An Update on New Generation Transcatheter Aortic Valves and Delivery Systems
Abstract
1. Introduction
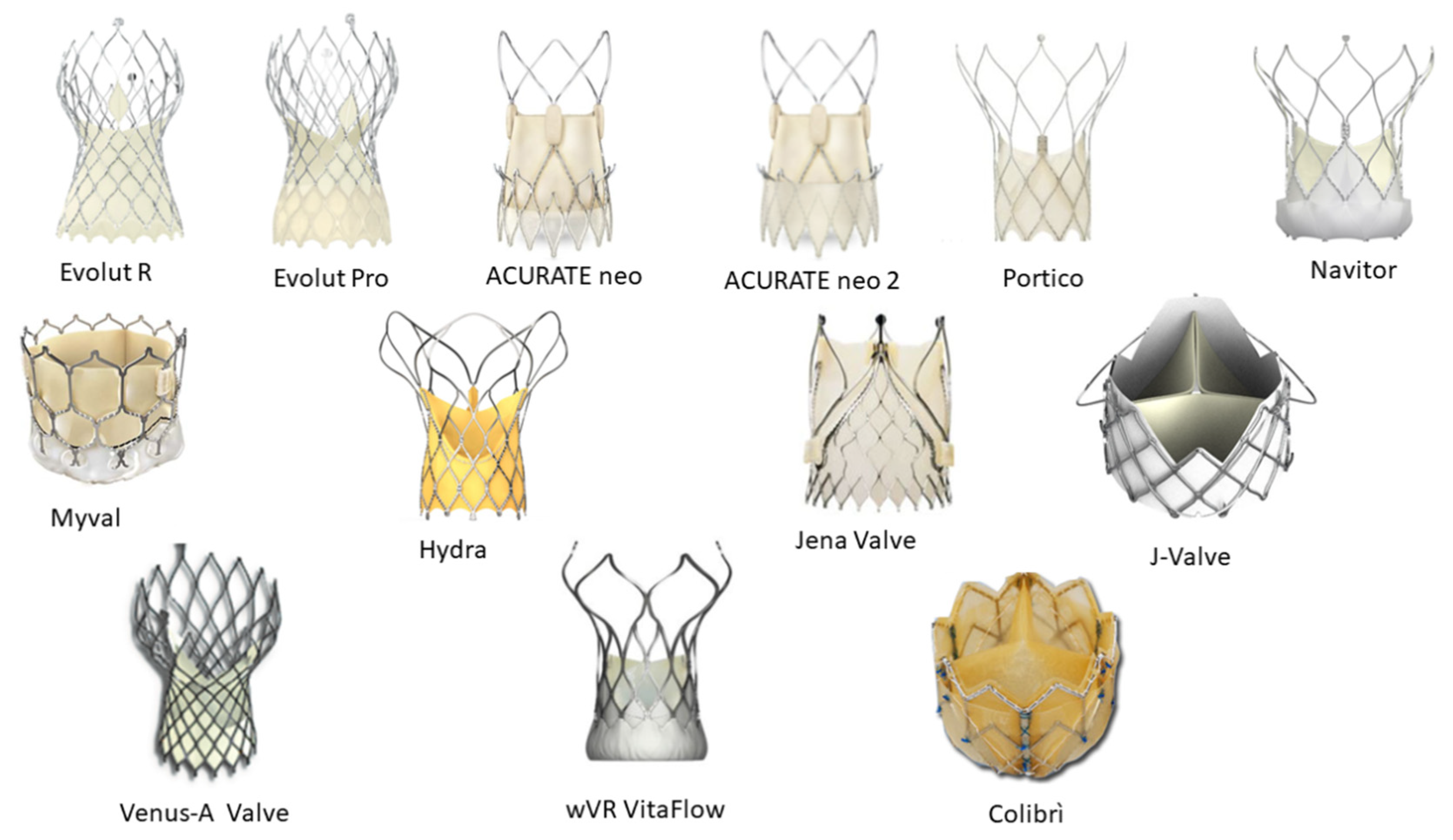
2. Transcatheter Heart Valves
2.1. Self-Expanding Transcatheter Heart Valves
2.1.1. ACURATE neo™
2.1.2. ACURATE NEO2™
2.2. Self-Expanding Transcatheter Heart Valves for Aortic Regurgitation
2.3. Balloon-Expandable Transcatheter Heart Valves
2.4. Investigational Devices
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Cahill, T.J.; Chen, M.; Hayashida, K.; Latib, A.; Modine, T.; Piazza, N.; Redwood, S.; Søndergaard, L.; Prendergast, B.D. Transcatheter aortic valve implantation: Current status and future perspectives. Eur. Heart J. 2018, 39, 2625–2634. [Google Scholar] [CrossRef]
- Tchetche, D.; Van Mieghem, N.M. New-generation TAVI devices: Description and specifications. EuroIntervention 2014, 10 (Suppl. U), U90–U100. [Google Scholar] [CrossRef]
- van Gils, L.; Tchetche, D.; Latib, A.; Sgroi, C.; Manoharan, G.; Mollmann, H.; Van Mieghem, N.M. TAVI with current CE-marked devices: Strategies for optimal sizing and valve delivery. EuroIntervention 2016, 12, Y22–Y27. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Adams, D.H.; Reardon, M.J.; Yakubov, S.J.; Kleiman, N.S.; Heimansohn, D.; Hermiller, J., Jr.; Hughes, G.C.; Harrison, J.K.; Coselli, J.; et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J. Am Coll. Cardiol. 2014, 63, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tolg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schäfer, U.; et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA 2014, 311, 1503–1514. [Google Scholar] [CrossRef]
- Chamandi, C.; Puri, R.; Rodriguez-Gabella, T.; Rodes-Cabau, J. Latest-Generation Transcatheter Aortic Valve Replacement Devices and Procedures. Can. J. Cardiol. 2017, 33, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Reardon, M.J.; Khabbaz, K.; Harrison, J.K.; Hughes, G.C.; Kodali, S.; George, I.; Deeb, G.M.; Chetcuti, S.; Kipperman, R.; et al. Early Clinical Outcomes after Transcatheter Aortic Valve Replacement Using a Novel Self-Expanding Bioprosthesis in Patients with Severe Aortic Stenosis Who Are Suboptimal for Surgery: Results of the Evolut R U.S. Study. JACC Cardiovasc. Interv. 2017, 10, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Mahtta, D.; Elgendy, I.Y.; Bavry, A.A. From CoreValve to Evolut PRO: Reviewing the Journey of Self-Expanding Transcatheter Aortic Valves. Cardiol. Ther. 2017, 6, 183–192. [Google Scholar] [CrossRef]
- Forrest, J.K.; Mangi, A.A.; Popma, J.J.; Khabbaz, K.; Reardon, M.J.; Kleiman, N.S.; Yakubov, S.J.; Watson, D.; Kodali, S.; George, I.; et al. Early Outcomes with the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve with Pericardial Wrap. JACC Cardiovasc. Interv. 2018, 11, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wyler von Ballmoos, M.C.; Reardon, M.J.; Williams, M.R.; Mangi, A.A.; Kleiman, N.S.; Yakubov, S.J.; Watson, D.; Kodali, S.; George, I.; Tadros, P.; et al. Three-Year Outcomes with a Contemporary Self-Expanding Transcatheter Valve from the Evolut PRO US Clinical Study. Cardiovasc. Revasc. Med. 2021, 26, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, K.; Piayda, K.; Afzal, S.; Kleinebrecht, L.; Makosch, M.; Hennig, I.; Quast, C.; Jung, C.; Polzin, A.; Westenfeld, R.; et al. The Latest Evolution of the Medtronic CoreValve System in the Era of Transcatheter Aortic Valve Replacement: Matched Comparison of the Evolut PRO and Evolut R. JACC Cardiovasc. Interv. 2018, 11, 2314–2322. [Google Scholar] [CrossRef]
- Grube, E.; Van Mieghem, N.M.; Bleiziffer, S.; Modine, T.; Bosmans, J.; Manoharan, G.; Linke, A.; Scholtz, W.; Tchétché, D.; Finkelstein, A.; et al. Clinical Outcomes with a Repositionable Self-Expanding Transcatheter Aortic Valve Prosthesis: The International FORWARD Study. J. Am. Coll. Cardiol. 2017, 70, 845–853. [Google Scholar] [CrossRef]
- Kalogeras, K.; Ruparelia, N.; Kabir, T.; Jabbour, R.; Naganuma, T.; Vavuranakis, M.; Nakamura, S.; Wang, B.; Sen, S.; Hadjiloizou, N.; et al. Comparison of the self-expanding Evolut-PRO transcatheter aortic valve to its predecessor Evolut-R in the real world multicenter ATLAS registry. Int. J. Cardiol. 2020, 310, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Cheng, W.; Waksman, R.; Satler, L.F.; Chakravarty, T.; Groh, M.; Abernethy, W.; Russo, M.J.; Heimansohn, D.; Hermiller, J.; et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): A randomised, controlled, non-inferiority trial. Lancet 2020, 396, 669–683. [Google Scholar] [CrossRef]
- Mas-Peiro, S.; Seppelt, P.C.; Weiler, H.; Mohr, G.L.; Papadopoulos, N.; Walther, T.; Zeiher, A.M.; Fichtlscherer, S.; Vasa-Nicotera, M. A Direct Comparison of Self-Expandable Portico versus Balloon-Expandable Sapien 3 Devices for Transcatheter Aortic Valve Replacement: A Case-Matched Cohort Study. J. Invasive Cardiol. 2019, 31, E199–E204. [Google Scholar]
- Fontana, G.P. A randomized trial of Portico vs. commercially available transcatheter aortic valves in patients with severe aortic stenosis. Presented at TCT 2019, San Francisco, CA, USA, 27 September 2019. Online resource. [Google Scholar]
- Chandra, P.; Jose, J.; Mattummal, S.; Mahajan, A.U.; Govindan, S.C.; Makhale, C.N.; Chandra, S.; Shetty, R.; Mohanan, S.; John, J.F.; et al. Clinical evaluation of the Hydra self-expanding transcatheter aortic valve: 6 month results from the GENESIS trial. Catheter. Cardiovasc. Interv. 2021, 98, 371–379. [Google Scholar] [CrossRef]
- Aidietis, A.; Srimahachota, S.; Dabrowski, M.; Bilkis, V.; Buddhari, W.; Cheung, G.; Nair, R.K.; Mussayev, A.A.; Mattummal, S.; Chandra, P.; et al. 30-Day and 1-Year Outcomes with HYDRA Self-Expanding Transcatheter Aortic Valve. JACC Cardiovasc. Inter. 2022, 15, 93–104. [Google Scholar] [CrossRef]
- Liao, Y.B.; Zhao, Z.G.; Wei, X.; Xu, Y.N.; Zuo, Z.L.; Li, Y.J.; Zheng, M.X.; Feng, Y.; Chen, M. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: Preliminary Experiences in China. Catheter. Cardiovasc. Interv. 2017, 89 (Suppl. 1), 528–533. [Google Scholar] [CrossRef]
- Wang, R.; Kawashima, H.; Mylotte, D.; Rosseel, L.; Gao, C.; Aben, J.P.; Abdelshafy, M.; Onuma, Y.; Yang, J.; Soliman, O.; et al. Quantitative Angiographic Assessment of Aortic Regurgitation After Transcatheter Implantation of the Venus A-valve: Comparison with Other Self-Expanding Valves and Impact of a Learning Curve in a Single Chinese Center. Glob. Heart 2021, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, W.; Wang, J.; Wu, Y.; Chen, M.; Modine, T.; Mylotte, D.; Piazza, N.; Ge, J. VitaFlow transcatheter valve system in the treatment of severe aortic stenosis: One-year results of a multicenter study. Catheter. Cardiovasc. Interv. 2020, 95, 332–338. [Google Scholar] [CrossRef]
- Mollmann, H.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schafer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1,000 patients enrolled in the SAVI TF registry. EuroIntervention 2018, 13, e1764–e1770. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schafer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. The SAVI-TF Registry: 1-Year Outcomes of the European Post-Market Registry Using the ACURATE neo Transcatheter Heart Valve under Real-World Conditions in 1,000 Patients. JACC Cardiovasc. Interv. 2018, 11, 1368–1374. [Google Scholar] [CrossRef]
- Lanz, J.; Kim, W.K.; Walther, T.; Burgdorf, C.; Mollmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Moriyama, N.; Vento, A.; Laine, M. Safety of Next-Day Discharge after Transfemoral Transcatheter Aortic Valve Replacement with a Self-Expandable Versus Balloon-Expandable Valve Prosthesis. Circ. Cardiovasc. Interv. 2019, 12, e007756. [Google Scholar] [CrossRef]
- Purita, P.A.M.; Tahoces, L.S.; Fraccaro, C.; Nai Fovino, L.; Kim, W.K.; Espada-Guerreiro, C.; De Backer, O.; Seiffert, M.; Nombela-Franco, L.; Gomez, R.M.; et al. Transcatheter treatment of native aortic valve regurgitation: Results from an international registry using the transfemoral ACURATE neo valve. Int. J. Cardiol. Heart Vasc. 2020, 27, 100480. [Google Scholar] [CrossRef]
- Mangieri, A.; Chieffo, A.; Kim, W.K.; Stefanini, G.G.; Rescigno, G.; Barbanti, M.; Tamburino, C.; Rück, A.; Pagnesi, M.; Linder, R.; et al. Transcatheter aortic valve implantation using the ACURATE neo in bicuspid and tricuspid aortic valve stenosis: A propensity-matched analysis of a European experience. EuroIntervention 2018, 14, e1269–e1275. [Google Scholar] [CrossRef]
- Hamm, K.; Reents, W.; Zacher, M.; Kerber, S.; Diegeler, A.; Schieffer, B.; Barth, S. Transcatheter aortic valve implantation using the ACURATE TA and ACURATE neo valves: A four-year single-centre experience. EuroIntervention 2017, 13, 53–59. [Google Scholar] [CrossRef]
- Mollmann, H.; Walther, T.; Siqueira, D.; Diemert, P.; Treede, H.; Grube, E.; Nickenig, G.; Baldus, S.; Rudolph, T.; Kuratani, T.; et al. Transfemoral TAVI using the self-expanding ACURATE neo prosthesis: One-year outcomes of the multicentre “CE-approval cohort”. EuroIntervention 2017, 13, e1040–e1046. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Nissen, H.; Mogensen, B.; Cuculi, F.; Fallesen, C.; Veien, K.T.; Brinkert, M.; Kobza, R.; Rück, A. Very low pacemaker rate following ACURATE neo transcatheter heart valve implantation. EuroIntervention 2017, 13, 1273–1280. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.; Steinbruchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Holzamer, A.; Kim, W.K.; Ruck, A.; Sathananthan, J.; Keller, L.; Cosma, J.; Bauer, T.; Nef, H.; Amat-Santos, I.J.; Brinkert, M.; et al. Valve-in-Valve Implantation Using the ACURATE Neo in Degenerated Aortic Bioprostheses: An International Multicenter Analysis. JACC Cardiovasc. Interv. 2019, 12, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Franzone, A.; Piccolo, R.; Siontis, G.C.M.; Lanz, J.; Stortecky, S.; Praz, F.; Roost, E.; Vollenbroich, R.; Windecker, S.; Pilgrim, T. Transcatheter Aortic Valve Replacement for the Treatment of Pure Native Aortic Valve Regurgitation: A Systematic Review. JACC Cardiovasc. Interv. 2016, 9, 2308–2317. [Google Scholar] [CrossRef]
- Mauri, V.; Kim, W.K.; Abumayyaleh, M.; Walther, T.; Moellmann, H.; Schaefer, U.; Conradi, L.; Hengstenberg, C.; Hilker, M.; Wahlers, T.; et al. Short-Term Outcome and Hemodynamic Performance of Next-Generation Self-Expanding Versus Balloon-Expandable Transcatheter Aortic Valves in Patients with Small Aortic Annulus: A Multicenter Propensity-Matched Comparison. Circ. Cardiovasc. Interv. 2017, 10, e005013. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Pellegrini, C.; Kim, W.K.; Holzamer, A.; Pilgrim, T.; Toggweiler, S.; Schäfer, U.; Blumenstein, J.; Deuschl, F.; Rheude, T.; et al. Transcatheter Valve SELECTion in Patients with Right Bundle Branch Block and Impact on Pacemaker Implantations. JACC Cardiovasc. Interv. 2019, 12, 1781–1793. [Google Scholar] [CrossRef]
- Husser, O.; Kim, W.K.; Pellegrini, C.; Holzamer, A.; Walther, T.; Mayr, P.N.; Joner, M.; Kasel, A.M.; Trenkwalder, T.; Michel, J.; et al. Multicenter Comparison of Novel Self-Expanding Versus Balloon-Expandable Transcatheter Heart Valves. JACC Cardiovasc. Interv. 2017, 10, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.; Reents, W.; Zacher, M.; Kerber, S.; Diegeler, A.; Schieffer, B.; Schreiber, M.; Lauer, B.; Kuntze, T.; Dahmer, M.; et al. Multicentre propensity-matched comparison of transcatheter aortic valve implantation using the ACURATE TA/neo self-expanding versus the SAPIEN 3 balloon-expandable prosthesis. EuroIntervention 2019, 15, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Bleiziffer, S.; Thiele, H.; Scholtz, S.; Hildick-Smith, D.; Cunnington, M.; Wolf, A.; Barbanti, M.; Tchetchè, D.; Garot, P.; et al. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients with Symptomatic Severe Aortic Stenosis: SCOPE 2 Randomized Clinical Trial. Circulation 2020, 142, 2431–2442. [Google Scholar] [CrossRef]
- Mollmann, H.; Holzhey, D.M.; Hilker, M.; Toggweiler, S.; Schafer, U.; Treede, H.; Joner, M.; Søndergaard, L.; Christen, T.; Allocco, D.J.; et al. The ACURATE neo2 valve system for transcatheter aortic valve implantation: 30-day and 1-year outcomes. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1912–1920. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. ACURATE Neo™ AS Aortic Bioprosthesis for Implantation Using the ACURATE neoTM AS TF Transfemoral Delivery System in Patients with Severe Aortic Stenosis. ClinicalTrials.gov Identifier: NCT02909556. Available online: https://clinicaltrials.gov/ct2/show/NCT02909556 (accessed on 25 June 2021).
- Buono, A. Short-Term Outcomes of a Novel Self-Expanding Device: ITAL-neo Registry. Presented at the Italian Congress of Interventional Cardiology; October 2021. Available online: https://www.dicardiology.com/article/acurate-neo2-tavr-valve-demonstrate-reduced-paravalvular-leak-and-low-permanent-pacemaker (accessed on 26 May 2021).
- Ruck, A. Results from the Early neo2 Registry Acurate neo2 TAVI Valve. Presented at the Congress for the European Association of Percutaneous Cardiovascular Interventions (EuroPCR); 2021. Available online: https://media.pcronline.com/diapos/EuroPCR2021/3890-20210518_0922_Clinical_Science_Ruck_Andreas_0000_(7805)/Ruck_Andreas_20211805_1408_VOD.pdf (accessed on 15 June 2021).
- ClinicalTrials.gov. Early neo2 Registry of the Acurate neo2 TAVI Prosthesis. ClinicalTrials.gov Identifier: NCT04810195. Available online: https://clinicaltrials.gov/ct2/show/NCT04810195 (accessed on 24 June 2021).
- Kempfert, J.; Rastan, A.J.; Mohr, F.W.; Walther, T. A new self-expanding transcatheter aortic valve for transapical implantation first in man implantation of the JenaValve. Eur. J. Cardiothorac. Surg. 2011, 40, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Treede, H.; Mohr, F.W.; Baldus, S.; Rastan, A.; Ensminger, S.; Arnold, M.; Kempfert, J.; Figulla, H.R. Transapical transcatheter aortic valve implantation using the JenaValve system: Acute and 30-day results of the multicentre CE-mark study. Eur. J. Cardiothorac. Surg. 2012, 41, e131–e138. [Google Scholar] [CrossRef][Green Version]
- Silaschi, M.C.L.; Wendler, O.; Schlingloff, F.; Kappert, U.; Rastan, A.J.; Baumbach, H.; Holzhey, D.; Eichinger, W.; Bader, R.; Treede, H. The JUPITER registry: One-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter. Cardiovasc. Interv. 2018, 91, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, Y.; Guo, Y.Q.; Hu, J.; Zhang, J.; Wei, X.; Tang, H.; Shi, Y. Transapical transcatheter aortic valve implantation using a new second-generation TAVI system—J-Valve™ for high-risk patients with aortic valve diseases: Initial results with 90-day follow-up. Int. J. Cardiol. 2015, 199, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Rao, R.S.; Chandra, P.; Goel, P.K.; Bharadwaj, P.; Joseph, G.; Jose, J.; Mahajan, A.U.; Mehrotra, S.; Sengottovelu, G.; et al. First-in-human evaluation of a novel balloon-expandable transcatheter heart valve in patients with severe symptomatic native aortic stenosis: The MyVal-1 study. EuroIntervention 2020, 16, 421–429. [Google Scholar] [CrossRef]
- Akyuz, A.R.; Konus, A.H.; Cirakoglu, O.F.; Sahin, S.; Kul, S.; Korkmaz, L. First experiences with a new balloon-expandable Myval transcatheter aortic valve: A preliminary study. Herz, 2021; 1–7, Advance online publication. [Google Scholar]
- Sharma, S.K.; Rao, R.S.; Chopra, M.; Sonawane, A.; Jose, J.; Sengottuvelu, G. Myval transcatheter heart valve system in the treatment of severe symptomatic aortic stenosis. Future Cardiol. 2021, 17, 73–80. [Google Scholar] [CrossRef]
- Kawashima, H.; Wang, R.; Mylotte, D.; Jagielak, D.; De Marco, F.; Ielasi, A.; Onuma, Y.; den Heijer, P.; Terkelsen, C.J.; Wijns, W.; et al. Quantitative Angiographic Assessment of Aortic Regurgitation after Transcatheter Aortic Valve Implantation among Three Balloon-Expandable Valves. Glob. Heart 2021, 16, 20. [Google Scholar] [CrossRef]
- Delgado-Arana, J.R.; Gordillo-Monge, M.X.; Halim, J.; De Marco, F.; Trani, C.; Martin, P.; Infusino, F.; Ancona, M.; den Heijer, P.; Bedogni, F.; et al. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart (Br. Card. Soc.), 2021; Advance online publication. [Google Scholar]
- Santos-Martinez, S.; Halim, J.; Castro-Mejía, A.; De Marco, F.; Trani, C.; Martin, P.; Infusino, F.; Ancona, M.; Moreno, R.; den Heijer, P.; et al. Myval versus alternative balloon- and self-expandable transcatheter heart valves: A central core lab analysis of conduction disturbances. Int. J. Cardiol. 2022; Advance online publication. [Google Scholar]
- Kawashima, H.; Soliman, O.; Wang, R.; Ono, M.; Hara, H.; Gao, C.; Zeller, E.; Thakkar, A.; Tamburino, C.; Bedogni, F.; et al. Rationale and design of a randomized clinical trial comparing safety and efficacy of myval transcatheter heart valve versus contemporary transcatheter heart valves in patients with severe symptomatic aortic valve stenosis: The LANDMARK trial. Am Heart J. 2021, 232, 23–38. [Google Scholar] [CrossRef]
- Fish, R.D.; Paniagua, D.; Urena, P.; Chevalier, B. The Colibri heart valve: Theory and practice in the achievement of a low-profile, pre-mounted, pre-packaged TAVI valve. EuroIntervention 2013, 9, S111–S114. [Google Scholar] [CrossRef]

| THVs | Company | Access Route | Sizes (mm) | Stent Frame | Delivery System Diameter (Fr) | Leaflets Tissue | Repositionable | Retrievable | PPM (%) | PVL (%) | Major Vascular Complications (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Evolut Pro | Medtronic | TAo, TV | 23, 26, 29 | Nitinol | 16 | Porcine | Yes | Yes | 10.8 *–11.9 * | 0 *–11 * | 0–10 |
| NEO2 | Boston Scientific | TA, TV | 23, 25, 27 | Nitinol | 14 | Porcine | Yes | No | 6.0 *–7.7 * | 2.7 *–3.5 * | 3.4 |
| Portico | Abbott | Tao, TV | 23, 25, 27, 29 | Nitinol | 18 | Bovine | Yes | Yes | 8.8 *–15.8 * | 3.4 *–5.7 * | 2.2–7.2 |
| Navitor | Abbott | 23, 25, 27, 29 | Nitinol | 14 | Bovine | Yes | Yes | 15 * | 0 * | 0.8 | |
| Myval | Meril Life Sciences | TV | 20, 23, 26, 29, 21.5, 24.5, 27.5,30.5, 32 | Ni-Co | 14 | Bovine | No | No | 5.8 *–8 * | 0 *–4 * | 0–3 |
| Hydra | Vascular Innovations | TV | 22, 26, 30 | Nitinol | 18 | Bovine | No | No | 7.5 * | 10 * | 2.5 |
| JenaValve | Jena Valve | TA | 23, 25, 27 | Nitinol | 32 | Porcine | Yes | Yes | 9.1 * | 0 | |
| J-valve | Jie Cheng Medical Technologies | TA | 21, 23, 25, 27 | Nitinol | 27 | Porcine | Yes | Yes | 2.3 *–4.8 ** | 0 | 0 |
| Venus-A valve | Venus Medtech | TV | 23, 26, 29, 32 | Nitinol | 19 | Porcine | Yes | No | 7.4 *–18.8 | 8.8 *–14.2 * | 6.2 |
| wVR VitaFlo | Microport | TV | 21, 24, 27, 30 | Nitinol | 16/18 | Bovine | Yes | No | 16.4 *–19.1 ** | 2 * | 1.8 |
| Colibri | Colibri Heart Valve | TV | 24 | Stainless Steel | 14 | “Dry” porcine | No | No | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santangelo, G.; Ielasi, A.; Pellicano, M.; Latib, A.; Tespili, M.; Donatelli, F. An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. J. Clin. Med. 2022, 11, 499. https://doi.org/10.3390/jcm11030499
Santangelo G, Ielasi A, Pellicano M, Latib A, Tespili M, Donatelli F. An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. Journal of Clinical Medicine. 2022; 11(3):499. https://doi.org/10.3390/jcm11030499
Chicago/Turabian StyleSantangelo, Gloria, Alfonso Ielasi, Mariano Pellicano, Azeem Latib, Maurizio Tespili, and Francesco Donatelli. 2022. "An Update on New Generation Transcatheter Aortic Valves and Delivery Systems" Journal of Clinical Medicine 11, no. 3: 499. https://doi.org/10.3390/jcm11030499
APA StyleSantangelo, G., Ielasi, A., Pellicano, M., Latib, A., Tespili, M., & Donatelli, F. (2022). An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. Journal of Clinical Medicine, 11(3), 499. https://doi.org/10.3390/jcm11030499







