Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis
Abstract
:1. Introduction
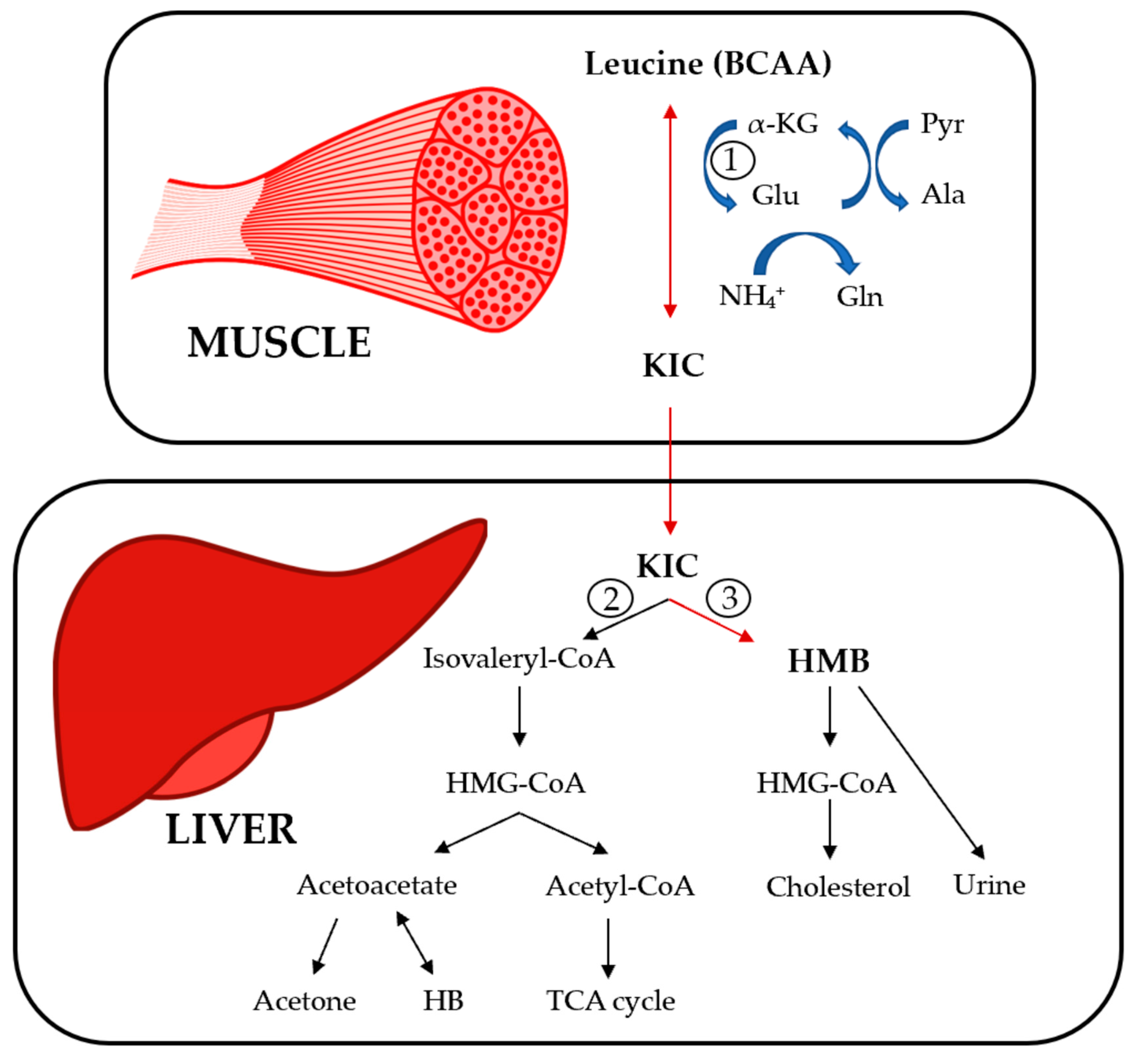
2. BCAA Metabolism
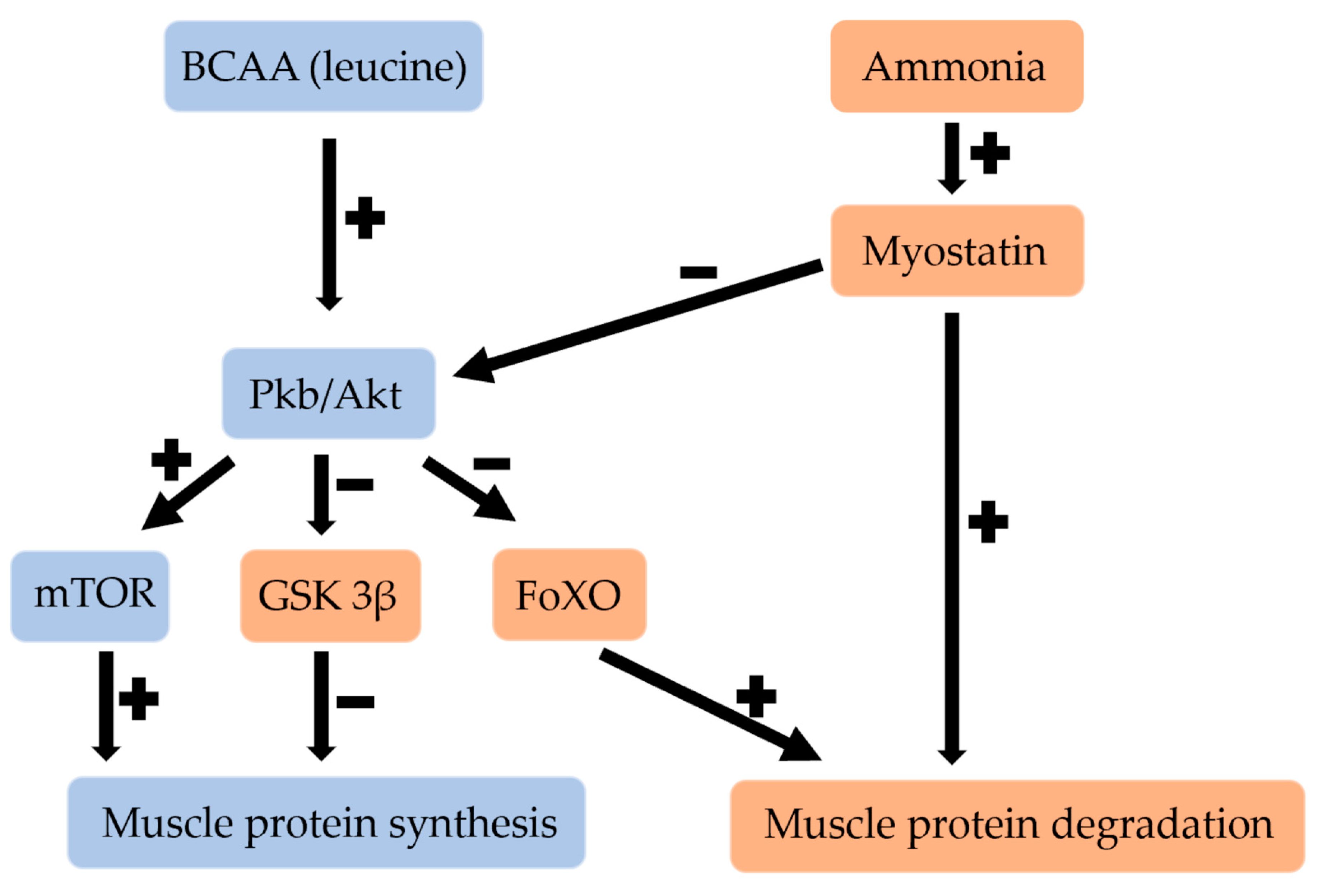
3. Effect of BCAAs and HMB on the Regulation of Muscle Growth
3.1. Effect of BCAAs on Skeletal Muscle
3.2. Effect of HMB on Skeletal Muscle
4. Clinical Nutrition in Liver Cirrhosis
4.1. Definitions and Clinical Impact of Nutrition in Liver Cirrhosis
4.2. Pathogenesis of Malnutrition in Liver Cirrhosis
4.3. Nutritional Assessment in Liver Cirrhosis
5. Metabolic Disorders in Liver Cirrhosis and Their Clinical Manifestation
6. Nutritional Role of BCAAs in Liver Cirrhosis
6.1. Effect on Body Composition and Muscular Strength
6.2. Effect on Serum Albumin
7. Clinical Role of BCAAs in Liver Cirrhosis
7.1. Effect on Complications of Liver Cirrhosis
7.2. Effect on Mortality and Quality of Life
8. Recommendations of the Main Scientific Societies Regarding BCAA Supplementation in Liver Cirrhosis
- -
- Patients with liver cirrhosis who present malnutrition and/or sarcopenia with BMI < 30 kg/m² who do not reach the recommended nutritional intake of 30–35 Kcal/Kg/day, nutritional supplementation can be chosen, preferably oral, and can be with a standard amino acid regimen or based on BCAAs.
- -
- Patients with liver cirrhosis who do not reach a protein intake of 1.2 g/Kg/day, or 1.5 g/Kg/day if they have sarcopenia, or have intolerance to animal proteins, can opt for replacement with vegetable proteins and BCAA supplementation.
- -
- Patients with liver cirrhosis and hepatic encephalopathy can be considered for oral BCAA supplementation to improve neuropsychiatric performance and to reach the recommended nitrogen intake, but their use intravenously for episodic overt hepatic encephalopathy is not supported.
- -
- Patients with liver cirrhosis and intolerance to animal proteins, vegetable proteins or oral BCAA (0.25 g/kg/day) supplementation should be used to facilitate adequate protein intake.
- -
- Long-term BCAA (0.25 g/kg/day) supplementation in advanced-stage liver cirrhosis may be prescribed to improve survival and quality of life.
- -
- After surgery or in the pre-liver transplant period, BCAA-based regimens have not been superior to other standard regimens with respect to morbidity and mortality.
- -
- BCAA supplementation is not recommended beyond emphasizing the importance of meeting daily overall protein targets from a diverse range of protein sources.
9. Effect of HMB in Liver Cirrhosis
9.1. Nutritional Role of HMB in Liver Cirrhosis
9.2. Clinical Role of HMB in Liver Cirrhosis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional support in cirrhotic patients with sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluud, L.L.; Dam, G.; Les, I.; Cordoba, J.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017, 5, CD001939. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Holecek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sport. Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 2017, 41, 80–85. [Google Scholar] [CrossRef]
- Girón, M.D.; Vílchez, J.D.; Salto, R.; Manzano, M.; Sevillano, N.; Campos, N.; Argilés, J.M.; Rueda, R.; López-Pedrosa, J.M. Conversion of leucine to β-hydroxy-β-methylbutyrate by α-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. J. Cachexia Sarcopenia Muscle 2015, 7, 68–78. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.O.; Austin, P.; Ballmer, P.; Biolo, G.I.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Plank, L.D.; McCall, J.L.; Gillanders, L.K.; McIlroy, K.; Gane, E.J. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: A comprehensive study. Am. J. Clin. Nutr. 2007, 85, 1257–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moctezuma-Velázquez, C.; García-Juárez, I.; Soto-Solís, R.; Hernández-Cortés, J.; Torre, A. Nutritional assessment and treatment of patients with liver cirrhosis. Nutrition 2013, 29, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Qi, X.; Guo, X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine 2016, 95, e2877. [Google Scholar] [CrossRef] [PubMed]
- Cañamares-Orbis, P.; Bernal-Monterde, V.; Sierra-Gabarda, O.; Casas-Deza, D.; Garcia-Rayado, G.; Cortes, L.; Lué, A. Impact of Liver and Pancreas Diseases on Nutritional Status. Nutrients 2021, 13, 1650. [Google Scholar] [CrossRef]
- Nutritional Status in Cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J. Hepatol. 1994, 21, 317–325. [Google Scholar]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.; Bannert, K.; Wiese, M.; Esau, S.; Sautter, L.F.; Ehlers, L.; Aghdassi, A.A.; Metges, C.C.; Garbe, L.-A.; Jaster, R.; et al. Molecular Mechanism Contributing to Malnutrition and Sarcopenia in Patients with Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 5357. [Google Scholar] [CrossRef]
- Aqel, B.A.; Scolapio, J.S.; Dickson, R.C.; Burton, D.D.; Bouras, E.P. Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clin. Gastroenterol. Hepatol. 2005, 3, 1095–1100. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients with Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Review article: Malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment. Pharmacol. Ther. 2019, 51, 64–77. [Google Scholar] [CrossRef] [PubMed]
- EASL. Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017, 23, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, M.; Chapman, B.; Hoermann, R.; Angus, P.W.; Testro, A.; Scodellaro, T.; Gow, P.J. Handgrip Strength Adds More Prognostic Value to the Model for End-Stage Liver Disease Score Than Imaging-Based Measures of Muscle Mass in Men with Cirrhosis. Liver Transplant. 2019, 25, 1480–1487. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Mitra, V.; Metcalf, J. Metabolic functions of the liver. Anaesth. Intensive Care Med. 2012, 13, 54–55. [Google Scholar] [CrossRef]
- Campollo, O.; Sprengers, D.; McIntyre, N. The BCAA/AAA ratio of plasma amino acids in three different groups of cirrhotics. Rev. Investig. Clínica; Organo Hosp. Enferm. Nutr. 1992, 44, 513–518. [Google Scholar]
- Espina, S.; Gonzalez-Irazabal, Y.; Sanz-Paris, A.; Lopez-Yus, M.; Garcia-Sobreviela, M.P.; del Moral-Bergos, R.; Garcia-Rodriguez, B.; Fuentes-Olmo, J.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy. Nutrients 2021, 13, 3764. [Google Scholar] [CrossRef]
- E Fischer, J.; Rosen, H.M.; Ebeid, A.M.; James, J.H.; Keane, J.M.; Soeters, P.B. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976, 80, 77–91. [Google Scholar]
- Deutsch-Link, S.; Moon, A.M.; Jiang, Y.; Barritt, A.S.; Tapper, E.B. Serum Ammonia in Cirrhosis: Clinical Impact of Hyperammonemia, Utility of Testing, and National Testing Trends. Clin. Ther. 2022, 44, e45–e57. [Google Scholar] [CrossRef]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Tsien, C.; Thapalaya, S.; Narayanan, A.; Weihl, C.C.; Ching, J.K.; Eghtesad, B.; Singh, K.; Fu, X.; Dubyak, G.; et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am. J. Physiol. Metab. 2012, 303, E983–E993. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Yoshimatsu, M.; Motomura, T.; Takeishi, K.; Toshima, T.; Muto, J.; Matono, R.; Taketomi, A.; Uchiyama, H.; Maehara, Y. Beneficial effects of supplementation with branched-chain amino acids on postoperative bacteremia in living donor liver transplant recipients. Liver Transplant. 2011, 17, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Ooi, P.; Gilmour, S.; Yap, J.; Mager, D. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin. Nutr. ESPEN 2018, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Bucsa, C.; Farcas, A.; Leucuta, D.-C.; Popa, S.-L.; Dumitrascu, D.L. Effects of Branched-Chain Amino Acids on Parameters Evaluating Sarcopenia in Liver Cirrhosis: Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 749969. [Google Scholar] [CrossRef] [PubMed]
- Konstantis, G.; Pourzitaki, C.; Chourdakis, M.; Kitsikidou, E.; Germanidis, G. Efficacy of branched chain amino acids supplementation in liver cirrhosis: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1171–1190. [Google Scholar] [CrossRef]
- Hernández-Conde, M.; Llop, E.; Gómez-Pimpollo, L.; Carrillo, C.F.; Rodríguez, L.; Brule, E.V.D.; Perelló, C.; López-Gómez, M.; Abad, J.; Martínez-Porras, J.L.; et al. Adding Branched-Chain Amino Acids to an Enhanced Standard-of-Care Treatment Improves Muscle Mass of Cirrhotic Patients with Sarcopenia: A Placebo-Controlled Trial. Am. J. Gastroenterol. 2021, 116, 2241–2249. [Google Scholar] [CrossRef]
- Lee, J.L.; Oh, E.S.; Lee, R.W.; Finucane, T.E. Serum Albumin and Prealbumin in Calorically Restricted, Nondiseased Individuals: A Systematic Review. Am. J. Med. 2015, 128, 1023.e1–1023.e22. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Kubota, T.; Horigome, R.; Kimura, N.; Honda, H.; Iwanaga, A.; Seki, K.; Honma, T.; Yoshida, T. Branched-chain amino acids to tyrosine ratio (BTR) predicts intrahepatic distant recurrence and survival for early hepatocellular carcinoma. Hepatogastroenterology 2013, 60, 2055–2059. [Google Scholar]
- TTajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koretz, R.L.; Avenell, A.; Lipman, T.O. Nutritional support for liver disease. Cochrane Database Syst. Rev. 2012, Cd008344. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Taniguchi, E.; Sata, M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr. Clin. Pract. 2013, 28, 580–588. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin. Gastroenterol. Hepatol. 2005, 3, 705–713. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M.; Peláez, R.B.; Irigoin, R.R. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-beta-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Lattanzi, B.; Giusto, M.; Albanese, C.; Mennini, G.; D’Ambrosio, D.; Farcomeni, A.; Corradini, S.G.; Rossi, M.; Merli, M. The Effect of 12 Weeks of β-Hydroxy-β-Methyl-Butyrate Supplementation after Liver Transplantation: A Pilot Randomized Controlled Study. Nutrients 2019, 11, 2259. [Google Scholar] [CrossRef] [Green Version]
- Lattanzi, B.; Bruni, A.; Di Cola, S.; Molfino, A.; De Santis, A.; Muscaritoli, M.; Merli, M. The Effects of 12-Week Beta-Hydroxy-Beta-Methylbutyrate Supplementation in Patients with Liver Cirrhosis: Results from a Randomized Controlled Single-Blind Pilot Study. Nutrients 2021, 13, 2296. [Google Scholar] [CrossRef]
- Espina, S.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Pérez-Matute, P.; Andrade, F.; Garcia-Rodriguez, B.; Carpéné, C.; Zakaroff, A.; Bernal-Monterde, V.; Fuentes-Olmo, J.; et al. Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients. Nutrients 2022, 14, 2344. [Google Scholar] [CrossRef] [PubMed]


| Decreased Caloric Intake | Malabsorption | Altered Metabolism |
|---|---|---|
| Hyporexia | Altered enterohepatic circulation of bile salts | Reduced glycogen synthesis |
| Early satiety | Bacterial overgrowth | Increased gluconeogenesis |
| Dysgeusia | Portosystemic shunting | Increased fatty acid oxidation |
| Diet unpalatability | Gastrointestinal dysmotility | Protein and fat breakdown |
| Alcohol abuse | Pancreatic enzyme deficiency | |
| Dietary restrictions | Enteropathy |
| Sarcopenia | |
|---|---|
| Method | Measurement |
| Manual anthropometry | MAMC |
| Ultrasound | Muscle thickness |
| BIA | Fat free mass |
| DXA | Fat free mass |
| CT | L3 SMI |
| Muscular strength | Handgrip strength |
| Study, Year | Study Type | Number of Studies Included | Population | BCAA Intervention | Comparison | Measurements | Main Findings |
|---|---|---|---|---|---|---|---|
| Konstantis et al., 2022 [37] | Meta-analysis | 20 | Adults with cirrhosis, including HCC | Hypo- or hyper- caloric formulas | Diet, snacks, M-DXT, L-ALB & casein | SMI and/or MAMC | Slight significant increase in BCAA group compared to control group |
| Tricipital skinfold | Decreasing trend in the BCAA group, without differences with the control | ||||||
| BMI | Significant increase in the BCAA compared to control | ||||||
| Ismaiel et al., 2022 [36] | Meta-analysis | 17 | Adults with cirrhosis, excluding HCC | Hypo- or hyper- caloric formulas | M-DXT, diet, L-ALB & No group of comparison | SMI and/or MAMC | Significant increase in BCAA group, without differences compared to control |
| Handgrip strength | Non-significant increase at the end of BCAA therapy and compared to control | ||||||
| Tricipital skinfold | Non-significant decrease with BCAA vs control therapy | ||||||
| Ooi et al., 2018 [35] | Systematic review | 40 | Children & adults with cirrhosis or hepatic failure, including HCC | Hypo- or hyper- caloric formulas | Diet, casein, etc & No group of comparison | Fat free mass | No variation in 75% of studies |
| Muscular strength | Increased in 75% of studies | ||||||
| Fat mass | No variation in 75% of studies | ||||||
| Body weight | No variation in 76% of studies |
| Study, Year | Study Type | Population | HMB Intervention | Comparison | Measurements | Main Findings |
|---|---|---|---|---|---|---|
| Lattanzi et al., 2019 [49] | Clinical trial | After liver transplantation (n = 22) | 3g daily of HMB dissolved in juice | Placebo | DXA (ASMI), anthropometry (MAMC) and hand grip strength | All increases only in HMB group |
| Lattanzi et al., 2021 [50] | Clinical trial | Compensated liver cirrhosis (n = 24) | 3g daily of HMB dissolved in water | Placebo | Quadriceps ultrasound, muscle function tests, BIA, and hand grip strength | Increase in the HMB group of quadriceps muscle mass and muscle function tests No variation in BIA parameters or in hand grip strength |
| Espina et al., 2022 [51] | Clinical trial | Decompensated liver cirrhosis (n = 43) | ONS with 1.5g of HMB, twice a day | ONS without HMB, twice a day | BIA (FM, FFM), anthropometry (weight, MAMC), and hand grip strength | Increase in both groups of weight and FM No variation in FFM and MAMC Upward trend in hand grip strength in HMB group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espina, S.; Sanz-Paris, A.; Bernal-Monterde, V.; Casas-Deza, D.; Arbonés-Mainar, J.M. Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis. J. Clin. Med. 2022, 11, 7337. https://doi.org/10.3390/jcm11247337
Espina S, Sanz-Paris A, Bernal-Monterde V, Casas-Deza D, Arbonés-Mainar JM. Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis. Journal of Clinical Medicine. 2022; 11(24):7337. https://doi.org/10.3390/jcm11247337
Chicago/Turabian StyleEspina, Silvia, Alejandro Sanz-Paris, Vanesa Bernal-Monterde, Diego Casas-Deza, and Jose Miguel Arbonés-Mainar. 2022. "Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis" Journal of Clinical Medicine 11, no. 24: 7337. https://doi.org/10.3390/jcm11247337
APA StyleEspina, S., Sanz-Paris, A., Bernal-Monterde, V., Casas-Deza, D., & Arbonés-Mainar, J. M. (2022). Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis. Journal of Clinical Medicine, 11(24), 7337. https://doi.org/10.3390/jcm11247337






