Impact of Short-Acting Disopyramide on Left Ventricular Mechanics Evaluated by Strain Analysis in Patients with Hypertrophic Obstructive Cardiomyopathy
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Study Protocol
2.3. Echocardiographic Examinations
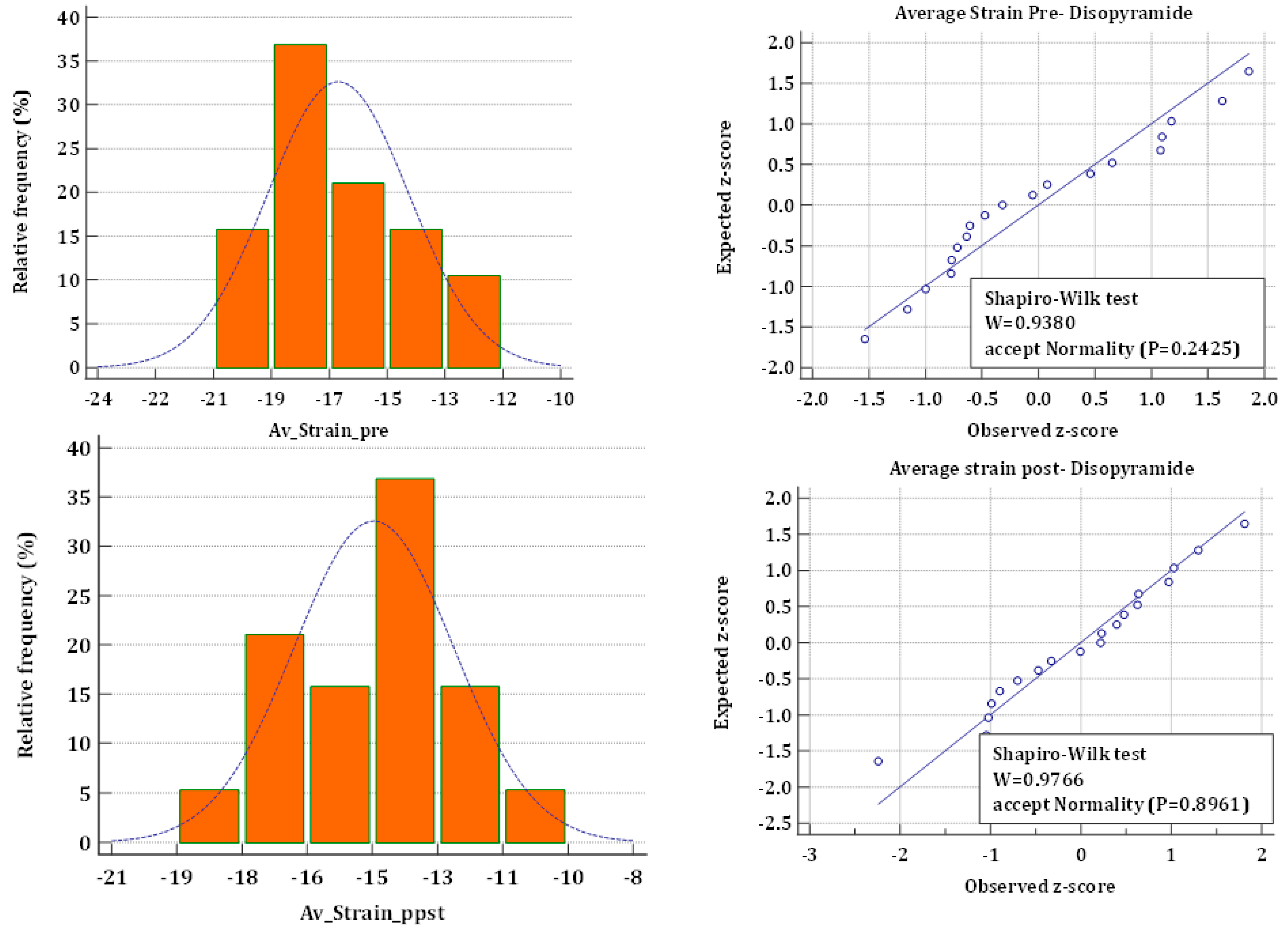
2.4. Strain Analysis
2.5. Statistical Analysis
3. Results
3.1. Conventional Echocardiography
3.2. Systolic Mechanical Imaging
3.3. Diastolic Mechanical Imaging (Reverse Rotation and Atrial Function)
3.4. Interobserver Variability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2011, 58, e212–e260. [Google Scholar] [PubMed] [Green Version]
- Authors/Task Force members; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Pollick, C.; Giacomini, K.M.; Blaschke, T.F.; Nelson, W.L.; Turner-Tamiyasu, K.; Briskin, V.; Popp, R.L. The cardiac effects of d- and l-disopyramide in normal subjects: A noninvasive study. Circulation 1982, 66, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowey, P.R.; Friedman, P.L.; Podrid, P.J.; Zielonka, J.; Lown, B.; Wynne, J.; Holman, B. Use of radionuclide ventriculography for assessment of changes in myocardial performance induced by disopyramide phosphate. Am. Heart J. 1982, 104, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Gottdiener, J.S.; Dibianco, R.; Bates, R.; Sauerbrunn, B.J.; Fletcher, R.D. Effects of disopyramide on left ventricular function: Assessment by radionuclide cineangiography. Am. J. Cardiol. 1983, 51, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.C.; Iskandrian, A.S.; Hakki, A.-H.; Kane, S.A.; Segal, B.L. Effect of Oral Disopyramide Therapy on Left Ventricular Function. Chest 1983, 83, 480–484. [Google Scholar] [CrossRef]
- Pollick, C.; Kimball, B.; Henderson, M.; Wigle, E. Disopyramide in hypertrophic cardiomyopathy I. Hemodynamic assessment after intravenous administration. Am. J. Cardiol. 1988, 62, 1248–1251. [Google Scholar] [CrossRef]
- Kenedi, P.P. Clinical Significance of the Negative Inotropic Effect of Disopyramide. J. Int. Med. Res. 1988, 16, 257–263. [Google Scholar] [CrossRef]
- Arakawa, M.; Miwa, H.; Noda, T.; Kagawa, K.; Nishigaki, K.; Ito, Y.; Kawada, T.; Hirakawa, S. Acute effects of conventional oral dose of disopyramide on left atrial and ventricular functions. Int. J. Clin. Pharmacol. Ther. Toxicol. 1993, 31, 253–259. [Google Scholar]
- Matsubara, H.; Nakatani, S.; Nagata, S.; Ishikura, F.; Katagiri, Y.; Ohe, T.; Miyatake, K. Salutary effect of disopyramide on left ventricular diastolic function in hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 1995, 26, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Carasso, S.; Woo, A.; Yang, H.; Schwartz, L.; Vannan, M.A.; Jamorski, M.; Linghorne, M.; Wigle, E.D.; Rakowski, H. Myocardial Mechanics Explains the Time Course of Benefit for Septal Ethanol Ablation for Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2008, 21, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F.; Bierig, S.M.; Budoff, M.J.; Desai, M.; Dilsizian, V.; Eidem, B.; Goldstein, S.A.; Hung, J.; Maron, M.S.; Ommen, S.R.; et al. American Society of Echocardiography clinical recommendation for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2011, 24, 473–498. [Google Scholar]
- Ozawa, K.; Funabashi, N.; Takaoka, H.; Kamata, T.; Kanaeda, A.; Saito, M.; Nomura, F.; Kobayashi, Y. Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int. J. Cardiol. 2015, 184, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Serri, K.; Reant, P.; Lafitte, M.; Berhouet, M.; Le Bouffos, V.; Roudaut, R.; Lafitte, S. Global and Regional Myocardial Function Quantification by Two-Dimensional Strain: Application in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 1175–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Sun, J.P.; Lever, H.M.; Popovic, Z.B.; Drinko, J.K.; Greenberg, N.L.; Shiota, T.; Thomas, J.D.; Garcia, M.J. Use of strain imaging in detecting segmental dysfunction in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2003, 16, 233–239. [Google Scholar] [CrossRef]
- Yang, H.; Carasso, S.; Woo, A.; Jamorski, M.; Nikonova, A.; Wigle, E.D.; Rakowski, H. Hypertrophy Pattern and Regional Myocardial Mechanics Are Related in Septal and Apical Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2010, 23, 1081–1089. [Google Scholar] [CrossRef]
- Dhillon, A.; Sweet, W.; Popovic, Z.; Smedira, N.G.; Thamilarasan, M.; Lytle, B.W.; Tan, C.; Starling, R.C.; Lever, H.M.; Moravec, C.S.; et al. Association of Noninvasively Measured Left Ventricular Mechanics With In Vitro Muscle Contractile Performance: A Prospective Study in Hypertrophic Cardiomyopathy Patients. J. Am. Heart Assoc. 2014, 3, e001269. [Google Scholar] [CrossRef] [Green Version]
- van Dockum, W.G.; Kuijer, J.P.; Götte, M.J.; Ten Cate, F.J.; Ten Berg, J.M.; Beek, A.M.; Twisk, J.W.; Marcus, J.T.; Visser, C.A.; van Rossum, A.C. Septal ablation in hypertrophic obstructive cardiomyopathy improves systolic myocardial function in the lateral (free) wall: A follow-up study using CMR tissue tagging and 3D strain analysis. Eur. Heart J. 2006, 27, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Popovic, Z.; Kwon, D.H.; Mishra, M.; Buakhamsri, A.; Greenberg, N.L.; Thamilarasan, M.; Flamm, S.D.; Thomas, J.D.; Lever, H.M.; Desai, M.Y. Association Between Regional Ventricular Function and Myocardial Fibrosis in Hypertrophic Cardiomyopathy Assessed by Speckle Tracking Echocardiography and Delayed Hyperenhancement Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1299–1305. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Arabadjian, M. A Primer of Disopyramide Treatment of Obstructive Hypertrophic Cardiomyopathy. Prog. Cardiovasc. Dis. 2012, 54, 483–492. [Google Scholar] [CrossRef]
| Measurement | Before Disopyramide | After Disopyramide | p-Value |
|---|---|---|---|
| BP dia (mmHg) | 74.84 ± 9.2 | 72.2 ± 10.9 | 0.1054 |
| BP sys (mmHg) | 136.5 ± 19.26 | 129.84 ± 18.3 | 0.1419 |
| Pulse (b/min) | 62.05 ± 10.88 | 60.1 ± 8.6 | 0.8206 |
| Systolic | |||
| EF Simpson (%) | 71.68 ± 5.4 | 70.1 ± 4.7 | 0.1499 |
| Rest gradient mmHg | 42.7 ± 28.4 | 43.47 ± 28.4 | 0.9742 |
| Gradients during Valsalva (mmHg) | 62.1 ± 30.6 | 62.26 ± 34.63 | 0.6025 |
| S ant (cm/s) | 7 ± 1.2 | 7.7 ± 2.2 | 0.9765 |
| S inf (cm/s) | 7.7 ± 1.6 | 7.6 ± 1.7 | 0.9743 |
| S lat (cm/s) | 8.6 ± 1.3 | 8.3 ± 1.3 | 0.6522 |
| S sep (cm/s) | 6.7 ± 1.5 | 6.6 ± 1.2 | 0.9523 |
| RV s (cm/s) | 13.46 ± 3.9 | 12.6 ± 2.9 | 0.3369 |
| TAPSE (cm) | 22.37 ± 3.3 | 22.68 ± 4.0 | 0.5898 |
| LA volume (ml) | 80.42 ± 23.3 | 76.17 ± 22.18 | 0.6165 |
| MR (grade) | 2.63 ± 0.8 | 2.68 ± 1 | 1 |
| RVSP (mmHg) | 17.47 ± 17.6 | 15.47 ± 17.55 | 0.1445 |
| Diastolic | |||
| E (cm/s) | 82.6 ± 20.2 | 85 ± 22.6 | 0.2 |
| A (cm/s) | 88.7 ± 26.6 | 83.5 ± 23 | 0.3114 |
| E/A | 1.0 ± 0.42 | 1.1 ± 0.47 | 0.2111 |
| Dec time (ms) | 232.1 ± 70.4 | 233 ± 79 | 0.774 |
| e’ lat (cm/s) | 7.1 ± 2 | 6.6 ± 1.8 | 0.1011 |
| e’ sep (cm/s) | 5.3 ± 1.04 | 5.33 ± 1.4 | 0.757 |
| E/e’ lat (cm/s) | 12.47 ± 5.3 | 12.94 ± 5.8 | 0.1042 |
| E/e’ sep (cm/s) | 15.8 ± 4.7 | 15.9 ± 5.8 | 0.1688 |
| Pul S (cm/s) | 57.3 ± 12.3 | 54 ±11.7 | 0.6046 |
| Pul D (cm/s) | 52.9 ± 17.2 | 56.3 ± 18.4 | 0.2959 |
| e’ ant (cm/s) | 6 ± 2 | 5.8 ± 2 | 0.587 |
| e’ inf (cm/s) | 5.75 ± 1.3 | 5.78 ± 1.8 | 0.9239 |
| Measurements | Before Disopyramide | After Disopyramide | p-Value |
|---|---|---|---|
| Longitudinal Mechanics | |||
| Bi-plane VVI LVEF | 53 ± 6 | 50 ± 5 | 0.017 |
| Global | |||
| 1. Average strain peaks | −17 ± 2 | −14 ± 2 | <0.0001 |
| 2. Global strain at AVC | −15 ± 2 | −13 ± 2 | <0.0001 |
| Global synchrony (%) | 92 ± 5 | 91 ± 5 | 0.26 |
| Segmental | |||
| 06-basal septal | −14 ± 5 | −10 ± 4 | 0.015 |
| 12-mid septal | −14 ± 5 | −13 ± 5 | 0.18 |
| 16-apical septal | −21 ± 8 | −21 ± 6 | 0.93 |
| 14-apical lateral | −17 ± 5 | −14 ± 5 | 0.021 |
| 09-mid lateral | −15 ± 6 | −13 ± 5 | 0.086 |
| 03-basal lateral | −20 ± 7 | −18 ± 5 | 0.24 |
| 05-basal inferior | −16 ± 6 | −13 ± 5 | 0.019 |
| 11-mid inferior | −12 ± 6 | −10 ± 5 | 0.31 |
| 15-apical inferior | −21 ± 4 | −20 ± 5 | 0.13382 |
| 13-apical anterior | −16 ± 5 | −11 ± 5 | 0.00012 |
| 08-mid anterior | −16 ± 3 | −15 ± 5 | 0.28 |
| 02-basal anterior | −18 ± 5 | −16 ± 5 | 0.13 |
| 04-basal posterior | −19 ± 6 | −16 ± 4 | 0.046 |
| 10-mid posterior | −14 ± 5 | −12 ± 5 | 0.28 |
| 14-apical posterior | −16 ± 6 | −15 ± 4 | 0.85 |
| 13-apical anteroseptal | −20 ± 5 | −18 ± 5 | 0.056 |
| 07-mid anterosepta | −18 ± 5 | −15 ± 6 | 0.064 |
| 01-basal anteroseptal | −14 ± 4 | −12 ± 5 | 0.057 |
| Circumferential Mechanics | |||
| Base Circ Strain | −22 ± 11 | −24 ± 6 | 0.38 |
| Mid Circ Strain | −24 ± 5 | −28 ± 6 | 0.97 |
| Apex Circ Strain | −36 ± 8 | −37 ± 7 | 0.98 |
| Global CS at AVC | −28 ± 8 | −27 ± 9 | 0.29 |
| Rotation (°) | |||
| Base | −8 ± 3 | −6 ± 3 | 0.094 |
| Mid | 1 ± 6 | 2 ± 5 | 0.56 |
| Apex | 11 ± 4 | 8 ± 4 | 0.036 |
| Twist (°) | 16 ± 5 | 12 ± 6 | 0.001 |
| FEARR (%) | 37 ± 14 | 35 ± 18 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yedidya, I.; Elbaz Greener, G.; Vaturi, M.; Sagie, A.; Amir, O.; Carasso, S.; Monakier, D. Impact of Short-Acting Disopyramide on Left Ventricular Mechanics Evaluated by Strain Analysis in Patients with Hypertrophic Obstructive Cardiomyopathy. J. Clin. Med. 2022, 11, 7325. https://doi.org/10.3390/jcm11247325
Yedidya I, Elbaz Greener G, Vaturi M, Sagie A, Amir O, Carasso S, Monakier D. Impact of Short-Acting Disopyramide on Left Ventricular Mechanics Evaluated by Strain Analysis in Patients with Hypertrophic Obstructive Cardiomyopathy. Journal of Clinical Medicine. 2022; 11(24):7325. https://doi.org/10.3390/jcm11247325
Chicago/Turabian StyleYedidya, Idit, Gabby Elbaz Greener, Mordehay Vaturi, Alik Sagie, Offer Amir, Shemy Carasso, and Daniel Monakier. 2022. "Impact of Short-Acting Disopyramide on Left Ventricular Mechanics Evaluated by Strain Analysis in Patients with Hypertrophic Obstructive Cardiomyopathy" Journal of Clinical Medicine 11, no. 24: 7325. https://doi.org/10.3390/jcm11247325





