Cardiac Surgery in Advanced Heart Failure
Abstract
:1. Introduction
2. Definition of Advanced Heart Failure
3. Temporary Mechanical Circulatory Support
3.1. New Evidence for the Intra-Aortic Balloon Pump
3.2. IMPELLA
3.3. Venoarterial Extracorporeal Membrane Oxygenation (VA-ECMO)
4. Long-Term Left-Ventricular Mechanical Circulatory Support (LT-MCS)
5. Heart Transplantation
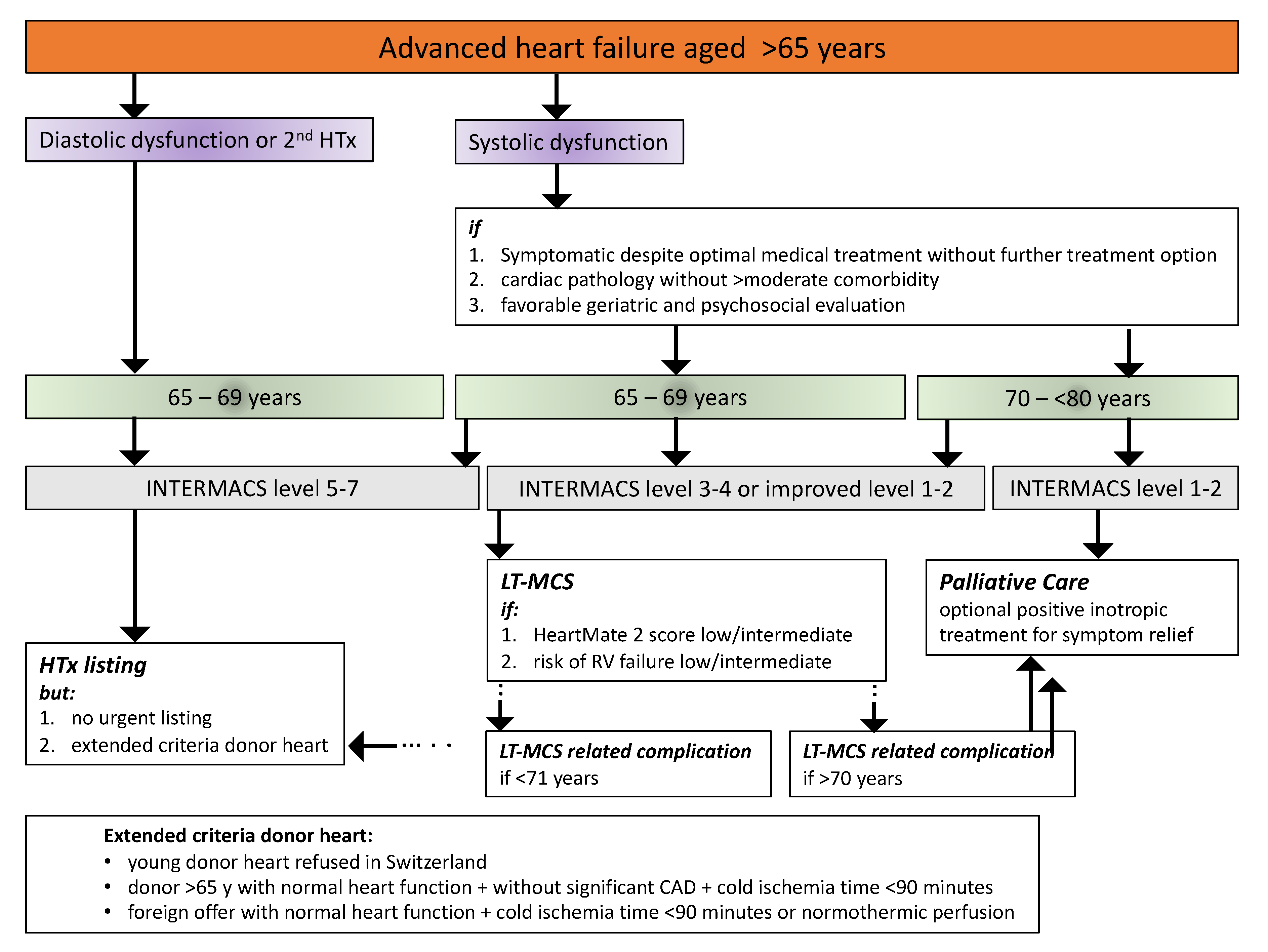
6. Taking Care of the Aged Advanced Heart Failure Patient in the French-Speaking Part of Switzerland
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions: ESC-HF-LT: 1-year follow-up. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xanthakis, V.; Enserro, D.M.; Larson, M.G.; Wollert, K.C.; Januzzi, J.L.; Levy, D.; Aragam, J.; Benjamin, E.J.; Cheng, S. Prevalence, Neurohormonal Correlates, and Prognosis of Heart Failure Stages in the Community. JACC Heart Fail. 2016, 4, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Claggett, B.; Loehr, L.; Chang, P.P.; Matsushita, K.; Kitzman, D.; Konety, S.; Kucharska-Newton, A.; Sueta, C.A.; Mosley, T.H.; et al. Heart Failure Stages Among Older Adults in the Community. Circulation 2017, 135, 224–240. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Bauchmach, A.; Bohm, B.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart 2021, 42, 1–128. [Google Scholar] [CrossRef]
- Abdurashidova, T.; Monney, P.; Tzimas, G.; Soborun, N.; Regamey, J.; Daux, A.; Barras, N.; Kirsch, M.; Müller, M.; Hullin, R. Non-severe aortic regurgitation increases short-term mortality in acute heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 3901–3909. [Google Scholar] [CrossRef]
- Yaku, H.; Ozasa, N.; Morimoto, T.; Inuzuta, Y.; Tamaki, A.; Yamatoto, Y.; Yoshikwaka, Y.; Kitai, T.; Tanighuci, R.; Jinnai, T.; et al. Demographics, management, and in-hospital outcome of hospitalized acute heart failure patients in contemporary real clinical practice in Japan. Circ. J. 2018, 82, 2811–2819. [Google Scholar] [CrossRef] [Green Version]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; Shah, A.N.; et al. The Global Health and Economic Burden of Hospitalizations for Heart Failure. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- German Heart Society. Deutscher Herzbericht 2017 Sektoren Uebergreifende Versorgungsanalyse zur Kardiologie, Herz-Chirurgie und Kinderherzmedizin; Deutschland. e.V. DH: Frankfurt, Germany, 2017. [Google Scholar]
- Maack, C.; Eschenhagen, T.; Hamdani, N.; Heinzel, F.R.; Lyon, A.R.; Manstein, D.J.; Metzger, J.; Papp, Z.; Tocchetti, C.G.; Yilmaz, M.B.; et al. Treatments targeting inotropy. Eur. Heart J. 2018, 40, 3626–3644. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Miller, P.E.; McCullough, M.; Desai, N.R.; Riello, R.; Psotka, M.; Böhm, M.; Allen, L.A.; Teerlink, J.R.; Rosano, G.M.; et al. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur. J. Heart Fail. 2019, 21, 1064–1078. [Google Scholar] [CrossRef]
- Barge-Caballero, E.; Almenar-Bonet, L.; Gonzalez-Vilchez, F.; Rodríguez, J.L.L.; González-Costello, J.; Segovia-Cubero, J.; Castel-Lavilla, M.A.; Jiménez, J.F.D.; Garrido-Bravo, I.P.; Rangel-Sousa, D.; et al. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: A nationwide Spanish registry. Eur. J. Heart Fail. 2017, 20, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Finnan, M.J.; Bakir, N.H.; Itoh, A.; Kotkar, K.D.; Pasque, M.K.; Damiano, R.J.; Moon, M.R.; Ewald, G.A.; Schilling, J.D.; Masood, M.F. 30 Years of Heart Transplant: Outcomes After Mechanical Circulatory Support From a Single Center. Ann. Thorac. Surg. 2021, 113, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Hannan, M.G.; Kuckucka, M.; de Jonge, N.; Loforte, A.; Lund, L.; Mohacsi, P.; Morshuis, M.; et al. 2019 EATCS expert consensus on long-term mechanical circulatory support. Eur. J. Cardio Thor. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Desch, S.; Thiele, H. Intra-aortic balloon counterpulsation—Does it work? Prog. Cardiovasc. Dis. 2020, 63, 623–629. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.; Dixon, S.; Massaro, J.; Palacois, I.; Maini, B.; Mulukatla, S. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: The PROTECT II study. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, H.; Zeymer, U.; Thelemann, N.; Neumann, F.-J.; Hauseleiter, J.; Abdel-Wahab, M.; Meyer-Sarei, R.; Fuernau, G.; Eitel, J.; Hambrecht, R.; et al. Intraaortic balloon pump in cardio—Genic shock complicating acute myocardial infarction: Long-term 6-year out- come of the randomized IABP-SHOCK II Trial. Circulation 2019, 139, 395–403. [Google Scholar] [CrossRef]
- Patel, M.R.; Smalling, R.W.; Thiele, H.; Huiman, X.; Zhou, Y.; Chandra, P.; Chew, D.; Cohen, D.; French, J.; Perera, D.; et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute ante-rior myocardial infarction without shock: The CRISP AMI randomized trial. JAMA 2011, 306, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Dhruva, S.S.; Ross, J.R.; Mortazavi, B.J.; Hurlay, N.Z.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.P.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarc-tion Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef]
- Huckaby, L.V.; Seese, L.M.; Mathier, M.A.; Hickey, G.W.; Kilic, A. Intra-aortic balloon pump bridging to heart transplantation: Impact of the 2018 allocation change. Circ. Heart Fail. 2020, 13, 206–213. [Google Scholar] [CrossRef]
- Bhimaraj, A.; Agrawal, T.; Duran, A.; Tamimi, O.; Amione-Guerra, J.; Trachtenberg, B.; Guha, A.; Hussain, I.; Kim, J.; Kassi, M.; et al. Percutaneous left axillary artery placement of intra-aortic balloon pump in advanced heart failure patients. JACC Heart Fail. 2020, 8, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The evolving landscape of Impella use in the United States among patients under-going percutaneous coronary intervention with mechanical circulatory support. Circulation 2020, 141, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lemor, A.; Hosseini Dehkordi, S.H.; Basir, M.B.; Villablanca, P.A.; Jain, T.; Koenig, G.C.; Alaswad, K.; Moses, J.W.; Kapur, N.K.; O'Neill, W. Impella versus extracorporeal membrane oxygenation for acute myocar-dial infarction cardiogenic shock. Cardiovasc. Revasc. Med. 2020, 21, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Swain, L.; Reyelt, L.; Bhave, S.; Qiao, X.; Thomas, C.J.; Zweck, E.; Crowley, P.; Boggins, C.; Esposito, M.; Chin, M.; et al. Transvalvular Ventricular Unloading Before Reperfusion in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 684–699. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: Results from an international, multicenter cohort study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Bastos, M.B.; Burkhoff, D.; Maly, J.; Daemen, J.; den Uil, C.; Amelot, K.; Lenzen, M.; Mahfoud, F.; Zijlstra, F.; Schreuder, J.J.; et al. Invasive left ventricle pressure–volume analysis: Overview and practical clinical im-plications. Eur. Heart J. 2020, 41, 1286–1297. [Google Scholar] [CrossRef]
- Varshney, A.S.; Berg, D.D.; Katz, J.N.; Baird-Zars, V.M.; Bohula, E.A.; Carnicelli, A.P.; Chaudhry, S.-P.; Guo, J.; Lawler, P.R.; Nativi-Nicolau, J.; et al. Use of Temporary Mechanical Circulatory Support for Management of Cardiogenic Shock Before and After the United Network for Organ Sharing Donor Heart Allocation System Changes. JAMA Cardiol. 2020, 5, 703–708. [Google Scholar] [CrossRef]
- Brunner, S.; Guenther, S.P.W.; Lackermair, K.; Peterss, S.; Orban, M.; Boulesteix, A.L.; Michel, S.; Hausleiter, J.; Massberg, S.; Hagl, C. Extracorporeal life support in cardiogenic shock complicating acute myo-cardial infarction. J. Am. Coll. Cardiol. 2019, 73, 2355–2357. [Google Scholar] [CrossRef]
- Lackermair, K.; Brunner, S.; Orban, M.; Peterss, S.; Orban, M.; Theiss, H.D.; Huber, B.C.; Juchem, G.; Born, F.; Boulesteix, A.-L.; et al. Outcome of patients treated with extracorporeal life support in cardiogenic shock complicating acute myocardial infarction: 1-year result from the ECLS-Shock study. Clin. Res. Cardiol. 2020, 110, 1412–1420. [Google Scholar] [CrossRef]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef]
- Donker, D.W.; Brodie, D.; Henriques, J.P.S.; Broomé, M. Left ventricular unloading during veno-arterial ECMO: A review of per-cutaneous and surgical unloading interventions. Perfusion 2019, 34, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Char, S.; Fried, J.; Melehy, A.; Mehta, S.; Ning, Y.; Kurlansky, P.; Takeda, K. Clinical efficacy of direct and indirect left ventricular unloading during venoarterial extracorporeal membrane oxygenation for primary cardiogenic shock. J. Thoracic. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Al-Fares, A.A.; Randhawa, V.K.; Englesakis, M.; McDomald, M.A.; Nagpal, A.D.; Estep, J.D.; Soltesz, E.G.; Fan, E. Optimal strategy and timing of left ventricular venting during veno-arterial extracorporeal life support for adults in cardiogenic shock: A systematic review and meta-analysis. Circul. Heart Fail. 2019, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lüsebrink, E.; Orban, M.; Kupka, D.; Scherer, C.; Hagl, C.; Zimmer, S.; Luedike, P.; Thiele, H.; Westermann, D.; Massberg, S.; et al. Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur. Heart J. 2020, 41, 3753–3761. [Google Scholar] [CrossRef]
- Udesen, N.J.; Møller, J.E.; Lindholm, M.G.; Eiskjær, H.; Schäfer, A.; Werner, N.; Holmvang, L.; Terkelsen, C.J.; Jensen, L.O.; Junker, A.; et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am. Heart J. 2019, 214, 60–68. [Google Scholar] [CrossRef]
- Testing the Value of Novel Strategy and its Cost Efficacy in Order to Improve the Poor Outcomes in Cardiogenic Shock—Tabular View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03813134 (accessed on 23 November 2021).
- Ostadal, P.; Rokyta, R.; Kruger, A.; Vondrakova, D.; Janotka, M.; Šmíd, O.; Šmalcová, J.; Hromadka, M.; Linhart, A.; Bělohlávek, J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): Rationale and design of the multicenter randomized trial. Eur. J. Heart Fail. 2017, 19, 124–127. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M. A prospective randomised trial of early LV venting using Impella CP for recovery in patients with cardiogenic shock managed with VA ECMO. ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03431467 (accessed on 29 November 2021).
- Felker, G.M.; Benza, R.L.; Chandler, A.B. Heart failure etiology and response to milrinone in decompensated heart fail-ure: Results from the OPTIME-CHF study. J. Am. Coll. Cardiol. 2003, 41, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Bonios, M.J.; Terrovitis, J.V.; Drakos, S.G.; Katsaros, F.; Pantsios, C.; Nanas, S.N.; Kanakadis, J.; Alexopoulos, G.; Toumanidis, S.; Anastasiou-Nana, M.; et al. Comparison of three different regimens of intermittent inotrope infusions for end stage heart failure. Int. J. Cardiol. 2012, 159, 225–229. [Google Scholar] [CrossRef]
- Papp, Z.; Agostini, P.J.; Alvarez, J.; Bettex, D.; Bouchez, S.; Brito, D.; Cerny, V.; Comin-Colet, J.; Crespo-Leiro, M.G.; Delgado, J.F.; et al. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Cardiovasc. Pharmacol. 2020, 76, 4–22. [Google Scholar] [CrossRef]
- Lund, L.H.; Edwards, L.B.; Dipchand, A.I.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; Yusen, R.D.; et al. The registry of the international society of heart and lung transplantation: Thir-ty-third adult heart transplantation report—2016; focus theme: Primary diagnostic indications for transplant. J. Heart. Lung Transpl. 2016, 35, 1158–1169. [Google Scholar] [CrossRef]
- Swisstransplant annual report, 2020, page 66. Available online: www.swisstransplant.org (accessed on 30 January 2022).
- Estep, J.D.; Starling, R.C.; Horstmanshof, D.A.; Milano, C.A.; Selzman, C.H.; Shah, K.B.; Loebe, M.; Moazami, N.; Long, J.W.; Stehlik, J.; et al. Risk assessment and comparative effectiveness of left ventric-ular assist device and medical management in ambulatory heart failure patients: Results from the ROADMAP study. J. Am. Coll. Cardiol. 2015, 66, 1747–1761. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.; Birks, E.; Guglin, M.; Lamba, H.; Frazier, O. Use of Ventricular Assist Devices and Heart Transplantation for Advanced Heart Failure. Circ. Res. 2019, 124, 1658–1678. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.E.S.; Graetz, T.J.; Emmert, D.A.; Avidan, M.S. Statistics of heart failure and mechanical circulatory support in 2020. Ann. Transl. Med. 2020, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Ambardekar, A.V.; Forde-McLean, R.C.; Kittleson, M.M.; Stewart, G.C.; Palardy, M.; Thibodeau, J.; DeVore, A.; Mountis, M.M.; Cadaret, L.; Teuteberg, J.J.; et al. High early event rates in patients with questionable eligibility for advanced heart failure therapies: Results from the Medical Arm of Mechanically Assisted Circulatory Support (Medamacs) Registry. J. Heart Lung Transpl. 2016, 35, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Cowger, J.; Shah, P.; Stulak, J.; Maltais, S.; Aaronson, K.D.; Kirklin, J.K.; Pagani, F.; Salerno, C. INTERMACS profiles and modifiers: Heterogeneity of patient classification and the impact of modifiers on predicting patient outcome. J. Heart Lung Transpl. 2015, 35, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, F.; Rogers, J.G. Left ventricular assist device therapy in advanced heart failure: Patient selection and outcomes. Eur. J. Heart Fail. 2019, 19, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, L.W.; Pagani, F.; Young, J.B.; Jessup, M.; Miller, L.; Kormos, R.L.; Naftel, D.C.; Ulisney, K.; Desvigne-Nickens, P.; Kirklin, J.K. INTERMACS Profiles of Advanced Heart Failure: The Current Picture. J. Heart Lung Transpl. 2009, 28, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving in-dications, outcomes, and scientific partnerships. Ann. Thorac. Surg. 2019, 107, 341–353. [Google Scholar] [CrossRef]
- Mehra, M.R.; Naka, Y.; Uriel, N.; Goldstein, D.J.; Cleveland, J.C.; Yuzefplskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 440–450. [Google Scholar] [CrossRef]
- Nowacka, A.; Hullin, R.; Tozzi, P.; Barras, N.; Regamey, J.; Yerly, P.; Rosner, L.; Marcucci, C.; Rusca, M.; Liaudet, L.; et al. Short-term single-center experience with the HeartMate 3 left ventricular assist de-vice for advanced heart failure. Eur. J. Cardiothorac. Surg. 2020, 58, 511–518. [Google Scholar] [CrossRef]
- Schaeffer, T.; Pfister, O.; Mork, C.; Mohacsi, P.; Rueter, F.; Scheifele, S.; Morgen, A.; Zenklusen, U.; Doebele, T.; Maurer, M.; et al. 5-year results of a newly implemented mechanical circulatory support program for terminal heart failure patients in a Swiss non-cardiac transplant university hospital. J. Cardiothorac. Surg. 2021, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Pagani, F.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transpl. 2017, 36, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson, R.M.; Stahovich, M.; Chillcott, S.; Baradarian, S.; Chammas, J.; Jaski, B.; Hoagland, P.; Dembitsky, W. Clinical Strategies and Outcomes in Advanced Heart Failure Patients Older Than 70 Years of Age Receiving the HeartMate II Left Ventricular Assist Device: A Community Hospital Experience. J. Am. Coll. Cardiol. 2011, 57, 2487–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamedali, B.; Yost, G.; Bhat, G. Obesity as a Risk Factor for Consideration for Left Ventricular Assist Devices. J. Card. Fail. 2015, 21, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Aittigrine, S.; Tozzi, P.; Hullin, R.; Yerly, P.; Regamey, J.; Rösner, L.; Rusca, M.; Kirsch, M.; Suter, M.; Mantziari, S. Laparoscopic sleeve gastrectomy for class III obesity in a patient with a left ventricular assist device (LVAD) Heartmate III. Surg. Obes. Relat. Dis. 2019, 15, 1420–1421. [Google Scholar] [CrossRef]
- Maurer, M.S.; Horn, E.; Reyentovich, A.; Dickson, V.V.; Pinney, S.; Goldwater, D.; Goldstein, N.E.; Jiminez, O.; Teruya, S.; Goldsmith, S.; et al. Can a left ventricular assist device in individuals with advanced systolic heart failure improve or reverse frailty? J. Am. Geriatr. Soc. 2017, 65, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Kormos, R.L.; Pagani, F.; Myers, S.L.; Stevenson, L.W.; Givertz, M.M.; Young, J.B. Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J. Heart Lung Transpl. 2013, 32, 1205–1213. [Google Scholar] [CrossRef]
- Hasin, T.; Topilsky, Y.; Schirger, J.A.; Li, Z.; Zhao, Y.; Boilson, B.A.; Clavell, A.L.; Rodeheffer, R.J.; Frantz, R.P.; Edwards, B.S.; et al. Changes in Renal Function After Implantation of Continuous-Flow Left Ventricular Assist Devices. J. Am. Coll. Cardiol. 2012, 59, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Uriel, N.; Naka, Y.; Colombo, P.C.; Farr, M.; Pak, S.W.; Cotarlan, V.; Albu, J.B.; Gallagher, D.; Mancini, D.; Ginsberg, H.N.; et al. Improved diabetic control in advanced heart failure patients treated with left ventric-ular assist devices. Eur. J. Heart. Fail. 2011, 13, 195–199. [Google Scholar] [CrossRef]
- Bansal, A.; Uriel, N.; Colombo, P.C.; Narisetty, K.; Long, J.E.; Bhimaraj, A.; Cleveland, J.C.; Goldstein, D.J.; Stulak, J.M.; Najjar, S.S.; et al. Effects of a fully magnetically levitated centrifugal -flow versus axial-flow left ven-tricular assist device on the von Willebrand factor: A prospective multicenter trial. J. Heart Lung Transpl. 2019, 38, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Calson, L.A.; Maynes, E.J.; Choi, J.H.; Hallett, A.M.; Horan, D.P.; Weber, M.P.; Deb, A.K.; Patel, S.; Samuels, L.E.; Morris, J.R.; et al. Characteristics and outcomes of gastrointestinal bleeding in patients with continu-ous-flow left ventricular assist devices: A systematic review. Artific. Organs 2020, 44, 1150–1161. [Google Scholar] [CrossRef]
- Hullin, R.; Regamey, J.; Yerly, P.; Aur, S.; Abdurashidova, T.; Rancati, V.; Tozzi, P.; Kirsch, M. Advanced heart failure: When and what to consider for left ventricular assist device implantation. Cardiovasc. Med. 2021, 24, w10079. [Google Scholar]
- Saeed, D.; Muslem, R.; Rasheed, M.; Caliskan, K.; Kalampoka, N.; Sipahi, F.; Lichtenberg, A.; Jawad, K.; Borger, M.; Huhn, S.; et al. Less invasive surgical implant strategy and right heart failure after LVAD implanta-tion. J. Heart Lung Transpl. 2021, 40, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-A.; Kim, H.-K.; Kim, Y.-J.; Cho, G.-Y.; Oh, S.; Sohn, D.-W. Role of pericardium in the maintenance of left ventricular twist. Heart 2010, 96, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Khalpey, Z.; Bin Riaz, I.; Marsh, K.M.; Ansari, M.Z.A.; Bilal, J.; Cooper, A.; Paidy, S.; Schmitto, J.D.; Smith, R.; Friedman, M.; et al. Robotic Left Ventricular Assist Device Implantation Using Left Thoracotomy Approach in Patients with Previous Sternotomies. ASAIO J. 2015, 61, e44–e46. [Google Scholar] [CrossRef] [PubMed]
- Khalpey, Z.; Sydow, N.; Slepian, M.J.; Poston, R. How to do it: Thoracoscopic left ventricular assist device implantation using robot assistance. J. Thorac. Cardiovasc. Surg. 2013, 147, 1423–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Burkhoff, D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Farag, J.; Woldendorp, K.; McNamara, N.; Bannon, P.G.; Marasco, S.F.; Loforte, A.; Potapov, E.V. Contemporary outcomes of continuous-flow biventricular assist devices. Ann. Cardiothorac. Surg. 2021, 10, 311–328. [Google Scholar] [CrossRef]
- Noly, P.E.; Ali, B.E.; Lamarche, Y.; Carrier, M. Carrier, Status, Indications, and Use of Cardiac Replacement Therapy in the Era of Mul-timodal Mechanical Approaches to Circulatory Support: A Scoping Review. Can. J. Cardiol. 2020, 36, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Hullin, R. Heart transplantation: Current practice and outlook to the future. Swiss Med. Wkly. 2014, 144. [Google Scholar] [CrossRef]
- Colvin, M.; Smith, J.M.; Hadley, N.; Uccellini, K.; Goff, R.; Foutz, J.; Israni, K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 Annual Data Report: Heart. Am. J. Transpl. 2020, 20, 340–426. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Tozzi, P.; Regamey, J.; Abdurashidova, T.; Meyer, P.; Lefol, K.; Pascual, M.; Yerly, P.; Aubert, V.; Aur, S.; et al. Has the Profile of Heart Transplantation Recipients changed within the last 3 decades? An analysis from the Lausanne Heart Transplantation Center. Swiss Med. Wkly. 2022, 152, w30108. [Google Scholar]
- Stern, L.K.; Velleca, A.; Nishihara, K.; Shen, A.; Zaliznyak, M.; Patel, J.; Hamilton, M.A.; Ramzy, D.; Esmalian, F.; Kobashigawa, J.A.; et al. Impact of the United Network for organ sharing 2018 donor heart allocation sys-tem on transplant morbidity and mortality. Clin. Transpl. 2021, 35, e14181. [Google Scholar] [CrossRef] [PubMed]
- Jawitz, O.K.; Fudim, M.; Raman, V.; Bryner, B.S. Reassessing Recipient Mortality Under the New Heart Allocation System: An Up-dated UNOS Registry Analysis. JACC Heart Fail. 2020, 8, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Menachem, J.N.; Schlendorf, K.H.; Mazurek, J.A.; Bichell, D.P.; Brinkley, D.; Frischhertz, B.P.; Mettler, B.A.; Shah, A.S.; Zalawadiya, S.; Book, W.; et al. Advanced Heart Failure in Adults with Congenital Heart Disease. JACC Heart Fail. 2019, 8, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Dipchand, A.I.; Goldfarb, S.; Levvey, B.J.; Meider, B.; Rossano, J.W.; Yusen, R.D.; et al. The registry of the International Society of Heart and Lung Transplantation: Thirty-second official adult heart transplantation report: 2015; Theme: Early graft failure. J. Heart Lung Transpl. 2015, 34, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Cedars, A.; Vanderpluym, C.; Koehl, D.; Cantor, R.; Kutty, S.; Kirklin, J.K. An Interagency Registry for mechanically assisted circulatory support (INTER-MACS) analysis of hospitalization, functional status, and mortality after mechanical circulatory support in adults with congenital heart disease. J. Heart Lung Transpl. 2018, 37, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Monda, E.; Lioncino, M.; Pacileo, R.; Rubino, M.; Cirillo, A.; Fusco, A.; Esposito, A.; Verrillo, F.; Di Fraia, F.; Mauriello, A.; et al. Advanced Heart Failure in Special Population—Pediatric Age. Heart Fail. Clin. 2021, 17, 673–683. [Google Scholar] [CrossRef]
- Khush, K.K.; Zaroff, J.G.; Nguyen, J.; Menza, R.; Goldstein, B.A. National decline in donor heart utilization with regional variabil-ity: 1995-2010. Am. J. Transpl. 2015, 15, 642–649. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transpl. 2010, 29, 914–956. [Google Scholar] [CrossRef]
- Kransdorf, E.P.; Kittleson, M.M.; Benck, L.R.; Patel, J.K.; Chung, J.S.; Esmailian, F.; Kearney, B.L.; Chang, D.H.; Ramzy, D.; Czer, L.S.C.; et al. Predicted heart mass is the optimal metric for size match in heart trans-plantation. J. Heart Lung Transpl. 2019, 38, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Holzhauser, L.; Imamura, T.; Bassi, N.; Fujino, T.; Nitta, D.; Kanelidis, A.J.; Narang, N.; Kim, G.; Raikhelkar, J.; Murks, C.; et al. Increasing heart transplant donor pool by liberalization of size matching. J. Heart Lung Transpl. 2019, 38, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Habal, M.V.; Clerkin, K.J.; Latif, F.; Restaino, S.W.; Zorn, E.; Takeda, K.; Naka, Y.; Yuzefpolskaya, M.; Farr, M.A.; et al. De novo human leukocyte antigen allosensitization in Heartmate 3 versus Heartmate II left ventricular assist device recipients. ASAIO J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohaissen, M.A.; Virani, S.A. Allosensitization in Heart Transplantation: An Overview. Can. J. Cardiol. 2014, 30, 161–172. [Google Scholar] [CrossRef]
- Chaidaroglou, A.; Armenis, I.; Gkouziouta, A.; Bonios, M.J.; Kogerakis, N.; Fragoulis, S.; Leontiadis, E.; Zarkalis, D.; Stavridis, G.; Kaklamis, L.; et al. The effect of paracorporeal pulsatile biventricular assist devices on allo-sensitization in adults: A comparison with left ventricular assist devices. Transplant. Immunol. 2021, 69, 101477. [Google Scholar] [CrossRef] [PubMed]
- Nwakanma, L.U.; Williams, J.A.; Weiss, E.S.; Russell, S.D.; Baumgartner, W.A.; Conte, J.V. Influence of Pretransplant Panel-Reactive Antibody on Outcomes in 8,160 Heart Transplant Recipients in Recent Era. Ann. Thorac. Surg. 2007, 84, 1556–1563. [Google Scholar] [CrossRef]
- Kransdorf, E.P.; Kittleson, M.M.; Patel, J.K.; Pando, M.J.; Steidley, D.E.; Kobashigawa, J.A. Calculated panel- reactive antibody predicts outcomes on the heart trans-plant waiting list. J. Heart Lung Transpl. 2017, 36, 787–796. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Alvarez, R.; Pelberg, J.; Yoon, E.; Alsammak, M.; Geier, S.S.; Ruggia-Check, C.; Hassler, J.; Hoosain, J.; Brisco, M.A.; et al. Serum dilutions as a predictive biomarker for peri-operative desensitization: An exploratory approach to transplanting sensitized heart candidates. Transpl. Immunol. 2020, 60, 101274. [Google Scholar] [CrossRef]
- Chih, S.; Patel, J. Desensitization strategies in adult heart transplantation-will persistence pay off? J. Heart Lung Transpl. 2016, 35, 962–972. [Google Scholar] [CrossRef] [Green Version]
- Kobashigawa, J.; Colvin, M.; Potena, L.; Dragun, D.; Crespo-Leiro, M.G.; Delgado, J.F.; Olymbios, M.; Parameshwar, J.; Patel, J.; Reed, E.; et al. The management of antibodies in heart transplantation: An ISHLT consensus document. J. Heart Lung Transpl. 2018, 37, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Al Saadi, T.; Lawrecki, T.; Narang, L.; Joshi, A.; Sciamanna, C.; Pauwaa, S.; Macaluso, G.; Tatooles, A.; Pappas, P.; Cotts, W.; et al. Outcomes of pre- heart transplantation desensitization in a series of highly sensi-tized patients bridged with left ventricular assist devices. J. Heart Lung Transpl. 2021, 40, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Starling, R.C.; Armstrong, B.; Bridges, N.D.; Eisen, H.; Givertz, M.M.; Kfoury, A.G.; Kobashigawa, J.; Ikle, D.; Morrison, J.; Pinney, S.; et al. Accelerated Allograft Vasculopathy With Rituximab After Cardiac Transplan-tation. J. Am. Coll. Cardiol. 2019, 74, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Yerly, P.; Rotman, S.; Regamey, J.; Aubert, V.; Aur, S.; Kirsch, M.; Hullin, R.; Pascual, M. Complement blockade with eculizumab to treat acute symptomatic humoral rejec-tion after heart transplantation. Xenotransplantation 2022, 10, e12726. [Google Scholar]
- Bretschneider, H.J.; Hübner, G.; Knoll, D.; Lohr, B.; Nordbeck, H.; Spieckermann, P.G. Myocardial resistance and tolerance to ischemia: Physiological and biochem-ical basis. J. Cardiovasc. Surg. 1975, 16, 241–620. [Google Scholar]
- Copeland, H.; Hayanga, J.A.; Neyrinck, A.; MacDonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor heart and lung procurement: A consensus statement. J. Heart Lung Transpl. 2020, 39, 501–517. [Google Scholar] [CrossRef]
- Naito, N.; Funamoto, M.; Pierson, R.N.; D'Alessandro, D.A. First clinical use of a novel hypothermic storage system for a long-distance donor heart procurement. J. Thorac. Cardiovasc. Surg. 2019, 159, e121–e123. [Google Scholar] [CrossRef]
- Guenthart, B.A.; Krishnan, A.; Koyano, T.; La Francessca, S.; Chan, J.; Alassar, A.; Macarthur, J.W.; Shudo, Y.; Hiesinger, W.; Woo, Y.J. Extended Static Hypothermic Preservation In Cardiac Transplantation: A Case Report. Transpl. Proc. 2021, 53, 2509–2511. [Google Scholar] [CrossRef]
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.W.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Twen-tieth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transpl. 2017, 36, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef]
- Schroder, J.N. Successful Utilization of Extended Criteria Donor (ECD) Hearts for Transplanation- Results of the OCS Heart Expand Trial to Evaluate the Effectiveness and Safety of the OCS Heart System to Preserve and Assess ECD Hearts for Transplantation. J. Heart Lung Transpl. 2019, 38, S42. [Google Scholar] [CrossRef]
- Sponga, S.; Bonetti, A.; Ferrara, V.; Beltrami, A.P.; Isola, M.; Vendramin, I.; Finato, N.; Ortolani, F.; Livi, U. Preservation by cold storage vs ex vivo normothermic perfusion of marginal donor hearts: Clinical, histopathologic, and ultrastructural features. J. Heart Lung Transpl. 2020, 39, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Prichard, R.A.; Connellan, M.B.; Dhital, K.K.; Macdonald, P.S. Long distance heart transplantation: A tale of two cities. Intern. Med. J. 2017, 47, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Stamp, N.L.; Shah, A.; Vincent, V.; Wright, B.; Wood, C.; Pavey, W.; Cokis, C.; Chih, S.; Dembo, L.; Larbalestier, R. Successful Heart Transplant after Ten Hours Out-of-body Time using the TransMedics Organ Care System. Heart Lung Circ. 2015, 24, 611–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transpl. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Piperata, A.; Caraffa, R.; Bifulco, O.; Avesani, M.; Gerosa, G.; Bottio, T. Heart transplantation in the new era of extended donor criteria. J. Card. Surg. 2021, 36, 4828–4829. [Google Scholar] [CrossRef]
- Weiss, J.; Beyeler, F.; Immer, F.F. Heart Swisstransplant Heart Working Group (STAH) Heart allocation and transplantation in Switzerland since the introduction of the Swiss Organ Allocation System (SOAS). Swiss Med. Wkly. 2014, 144, w14057. [Google Scholar] [CrossRef]
- Cowger, J.; Sundareswaran, K.; Rogers, J.G.; Park, S.J.; Pagani, F.D.; Bhan, G.; Jask, B.; Farrar, D.J.; Slaughter, M.S. Predicting survival in patients receiving continuous flow left ventricular assist devices: The HeartMate II risk score. J. Am. Coll. Cardiol. 2013, 61, 313–321. [Google Scholar] [CrossRef] [Green Version]

| Level | Time to MCS |
|---|---|
| “crash and burn”: critical cardiogenic shock | within hours |
| “progressive decline”: inotrope dependence with progressive decline | within few days |
| “stable inotrope-dependent”: clinical stability with mild to moderate dose of intravenous inotropes or patients on temporary circulatory support without inotropes | within a few weeks |
| “recurrent advanced heart failure”: “recurrent” rather than “refractory” decompensation | within weeks to months |
| “exertion intolerant”: clinical stability, comfortable at rest but intolerant to exercise | variable |
| “exertion limited”: clinical stability, able to do mild activity but presentation of fatigue within a few minutes on any meaningful physical activity | variable |
| “advanced nyha 3”: clinical stability with a reasonable but variable level of physical activity and without recent decompensation | variable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hullin, R.; Meyer, P.; Yerly, P.; Kirsch, M. Cardiac Surgery in Advanced Heart Failure. J. Clin. Med. 2022, 11, 773. https://doi.org/10.3390/jcm11030773
Hullin R, Meyer P, Yerly P, Kirsch M. Cardiac Surgery in Advanced Heart Failure. Journal of Clinical Medicine. 2022; 11(3):773. https://doi.org/10.3390/jcm11030773
Chicago/Turabian StyleHullin, Roger, Philippe Meyer, Patrick Yerly, and Matthias Kirsch. 2022. "Cardiac Surgery in Advanced Heart Failure" Journal of Clinical Medicine 11, no. 3: 773. https://doi.org/10.3390/jcm11030773
APA StyleHullin, R., Meyer, P., Yerly, P., & Kirsch, M. (2022). Cardiac Surgery in Advanced Heart Failure. Journal of Clinical Medicine, 11(3), 773. https://doi.org/10.3390/jcm11030773





