 84 (74–95) | 80 (70–91) | - | 0.22 |
| LVEF CMR, % | 57 (52–62) | 57 (53–62) | 59 (51–62) | - | 0.69 |
| RVEF CMR, % | 58 (54–63) | 58 (53–62) | 59 (55–65) | - | 0.12 |
| Presence of myocardial edema on CMR | 190 (58) | 145 (61) | 50 (47) | 81 | 0.014 |
| Presence of myocardial LGE on CMR | 288 (88) | 210 (90) | 78 (84) | 83 | 0.17 |
| Lateral wall LGE | 37 (17) | 31 (20) | 6 (9) | 190 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baritussio, A.; Cheng, C.-y.; Lorenzoni, G.; Basso, C.; Rizzo, S.; De Gaspari, M.; Fachin, F.; Giordani, A.S.; Ocagli, H.; Pontara, E.; et al. A Machine-Learning Model for the Prognostic Role of C-Reactive Protein in Myocarditis. J. Clin. Med. 2022, 11, 7068. https://doi.org/10.3390/jcm11237068
Baritussio A, Cheng C-y, Lorenzoni G, Basso C, Rizzo S, De Gaspari M, Fachin F, Giordani AS, Ocagli H, Pontara E, et al. A Machine-Learning Model for the Prognostic Role of C-Reactive Protein in Myocarditis. Journal of Clinical Medicine. 2022; 11(23):7068. https://doi.org/10.3390/jcm11237068
Chicago/Turabian StyleBaritussio, Anna, Chun-yan Cheng, Giulia Lorenzoni, Cristina Basso, Stefania Rizzo, Monica De Gaspari, Francesco Fachin, Andrea Silvio Giordani, Honoria Ocagli, Elena Pontara, and et al. 2022. "A Machine-Learning Model for the Prognostic Role of C-Reactive Protein in Myocarditis" Journal of Clinical Medicine 11, no. 23: 7068. https://doi.org/10.3390/jcm11237068
APA StyleBaritussio, A., Cheng, C.-y., Lorenzoni, G., Basso, C., Rizzo, S., De Gaspari, M., Fachin, F., Giordani, A. S., Ocagli, H., Pontara, E., Cattini, M. G. P., Bison, E., Gallo, N., Plebani, M., Tarantini, G., Iliceto, S., Gregori, D., Marcolongo, R., & Caforio, A. L. P. (2022). A Machine-Learning Model for the Prognostic Role of C-Reactive Protein in Myocarditis. Journal of Clinical Medicine, 11(23), 7068. https://doi.org/10.3390/jcm11237068









