Abstract
Objective. To assess the effects of neurostimulation (i.e., neuromuscular electrical stimulation (NMES) and pharyngeal electrical stimulation (PES)) in people with oropharyngeal dysphagia (OD). Methods. Systematic literature searches were conducted to retrieve randomised controlled trials in four electronic databases (CINAHL, Embase, PsycINFO, and PubMed). The methodological quality of included studies was assessed using the Revised Cochrane risk-of-bias tool for randomised trials (RoB 2). Results. In total, 42 studies reporting on peripheral neurostimulation were included: 30 studies on NMES, eight studies on PES, and four studies on combined neurostimulation interventions. When conducting meta analyses, significant, large and significant, moderate pre-post treatment effects were found for NMES (11 studies) and PES (five studies), respectively. Between-group analyses showed small effect sizes in favour of NMES, but no significant effects for PES. Conclusions. NMES may have more promising effects compared to PES. However, NMES studies showed high heterogeneity in protocols and experimental variables, the presence of potential moderators, and inconsistent reporting of methodology. Therefore, only conservative generalisations and interpretation of meta-analyses could be made. To facilitate comparisons of studies and determine intervention effects, there is a need for more randomised controlled trials with larger population sizes, and greater standardisation of protocols and guidelines for reporting.
1. Introduction
The aerodigestive tract facilitates the combined functions of breathing, vocalising, and swallowing. Any dysfunction in this system may lead to oropharyngeal dysphagia (OD) or swallowing problems [1]. OD can be the result of underlying diseases such as stroke or a progressive neurological disease (e.g., Parkinson’s disease, multiple sclerosis) or an adverse effect after head and neck oncological interventions (e.g., radiation or surgery) or intensive care treatment (e.g., intubation and tracheostomy). Prevalence estimates of OD have been reported to be as high as 50% in cerebral palsy [2], 80% in stroke and Parkinson’s disease, and over 90% in people with community-acquired pneumonia [3]. OD can have a severe impact on a person’s health as it may lead to dehydration, malnutrition, and even death. Research has identified inverse bidirectional relationships between decreased health-related quality of life and increased OD severity [4].
Traditional OD therapy may include physical interventions such as: bolus modification and management (e.g., adjusting the viscosity, volume, temperature and/or acidity of food and drinks); oromotor exercises; body and head postural adjustments; and swallow manoeuvres (e.g., manoeuvres to improve food propulsion into the pharynx and airway protection) [1]. Therapy may also include sensory stimulation, which involves applying techniques like thermal stimulation and chemical stimulation using natural agonists of polymodal sensory receptors (e.g., capsaicin, the spicy component of peppers) [5].
Another type of stimulation considered to be beneficial for promoting rehabilitation of swallowing dysfunction is acupuncture. This practice emerged from traditional Chinese medicine and exerts therapeutic effects by inserting thin needles at strategic places, termed acupuncture points, on the body surface aiming to rebalance the flow of energy or life force (‘qi’). Needles are then activated through specific manual movements or electrical stimulation. Although stimulation of acupuncture points seems to be associated with places where nerves, muscles, and connective tissues may be stimulated [6], their intrinsic mechanisms are still part of a continuing scientific debate on acupuncture.
Recently, an increasing number of studies have been published on alternative interventions aiming to enhance neural plasticity by using non-invasive brain stimulation (NIBS) techniques. Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are cortically or centrally applied NIBS techniques. Using electromagnetic induction, rTMS results in depolarisation of post-synaptic connections, whereas tDCS uses direct electrical current to shift the polarity of nerve cells [7]. Alternatively, electrical stimulation techniques like pharyngeal electrical stimulation (PES) and neuromuscular electrical stimulation (NMES) target the peripheral neural pathways [8]. NMES aims to strengthen muscular contractions during swallowing and uses stimulation by electrodes placed on the skin over the anterior neck muscles to activate sensory pathways [9,10,11]. In contrast, PES has been shown to drive neuroplasticity in the pharyngeal motor cortex through direct stimulation of the pharyngeal mucosa via intraluminal catheters [7].
Over the past decade, several reviews have been published on the effects of neurostimulation in patients with OD. Most of these reviews focused on selected types of neurostimulation: NMES [10,12], rTMS [13,14], tDCS [15], or rTMS and tDCS [16,17]. Only two systematic reviews included both cortical (rTMS and tDCS) and peripheral neurostimulation (PES and NMES) [18,19]. All reviews targeted interventions in post-stroke populations except one review that broadened inclusion criteria to patients with acquired brain injury including stroke [16]. To date, all systematic reviews on neurostimulation as a treatment for OD set boundaries for inclusion based on medical diagnoses.
The aim of this systematic review is to determine the effects of neurostimulation in people with OD without excluding populations based on medical diagnoses. Findings are based on the highest level of evidence only, namely randomised controlled trials (RCTs), and summarised by conducting meta-analyses. The results of this review will be presented in two companion papers. This paper (Part I) reports on pharyngeal and neuromuscular electrical stimulation (PES and NMES) while the second paper (Part II) will report on brain stimulation (i.e., rTMS and tDCS).
2. Methods
The methodology and reporting of this systematic review were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement and checklist (Supplementary Tables S1 and S2) which aim to enhance the essential and transparent reporting of systematic reviews [20,21]. The protocol for this review was registered at PROSPERO, the international prospective register of systematic reviews (registration number: CRD42020179842).
2.1. Information Sources and Search Strategies
Literature searches to identify studies were conducted on 6 March 2021, across four databases: CINAHL, Embase, PsycINFO, and PubMed. Publication dates of coverage ranged from 1937–2021, 1902–2021, 1887–2021, and 1809–2021, respectively. Additional searches, including checking the reference lists of eligible articles, were performed. Two main categories of terms were used in combination: (1) dysphagia and (2) randomised control trials. Search strategies were performed in all four electronic databases using subheadings (e.g., MeSH and Thesaurus terms) and free text terms. The full electronic search strategies for each database are reported in Table 1. To identify other literature beyond that found using these strategies, the reference lists of each eligible article were checked.

Table 1.
Search strategies.
2.2. Inclusion and Exclusion Criteria
Studies were included in this systematic review if they met the following criteria: (1) participants had a diagnosis of oropharyngeal dysphagia; (2) the study included non-invasive neurostimulation interventions aimed at reducing swallowing or feeding problems; (3) the study included a control group or comparison intervention group; (4) participants were randomly assigned to one of the study arms or groups; and (5) the study was published in the English language.
Interventions such as non-electrical peripheral stimulation (e.g., air-puff or gustatory stimulation), pharmacological interventions and acupuncture, were considered out of the scope of this review, and thus were excluded. Invasive techniques and/or those that did not specifically target OD (i.e., deep-brain stimulation studies after neurosurgical implementation of a neurostimulator) were also excluded. Conference abstracts, doctoral theses, editorials, and reviews were excluded.
Finally, only studies reporting on peripheral neurostimulation (i.e., PES and NMES) were included in this review (Part I). Studies on brain neurostimulation (i.e., rTMS and tDCS) will be reported on in a companion paper (Part II).
3. Systematic Review
3.1. Methodological Quality and Risk of Bias
The methodological quality of the included studies was assessed using the Revised Cochrane risk-of-bias tool for randomised trials (RoB 2) [22]. The RoB 2 tool identifies five domains to consider when assessing where bias may have been introduced into a randomised trial: (1) bias arising from the randomisation process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; and (5) bias in selection of the reported result. The RoB 2 gives a series of signalling questions for each domain whose answers give a judgement (i.e., “low risk of bias,” “some concerns,” or “high risk of bias”), which can be evaluated to determine a study’s overall risk of bias [22].
3.2. Data Collection Process
A data extraction form was created to extract data from the included studies under the following categories: participant diagnosis, inclusion and exclusion criteria, sample size, age, gender, intervention goal, intervention agent/delivery/dosage, outcome measures, and treatment outcome.
3.3. Data, Items and Synthesis of Results
Titles and abstracts of included studies were screened for eligibility by two independent reviewers, after which the eligibility of selected original articles was assessed by these same two reviewers. If agreement could not be reached between the first two reviewers, a third reviewer was consulted to reach consensus. Two independent researchers also assessed the methodological study quality and, where necessary, consensus was reached with involvement of a third reviewer. As none of the reviewers have formal or informal affiliations with any of the authors of the included studies, no evident bias in article selection or methodological study quality rating was present.
Data points across all studies were extracted using comprehensive data extraction forms. Risk of bias per individual study was assessed using the RoB 2 tool [22]. Data were extrapolated and synthesized using the following categories: participant characteristics, inclusion criteria, intervention conditions, outcome measures and intervention outcomes. Effect sizes and significance of findings were the main summary measures for assessing treatment outcome.
4. Meta-Analysis
Data Analysis. Data were extracted from each study to compare the effect sizes for the following: (1) pre-post outcome measures of OD and (2) mean difference between neurostimulation and comparison controls in outcome measures from pre- to post-intervention. Control groups may receive no treatment, sham stimulation and/or traditional dysphagia therapy (DT; e.g., bolus modification, oromotor exercises, body and head postural adjustments, and swallow manoeuvres). Only studies using instrumental assessment (e.g., videofluoroscopic swallow study (VFSS) or fiberoptic endoscopic evaluation of swallowing (FEES)) to confirm OD were included.
Data collected using outcome measures based on visuoperceptual evaluation of instrumental assessment were preferred over clinical non-instrumental assessments. Oral intake measures were only included if no other clinical data were available, whereas screening tools and patient self-report measures were excluded from meta-analyses altogether. When selecting outcome measures for meta-analyses, reducing heterogeneity between studies was a priority. Consequently, measures other than the authors’ primary outcomes may have been preferred if these measures contributed to greater homogeneity.
To compare effect sizes, group means, standard deviations, and sample sizes for pre- and post-measurements, data were entered into Comprehensive Meta-Analysis Version 3.3.070 [23]. If only non-parametric data were available (i.e., medians, interquartile ranges), data were converted into parametric data for meta-analytic purposes. Studies with multiple intervention groups were analysed separately for each experimental-control comparison. If studies included the same participants, only one study was included in the meta-analysis. For studies providing insufficient data for meta-analysis, authors were contacted by e-mail to request additional data.
Effect sizes were calculated in Comprehensive Meta-Analysis using a random-effects model since it was unlikely that studies would have similar true effects due to variations in sampling, participant characteristics, intervention approaches, and outcome measurements. Heterogeneity was estimated using the Q statistic to determine the spread of effect sizes about the mean and I2 was used to estimate the ratio of true variance to total variance. I2-values of less than 50%, 50% to 74%, and higher than 75% denote low, moderate, and high heterogeneity, respectively [24]. Effect sizes were generated using the Hedges’ g formula for standardized mean difference with a confidence interval of 95%. Effects sizes were interpreted using Cohen’s d convention as follows: g ≤ 0.2 as no or negligible effect; 0.2 < g ≤ 0.5 as small effect; 0.5 < g ≤ 0.8 as moderate effect; and g > 0.8 as large effect [25].
Forest plots of effect sizes for OD outcome scores were generated for PES and NMES separately: (1) pre-post neurostimulation and (2) neurostimulation interventions versus comparison groups. Subgroup analyses were used to explore effect sizes as a function of various moderators depending on neurostimulation type. For example, outcome measures, medical diagnoses, total treatment duration, total neurostimulation time, and stimulation characteristics (e.g., pulse duration, pulse rate, electrode configuration). To account for the possibility of spontaneous recovery during the intervention period, only between-subgroup meta-analyses were conducted using post-intervention data.
Comprehensive Data Analysis software was utilized to evaluate publication bias. The Begg and Muzumdar’s test [26] was used to calculate the rank correlation between the standardised effect size and the ranks of their variances. The Begg and Muzumdar test calculates both a tau and a two tailed p value, with values of close to zero indicating no correlation, while results closer to 1 suggest a correlation. Where asymmetry is the result of publication bias, high standard error values would correspond with larger effect sizes. Where larger effects correspond to low values, tau would be positive (with the inverse also being true). Conversely, when larger effects correspond to high values, tau would be negative.
Publication bias was also evaluated utilising a fail-safe N test. This measure addresses the question of how many omitted studies would be necessary to nullify the effect. It refers to the number of studies where the effect size was zero being included in the meta-analysis prior to the result becoming statistically insignificant [27]. When this value is comparably low, there may be reason to treat the results with caution. When the value is comparably high, however, it can be reasonably concluded that the treatment effect is not nil, although it may be increased due to the omission of some studies.
5. Results
5.1. Study Selection
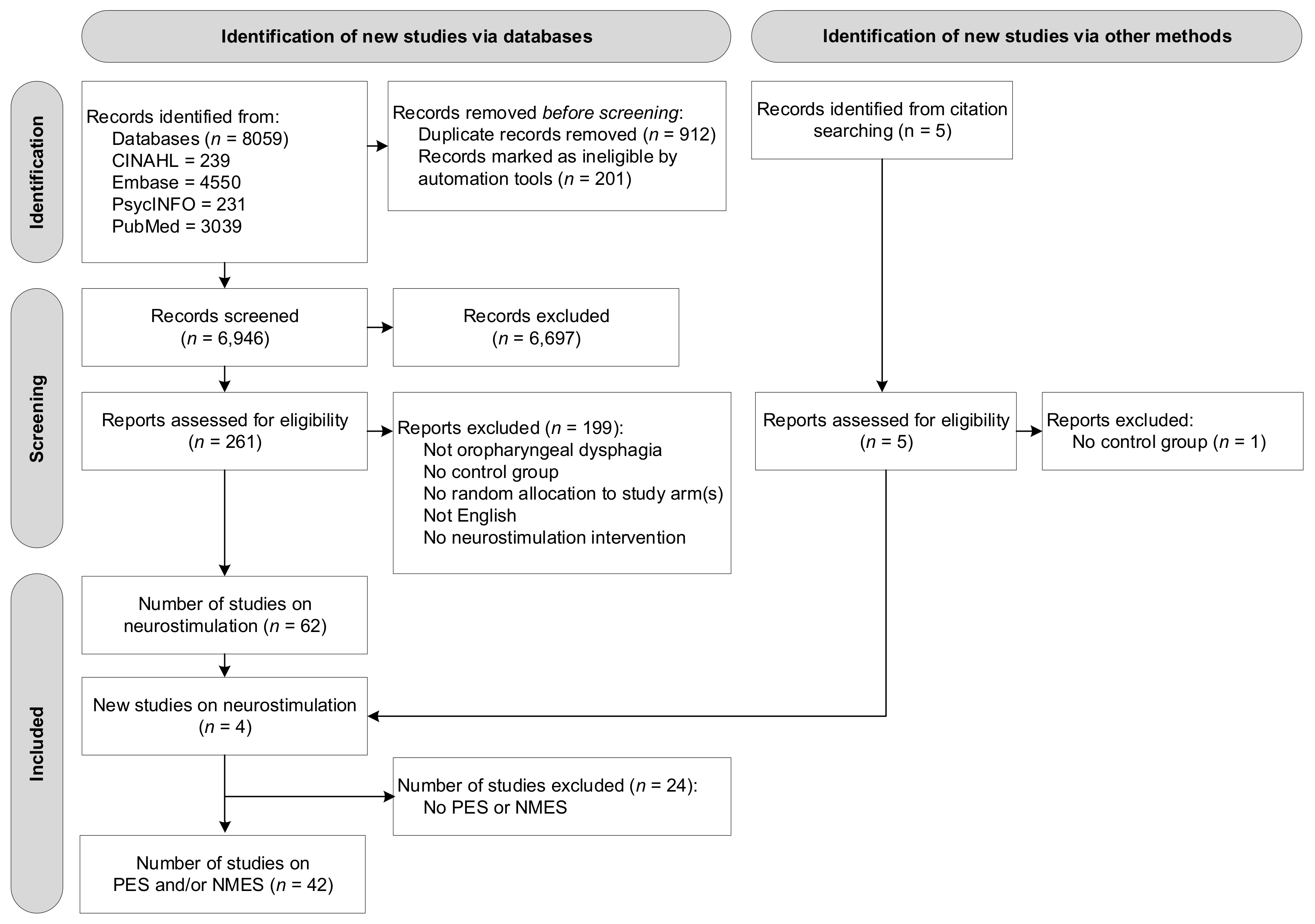
A total of 8059 studies were identified through subject heading and free text searches from the four databases: CINAHL (n = 239), Embase (n = 4550), PsycINFO (n = 231), and PubMed (n = 3039). Removing duplicate titles and abstracts (n = 1113) left a total of 6946 records. A total of 261 original articles were assessed at a full-text level, with articles grouped based on type of intervention. Four additional studies were found through reference checking of the included articles. At this stage, no studies were excluded based on type of intervention (e.g., behavioural intervention, neurostimulation). Of the reviewed 261 articles, 58 studies on neurostimulation were identified that satisfied the inclusion criteria. As this systematic review reports on PES and NMES interventions only, a final number of 42 studies reporting on peripheral neurostimulation were included in this review. Figure 1 presents the flow diagram of the reviewing process according to PRISMA.
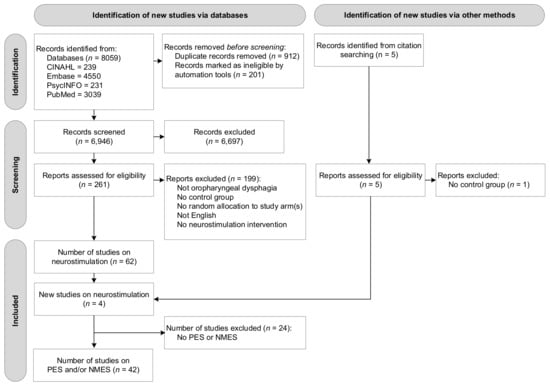
Figure 1.
Flow diagram of the reviewing process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
5.2. Description of Studies
All included studies are described in detail within Table 2 and Table 3. Specifically, Table 2 presents data on study characteristics including methodological study quality, inclusion and exclusion criteria, and details on participant groups. The following information is provided for all study groups (control and intervention groups): medical diagnosis, sample size, age and gender. Table 3 reports on intervention goals of included studies, intervention components, outcome measures, intervention outcomes, as well as main conclusions.

Table 2.
Study characteristics of studies on NMES and PES interventions for people with oropharyngeal dysphagia.

Table 3.
Outcome of NMES and PES interventions for people with oropharyngeal dysphagia.
Peripheral Neurostimulation Interventions. Across the 42 included studies, 30 studies reported on NMES and eight studies reported on PES. Four studies used another type of neurostimulation (i.e., rTMS) in addition to NMES or PES, either within the same group or different treatment groups.
Participants (Table 2). The 42 studies included a total of 2281 participants (mean 54.3; SD 39.1). The sample sizes ranged from the smallest sample of 16 participants [60,61] to the largest sample of 162 participants [36]. By intervention type, samples were characterized as follows: NMES total 1706, mean 56.9, SD 38.9, range 18–135; PES total 410, mean 51.3, SD 49.0, range 16–162; and combined neurostimulation total 165, mean 41.3, SD 19.3, range 18–64. The mean age of participants across all studies was 61.8 years (SD 15.3), with one study reporting age range only (65–93 years) [61]. Participant mean age across all studies ranged from 4.2 years [54] to 84.4 years [39]. The mean age of participants by intervention group was: NMES 60.9 years (SD 16.9), PES 64.7 years (SD 11.9), and combined neurostimulation 63.8 years (SD 6.4).
Across all studies, 61.0% (SD 13.5) of participants were male and one study did not report gender distribution [30]. Percentage of males by intervention group was NMES 62.6% (SD 14.0), PES 56.7% (SD 9.6), and other/combined 65.4% (SD 12.3). Most studies included stroke patients (n = 31), while three studies included mixed populations [28,41,43] and one study reported OD without further underlying medical diagnosis [39]. Other diagnoses by intervention group were: Parkinson’s disorder (n = 2)[32,46], cerebral palsy (n = 2) [50,54], and head and neck cancer (n = 2) [36,48] in NMES; and multiple sclerosis (n = 1) [63] in PES.
Across the 42 studies, VFSS was most frequently used to confirm participant’s diagnosis of OD (n = 31), whereas six studies used FEES [49,53,54,60,64,65]. Several of these studies combined instrumental assessment with either a screen (n = 2) [58,65] or clinical assessment (n = 6) [49,50,53,54,55,68]. One study used either clinical assessment or VFSS [50]. One study used a single screen [56], three studies used clinical assessment only [35,38,59], and one study used both [33]. The studies were conducted across 14 countries, with studies most frequently conducted in Korea (n = 11), China (n = 7), the UK (n = 7), Spain (n = 4), Italy (n = 2), Turkey (n = 2), and Germany (n = 2).
Outcome Measures (Table 2). Outcomes measures varied greatly across all studies included in the review, covering several domains within the area of OD. The Penetration Aspiration Score was the most reported outcome measure (PAS; 18 studies), followed by Functional Oral Intake Scale (FOIS; 12 studies), Functional Dysphagia Scale (FDS; 5 studies), Dysphagia Severity Rating Scale (DSRS; 5 studies), Swallowing Quality of Life questionnaire (SWAL-QOL; 4 studies), and Dysphagia Outcome and Severity Scale (DOSS; 3 studies).
NMES Intervention (n = 30: Table 2 and Table 3). In total, 22 studies included two study arms or groups, whereas eight studies included three groups [31,32,33,34,38,40,55,57]. All but five NMES studies [29,39,43,53,54] combined neurostimulation with simultaneous DT consisting of a wide range of behavioural interventions (e.g., head and body positioning, bolus modification, oromotor exercises, or swallow manoeuvres). Six studies included a NMES only group without DT [29,33,38,39,43,55], with five of these studies using NMES at motor stimulation level [29,33,38,43,55] and one study using NMES at sensory stimulation level [39]. An additional seven studies included a treatment arm with NMES at sensory stimulation level combined with DT [32,44,45,46,53,54,57]. All other participants in NMES groups received stimulation at motor level. Five studies compared different NMES electrode positions [28,34,40,41,42] and seven studies included a sham stimulation group [36,39,48,50,52,53,54].
Control groups included mostly sham NMES stimulation and/or DT. Only one study included a control group receiving neither DT nor NMES [30], and one study included usual care across different healthcare settings as the comparison group [51].
PES Intervention (n = 8: Table 2 and Table 3). All eight studies compared PES to a sham version of the treatment [58,59,60,61,62,63,64,65]. None of the studies included other treatment groups (e.g., DT) or control groups (e.g., usual care or no treatment).
Combined Neurostimulation Interventions (n = 4: Table 2 and Table 3). Three studies in the combined intervention group compared three different treatments. Of these, one study compared PES, paired associative stimulation (PAS) and rTMS [68], a second study compared DT, rTMS combined with DT, and NMES combined with DT [67], and a third study compared rTMS, PES and capsaicin stimulation [66]. A fourth study combined NMES stimulation with sham rTMS or rTMS stimulating different hemispheres (ipsilesional, contralesional or bilateral) [69].
5.3. Risk of Bias Assessment and Methodological Quality
The tau values from the Begg and Mazumdar rank correlation were 0.101 (two-tailed p = 0.589) and < 0.000 (two-tailed p > 0.999) for NMES and PES, respectively. The NMES meta-analysis incorporates data from 16 studies, which yielded a z-value of 4.107 (two-tailed p < 0.001). The fail-safe N is 55 indicating 55 ‘null’ studies need to be located and included for the combined two-tailed p-value to exceed 0.050. Therefore, there would need to be 3.4 missing studies for every observed study for the effect to be nullified. The PES meta-analysis incorporates data from five studies yielding a z-value of 1.156 (two-tailed p < 0.248). Since the combined result is not statistically significant, the fail-safe N (which addresses the concern that the observed significance may be spurious) is not relevant. Both of these procedures (i.e., Begg and Mazumdar rank correlation and fail-safe N) indicate the absence of publication bias.
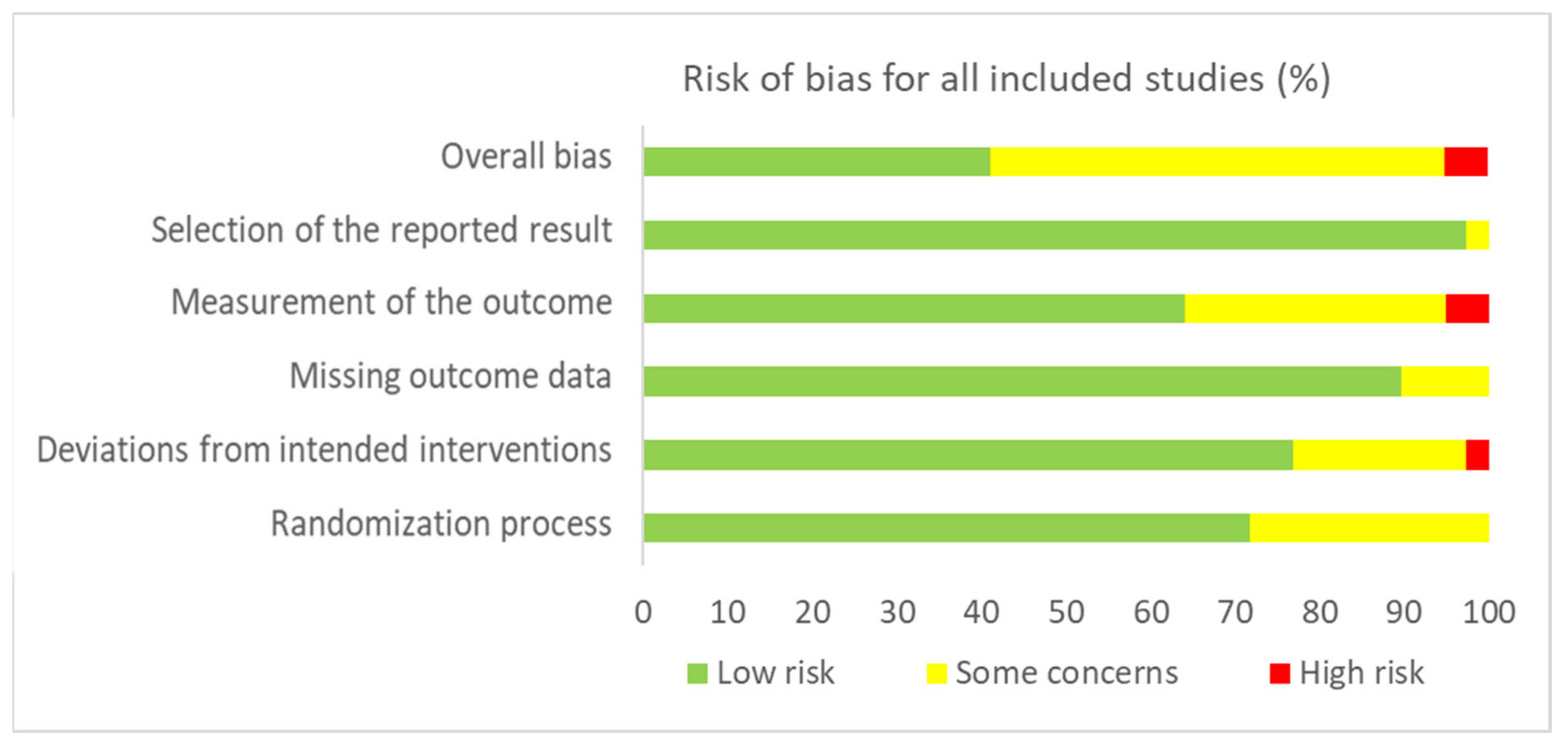
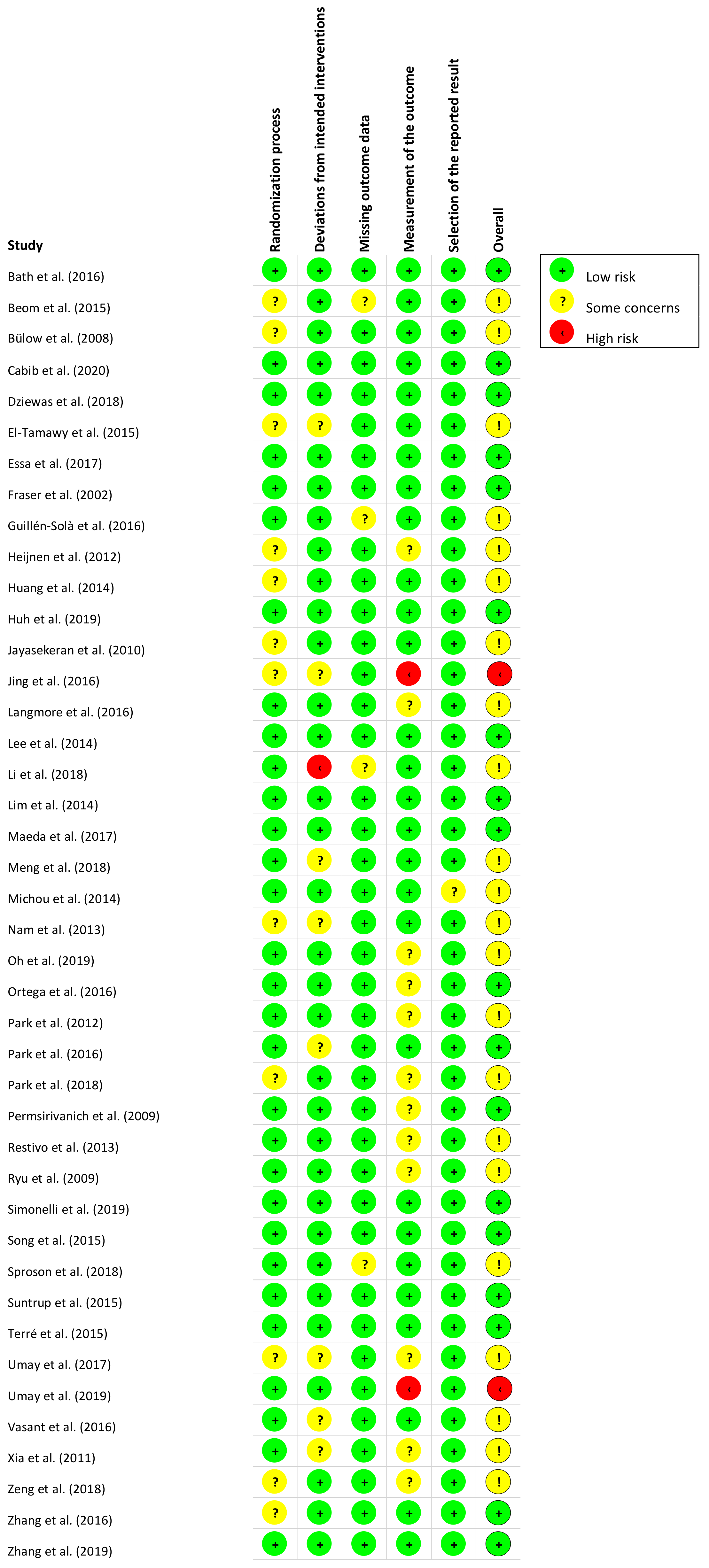
Figure 2 and Figure 3 present, respectively, the risk of bias summary per domain for all included studies combined and for individual studies. The majority of studies had low risk of bias with very few exceptions.
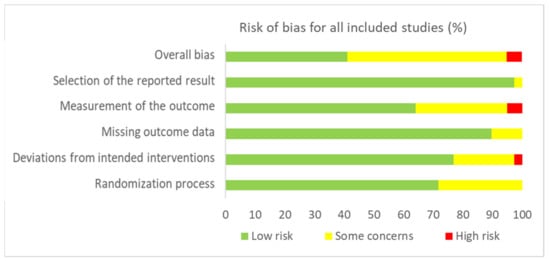
Figure 2.
Risk of bias summary for all included studies (n = 42) in accordance with RoB-2.
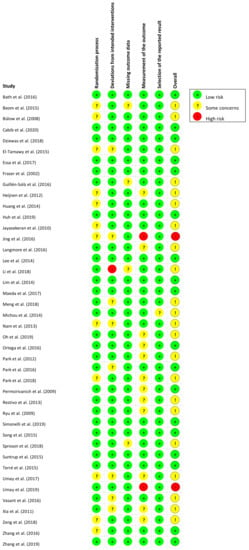
Figure 3.
Risk of bias summary for individual studies (n = 42) in accordance with RoB-2.
6. Meta-Analysis: Effects of Interventions
6.1. Neuromuscular Electrical Stimulation (NMES) Meta-Analysis
Eleven studies were included in the NMES meta-analysis [28,29,34,37,40,42,45,47,49,51,55], of which six studies included two or three different intervention groups [28,34,40,42,45,55]. A total of 20 studies were excluded from meta-analysis for the following reasons: in three studies, OD diagnosis was not confirmed by instrumental assessment (VFSS or FEES); five studies provided insufficient data for meta-analyses; and, twelve studies were excluded to reduce heterogeneity: six studies including subject populations with medical diagnoses other than stroke (i.e., children with cerebral palsy, head and neck cancer patients, patients with Parkinson’s disease, and elderly), five studies because of outcome measures (e.g., kinematic or biomechanical variables in VFS recordings), and one study using sensory NMES stimulation.
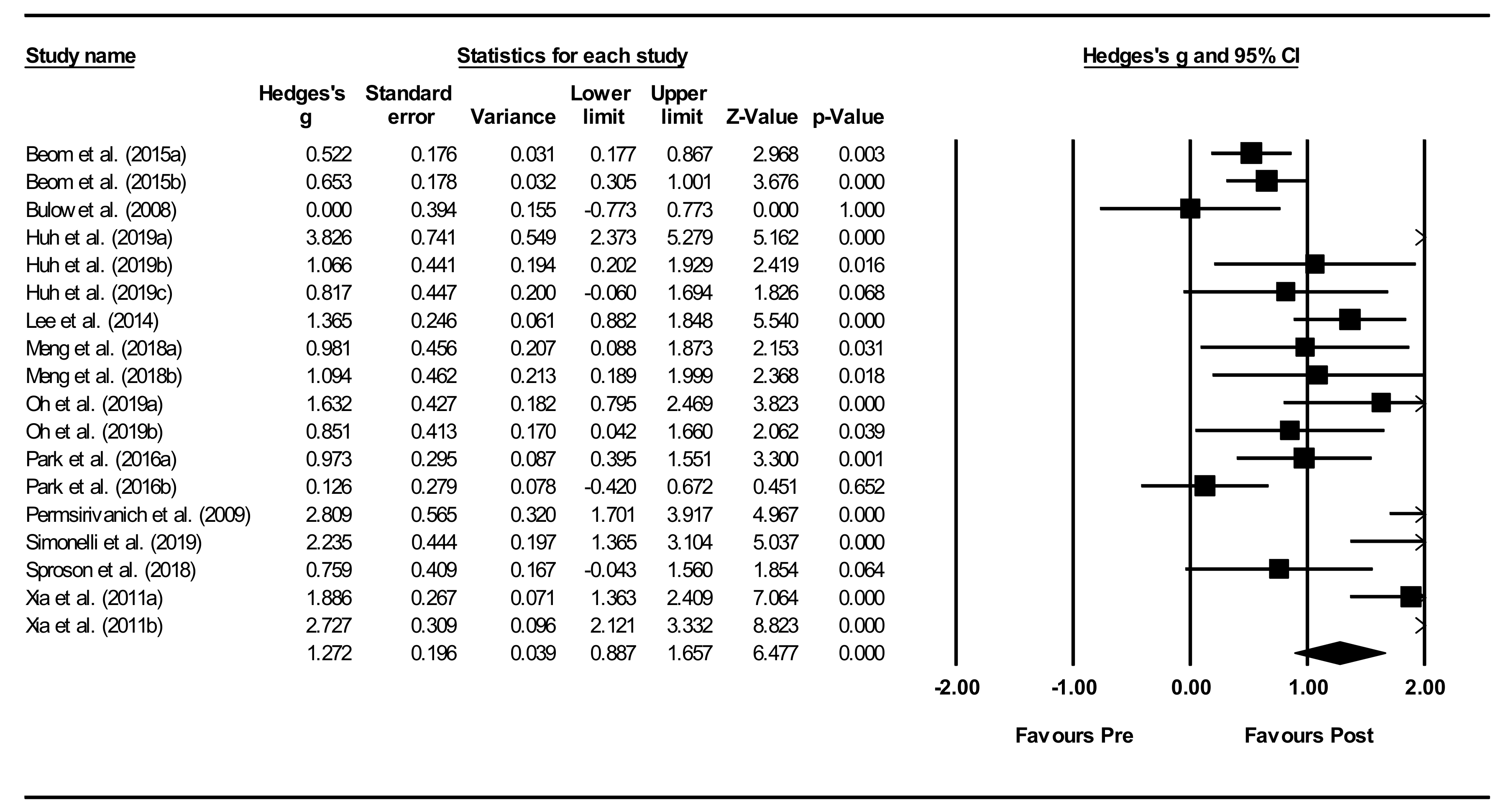
Overall within-group analysis (Figure 4). A significant, large pre-post intervention effect size was calculated using a random-effects model (z(17) = 6.477, p < 0.001, Hedges’ g = 1.272, and 95% CI = 0.887–1.657). Pre-post intervention effect sizes ranged from 0.000 to 3.826. In 13 of the 18 NMES intervention groups, effect sizes were large (Hedges’ g > 0.8), indicating that NMES accounted for a significant proportion of standardized mean difference for these studies. Between-study heterogeneity was significant (Q(17) = 106.7, and p < 0.001), with I2 showing that heterogeneity accounted for 84.1% of variation in effect sizes across studies.
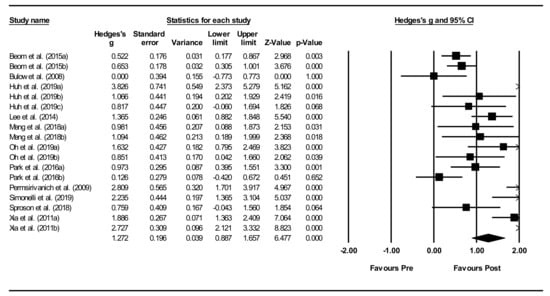
Figure 4.
Neuromuscular electrical stimulation (NMES) within intervention group pre-post meta-analysis [28,29,34,37,40,42,45,47,49,51,55]. Note. Refer to Table 2 for explanation of the subgroups.
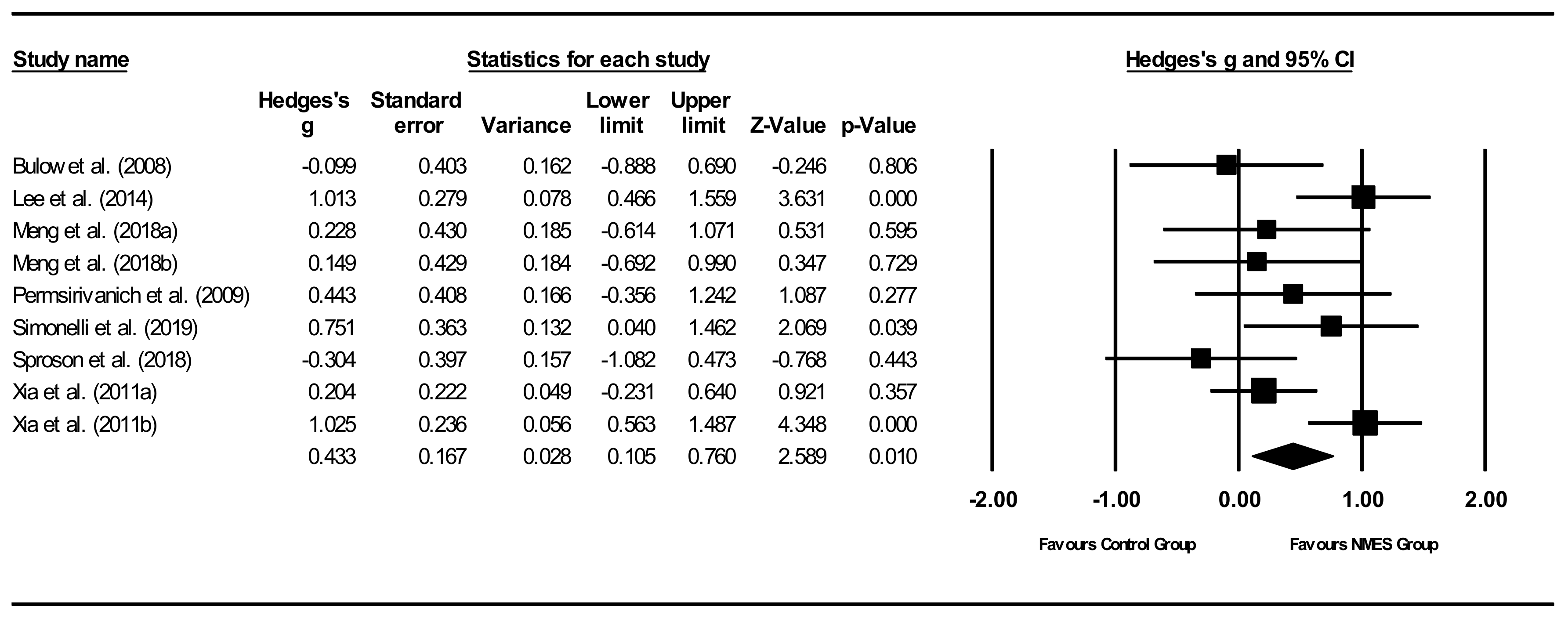
Overall between-group analysis (Figure 5). A significant, small post-intervention between-group total effect size in favour of NMES was calculated using a random-effects model (z(8) = 2.589, p = 0.010, Hedges’ g = 0.433, and 95% CI = 0.105–0.760). Between-study heterogeneity was significant (Q(8) = 18.0, and p = 0.021), with I2 showing that heterogeneity accounted for 55.6% of variation in effect sizes across studies.
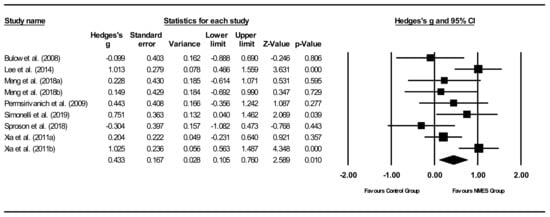
Figure 5.
NMES between group post meta-analysis [29,37,40,47,49,51,55]. Note. Refer to Table 2 for explanation of the subgroups.
Between-subgroup analyses. Subgroup analyses (Table 4) were conducted to compare diagnostic groups. Treatment effects were highest (moderate) for stroke patients, while other groups showed no significant effect sizes. For all other subgroup analyses, only stroke patients were included to improve homogeneity between studies. Subgroup analyses between studies compared intervention types (NMES, NMES + DT), time between pre- and post-intervention measurement, outcome measures, total stimulation times, electrodes configurations, pulse durations, and pulse rates (Table 4). NMES as an adjunctive treatment to DT showed significant, moderate positive treatment effects, whereas NMES alone showed non-significant effects. Effect sizes comparing time between pre- and post-treatment measurements showed no clear results. Although no effects could be identified at 2 weeks, a significant, positive effect size was found at 7 weeks. When comparing effect sizes based on outcome measures, the only significant effect found was a significant, large effect size for oral intake. The non-significant effects sizes for visuoperceptual evaluation of instrumental assessment ranged between negligible negative to moderate positive effects. Total stimulation time subgroup analyses showed significant, moderate positive treatment effects for longer stimulation times (>100 min). Shorter stimulation times did not result in significant effects. Comparisons for electrode configurations showed significant, moderate positive effects sizes for infrahyoid configuration. Electrode configuration based on patients’ characteristics, including OD outcome scores, indicated non-significant moderate effects, whereas both suprahyoid combined with infrahyoid and suprahyoid configurations resulted in negligible effects. Final comparisons between studies using different pulse durations did not suggest a linear relationship, whereas pulse rate comparisons indicated that studies using higher frequencies showed increased significant, positive moderate effect sizes.

Table 4.
Between subgroup meta-analyses for NMES and pharyngeal electrical stimulation (PES) comparing intervention groups of included studies.
6.2. Pharyngeal Electrical Stimulation (PES) Meta-Analysis
Five studies using PAS in adult stroke patients were included in the meta-analyses [58,62,65,66,68]. Three studies were excluded from meta-analyses for the following reasons: overlap in participant population between studies, insufficient data for meta-analyses, and no confirmation of OD diagnosis prior to treatment.
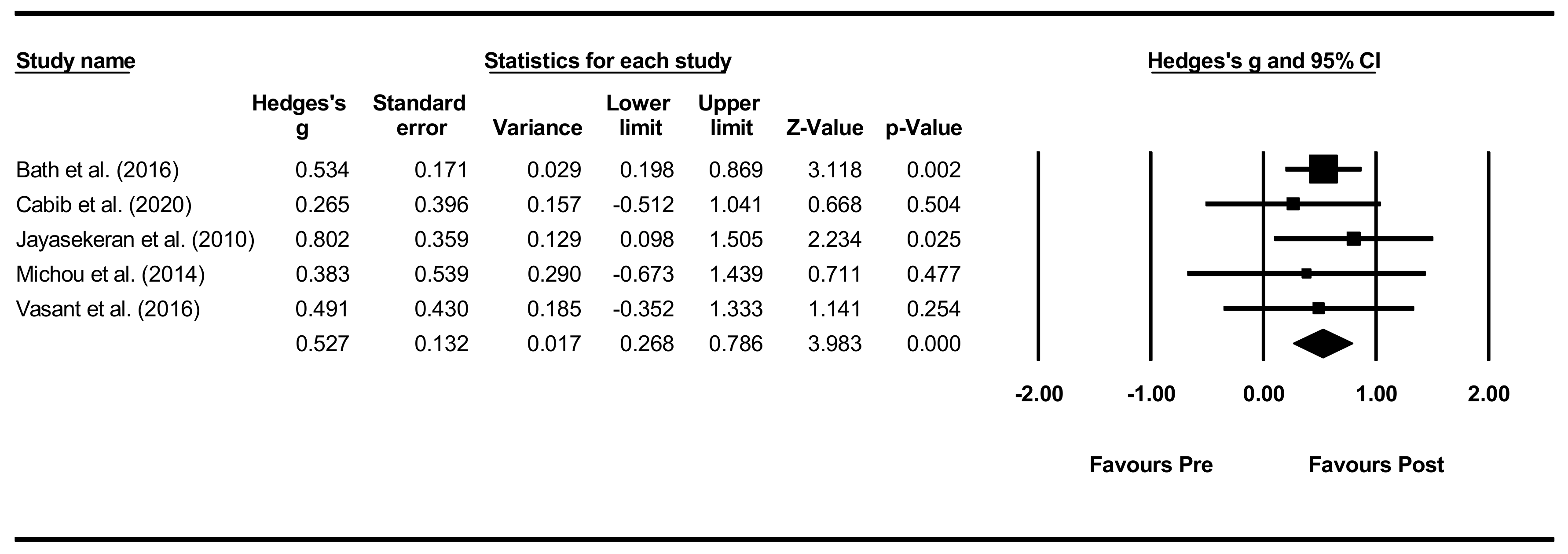
Overall within-group analysis. The pre-post intervention effect sizes for the included studies ranged from 0.265 (small effect) [66] to 0.802 (large effect) [62], with an overall moderate effect size of 0.527 (Figure 6). As one study, however, did not provide PAS data for all included participants [65], a sensitivity analysis was conducted for both PAS and DSRS, indicating minimal differences in effect sizes.
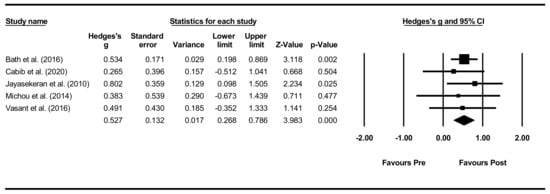
Figure 6.
PES within intervention group pre-post meta-analysis [58,62,65,66,68].
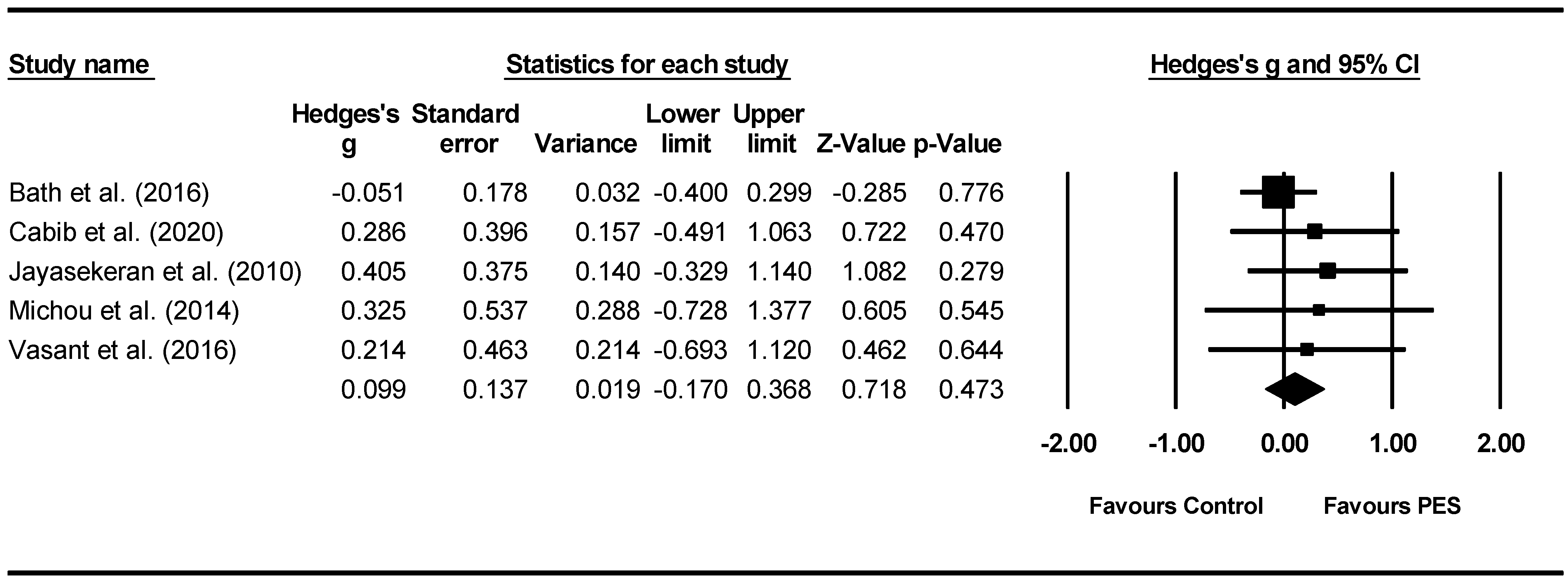
Overall between-group analysis. A non-significant post-intervention between-group total effect size in favour of PES was found using a random-effects model (z(4) = 0.718, p = 0.473, Hedges’ g = 0.099, and 95% CI = −0.170–0.368), suggesting no improvement in PAS outcomes following PES neurostimulation (Figure 7). Between-study heterogeneity was non-significant (Q(4) = 1.8, and p = 0.766).
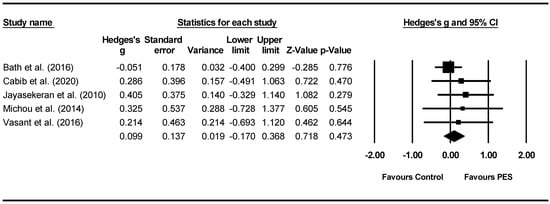
Figure 7.
PES between group post meta-analysis [58,62,65,66,68].
Between-subgroup analyses. Subgroup analyses were conducted (Table 4) comparing total stimulation time between studies, favouring shorter stimulation times (z(1) = 0.940, p = 0.347, Hedges’ g = 0.300, and 95% CI = −0.325–0.925).
7. Discussion
This study (Part I) aimed to determine the effects of PES and NMES in people with OD without excluding populations based on medical diagnoses. To base findings on the highest level of evidence, only RCTs were included. This systematic review and meta-analysis were conducted using PRISMA procedures as a guide.
7.1. Systematic Review Findings
When comparing RCTs in pharyngeal and neuromuscular electrical stimulation (i.e., PES and NMES), various methodological problems became apparent. Some studies did not define OD or used divergent definitions, whereas other studies applied different inclusion criteria. Most studies included patients with confirmed OD by instrumental assessment, but several studies used screening, patient self-report or clinical assessments instead. Consequently, participant characteristics may differ widely between studies. Despite most studies included stroke patients, meta-analysis comparing diagnostic groups other than stroke was possible for NMES, however this could not be conducted for PES.
Furthermore, the great variety in outcome measures also restricted comparisons by meta-analysis. As heterogeneity between studies indicates that no estimated overall effect by meta-analysis should be determined, combining studies targeting different domains within the area of OD will have similar implications. For instance, meta-analyses based on both patients’ self-reported health-related quality of life and visuoperceptual evaluation of instrumental assessments would very likely lead to inappropriate estimated overall effects. Thus, to reduce heterogeneity between outcome measures, some studies were excluded from the meta-analysis. This strong focus on reducing heterogeneity between studies when performing meta-analysis also implies that data other than the authors’ primary outcomes may have been preferably included in this analysis. For example, the primary outcome for Dziewas, Stellato, Van Der Tweel, Walther, Werner, Braun, Citerio, Jandl, Friedrichs, Nötzel, Vosko, Mistry, Hamdy, McGowan, Warnecke, Zwittag and Bath [59] and Suntrup, Marian, Schröder, Suttrup, Muhle, Oelenberg, Hamacher, Minnerup, Warnecke and Dziewas [64] was readiness for decannulation, which was considered too different from outcomes in the other included studies.
All eight PES studies compared neurostimulation with sham stimulation. However, among the 30 NMES studies, the comparison group variably consisted of usual care, DT, another dysphagia treatment or a combination of treatments. In contrast to PES studies that did not include any DT groups, most NMES studies combined neurostimulation with simultaneous DT. However, DT consisted of a wide range of behavioural interventions, using different treatment dosages, timings, and durations. Moreover, DT was referred to by many different names and acronyms (e.g., dysphagia training, behavioural intervention, classic treatment, or standard care). This suggest that care should be taken with the use of DT as an overarching term to group many different behavioural interventions to estimate overall effect sizes in meta-analyses.
Furthermore, RCTs are characterised by random allocation of participants to intervention groups and blinding or masking the nature of treatment for participants. However, in neurostimulation studies, blinding is frequently not feasible and participants may identify what treatment arm they have been assigned to (e.g., the presence of neurostimulation equipment, the experience of active stimulation). Also, since neurostimulation thresholding in PES is frequently applied in all groups to mask treatment assignment, patients receiving sham stimulation would still have been exposed to a certain level of neurostimulation during thresholding. Those studies not using thresholding in sham groups (e.g., [59,64]) might show larger treatment effect differences when comparing neurostimulation versus sham stimulation.
7.2. NMES
When considering meta-analyses for NMES, the highest effect sizes were found for stroke populations. As existing reviews in NMES [10,12,18,19] excluded other patient populations, no comparisons could be made between clinical populations. In addition, only two reviews conducted meta-analyses [18,19] selecting studies using different inclusion criteria (e.g., excluding comparison groups with active treatment components [18] or excluding chronic stroke patients [19]). Reviews may also prefer different outcome data for meta-analyses, especially in the case of RCTs using a large battery of assessments. As such, total numbers of included studies vary per review, but comparisons between reviews may be falsely estimated due to differences in methodology.
In this systematic review, a wide range in effect sizes was found in NMES RCTs depending on outcome measures used. However, oral intake scales showed highest effects sizes when compared to visuoperceptual evaluation of instrumental assessment or clinical assessment. This might be explained by NMES treatment usually taking place over consecutive weeks, in contrast to other neurostimulation techniques (e.g., PES or rTMS) that may be restricted to limited sessions over a few days only.
The great heterogeneity between DT groups also impeded comparisons between NMES only, NMES plus DT, and DT-only groups. No RCTs provided adequate DT group data to be included in the meta-analysis. For NMES groups, only two studies were included. As a result, information about the effects of DT is lacking. The negligible effect sizes found for NMES without DT were based on only two studies and the moderate effect sizes for combined NMES and DT were based on a total of seven studies.
Most studies performed NMES at motor stimulation level, whereas only a few studies included a group receiving NMES at sensory stimulation level. As none of these latter studies could be included in meta-analyses, no further details are available on comparisons between effect sizes for sensory versus motor stimulation. Also, terminology was confusing as sensory stimulation was sometimes referred to as sham stimulation [39].
NMES studies showed marked variation in the technical parameters and protocols applied. When comparing electrode configurations, both hyoid and combined hyoid and suprahyoid configurations showed negligible effects, whereas infrahyoid configurations resulted in moderate effects. A study using patient-dependent configurations showed promising results as well [55]. However, it remained unclear which criteria were used to decide on individual configurations. Furthermore, reporting on many technical parameters proved to be either incomplete or unclear for several studies (e.g., data on pulse duration, pulse rate, or stimulation time). As technical parameters may depend on medical device manufacturers, comparisons between brands may be warranted. For example, when considering pulse duration, a clear distinction in effect sizes is found between one study using a lower pulse rate—indicating a negative effect size—versus eight studies using higher pulse rates with moderate effect sizes.
7.3. PES
Compared to NMES, fewer PES studies were identified and thus a more limited meta-analysis was conducted. RCTs included stroke populations, except for one study that included patients with multiple sclerosis [63]. All studies compared active PES with sham treatment in stroke patients and used mostly visuoperceptual evaluation of radiographic recordings of the swallowing act as an outcome measure. Meta-analysis identified a non-significant post-intervention between-group total effect size in favour of PES. This finding seemed in line with findings by Chiang, Lin, Hsiao, Yeh, Liang and Wang [19], but this comparison is limited as it is based on only two studies. Additionally, Bath, Lee and Everton [18] reported that PES studies did not show an effect for many outcome measures (e.g., post-treatment proportions of participants with dysphagia, swallowing ability, penetration and aspiration scores or nutrition). However, in contrast to previous reviews, Cheng, Sasegbon and Hamdy [7] found a significant, moderate effect size in favour of PES when conducting meta-analysis. Again, inclusion criteria between reviews differed. For example, two studies [59,64] were excluded from meta-analysis in this review as well as the reviews by Chiang, Lin, Hsiao, Yeh, Liang and Wang [19] and Bath, Lee and Everton [18], but were included in the review by Cheng, Sasegbon and Hamdy [7]. This may have impacted the overall effect size as both PES studies showed significant treatment effects.
7.4. Moderators
Differences between NMES and PES studies made comparisons between RCTs difficult and hindered meta-analyses. Studies used different participant inclusion criteria in relation to underlying medical diagnoses or chronicity of stroke and used a large variety of outcome measures covering different domains within the area of OD. Outcome measures may also lack responsiveness, thus lack sensitivity to change during treatment. Moreover, studies varied significantly in technical parameters of neurostimulation. The number of studies and participants restricted the ability of statistical analyses to consider how each variable may have impacted the effects of neurostimulation.
Studies frequently neglected to report on potential moderators of stimulation effects in sufficient detail. For example, stroke severity and OD severity are inextricably linked and may moderate stimulation effects, yet only very few studies provided data on stroke severity. Similar problems occur when the chronicity of a stroke is not reported or the possibility of spontaneous recovery is ignored. This is especially true during NMES treatment, which may span a period of several weeks. In addition, no consensus was reached regarding the optimal moment for outcome measurement. Consequently, in this review, between-subgroup meta-analyses were conducted using post-intervention data only, so that the possibility of spontaneous recovery during the intervention period was taken into consideration.
7.5. Limitations
Despite a rigorous reviewing process following PRISMA guidelines and the use of RoB 2 to reduce bias, this review is subject to some limitations. Only RCTs published in English were included in this current study. Thus, some RCTs may have been excluded based on language criteria when their findings could have contributed to the current meta-analysis. Furthermore, meta-analyses included mostly stroke studies, thereby not providing effect sizes for other diagnostic patient populations. However, the main limitation of this review originates from the high degree of heterogeneity between studies, making comparisons across studies challenging. As such, generalisations and meta-analyses should be interpreted with care.
8. Conclusions
Meta-analyses for RCTS in NMES found a significant, large pre-post intervention effect size and significant, small post-intervention between-group effect size in favour of NMES. For PES studies, the meta-analyses showed a significant, moderate effect size for pre-post intervention, whereas overall between-group analysis did not result in significant treatment effects. Based on these results, NMES seems to have a more promising outcome compared to PES. However, only careful generalisations and interpretations of these meta-analyses can be made due to the NMES studies showing high heterogeneity in protocols and experimental variables, including potential moderators, and featuring inconsistent methodological reporting.
There is a need for more RCTs with larger sample sizes in addition to the standardisation of protocols and guidelines for reporting. These changes would better facilitate comparisons of studies and help to determine intervention effects more definitively. Delphi studies involving international experts might allow for a consensus to be reached, thus supporting future research, comparability and generalisability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11030776/s1, Table S1: PRISMA 2020 for Abstracts Checklist; Table S2: PRISMA 2020 Checklist.
Author Contributions
Conceptualization: R.S., R.C., A.-L.S., L.B., S.H. Formal analysis: R.S., R.C. Methodology: R.S., R.C. Project administration: R.S., R.C. Validation: R.S., R.C. Writing—review & editing: R.S., R.C., A.-L.S., L.B., S.H., B.J.H., L.R., S.W.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Speyer, R. (Ed.) Behavioural Treatment of Oropharyngeal Dysphagia; Springer: Berlin, Germany, 2018. [Google Scholar]
- Speyer, R.; Cordier, R.; Kim, J.H.; Cocks, N.; Michou, E.; Wilkes-Gillan, S. Prevalence of drooling, feeding and swallowing problems in cerebral palsy across the lifespan: Systematic review and meta-analysis. Dev. Med. Child Neurol. 2019, 61, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Speyer, R.; Kertscher, B.; Denman, D.; Swan, K.; Cordier, R. Health-related quality of life in oropharyngeal dysphagia. Dysphagia 2018, 33, 141–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wu, L.; Fang, Q.; Shen, M.; Zhang, L.; Liu, X. Effects of capsaicin on swallowing function in stroke patients with dysphagia: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-González, S.; Torres-Castillo, S.; López-Gómez, R.E.; Estrada, I.J. Acupuncture points and their relationship with multireceptive fields of neurons. J. Acupunct. Meridian Stud. 2017, 10, 81–89. [Google Scholar] [CrossRef]
- Cheng, I.; Sasegbon, A.; Hamdy, S. Effects of neurostimulation on poststroke dysphagia: A synthesis of current evidence from randomised controlled trials. Neuromodulation Technol. Neural Interface 2021, 24, 1388–1401. [Google Scholar] [CrossRef]
- Michou, E.; Sasegbon, A.; Hamdy, S. Neurostimulation for the treatment of dysphagia after stroke: Behavioural treatment of oropharyngeal dysphagia. In Dysphagia: Diagnosis and Treatment, 2nd ed.; Ekberg, O., Ed.; Medical Radiology: Diagnostic Imaging; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Carnaby, G.D.; LaGorio, L.; Silliman, S.; Crary, M. Exercise-based swallowing intervention (McNeill Dysphagia Therapy) with adjunctive NMES to treat dysphagia post-stroke: A double-blind placebo-controlled trial. J. Oral Rehabil. 2020, 47, 501–510. [Google Scholar] [CrossRef]
- Alamer, A.; Melese, H.; Nigussie, F. Effectiveness of Neuromuscular Electrical Stimulation on Post-Stroke Dysphagia: A Systematic Review of Randomized Controlled Trials. Clin. Interv. Aging 2020, 15, 1521–1531. [Google Scholar] [CrossRef]
- Baijens, L.W.J.; Speyer, R.; Passos, V.L.; Pilz, W.; Van Der Kruis, J.; Haarmans, S.; Desjardins-Rombouts, C. Surface electrical stimulation in dysphagic parkinson patients: A randomized clinical trial. Laryngoscope 2013, 123, E38–E44. [Google Scholar] [CrossRef]
- Clark, H.; Lazarus, C.; Arvedson, J.; Schooling, T.; Frymark, T. Evidence-Based Systematic Review: Effects of Neuromuscular Electrical Stimulation on Swallowing and Neural Activation. Am. J. Speech Lang. Pathol. 2009, 18, 361–375. [Google Scholar] [CrossRef] [Green Version]
- Dionisio, A.; Duarte, I.C.; Patricio, M.; Castelo-Branco, M. Transcranial magnetic stimulation as an intervention tool to recover from language, swallowing and attentional deficits after stroke: A systematic review. Cerebrovasc. Dis. 2018, 18, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xing, G.; Guoqiang, X.; Jin, Y.; Tang, Q.; He, B.; McClure, M.A.; Liu, H.; Chen, H.; Mu, Q. Repetitive transcranial magnetic stimulation as an alternative therapy for dysphagia after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 289–298. [Google Scholar] [CrossRef]
- Marchina, S.; Pisegna, J.M.; Massaro, J.M.; Langmore, S.E.; McVey, C.; Wang, J.; Kumar, S. Transcranial direct current stimulation for post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2021, 268, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Momosaki, R.; Kinoshita, S.; Kakuda, W.; Yamada, N.; Abo, M. Noninvasive brain stimulation for dysphagia after acquired brain injury: A systematic review. J. Med. Investig. 2016, 63, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisegna, J.M.; Kaneoka, A.; Pearson, W.G.; Kumar, S.; Langmore, S.E. Effects of non-invasive brain stimulation on post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Clin. Neurophysiol. 2016, 127, 956–968. [Google Scholar] [CrossRef] [Green Version]
- Bath, P.M.; Lee, H.S.; Everton, L.F. Swallowing therapy for dysphagia in acute and subacute stroke (Review). Cochrane Database Syst. Rev. 2018, 10, CD000323. [Google Scholar]
- Chiang, C.-F.; Lin, M.-T.; Hsiao, M.-Y.; Yeh, Y.-C.; Liang, Y.-C.; Wang, T.-G. Comparative Efficacy of Noninvasive Neurostimulation Therapies for Acute and Subacute Poststroke Dysphagia: A Systematic Review and Network Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 739–750.e4. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; McKenzie, J.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis; Biostat: Englewood, NJ, USA, 2014; Volume 3. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R. The file drawer problem and tolerance for null results. Psychol. Bull. 1979, 86, 638–664. [Google Scholar] [CrossRef]
- Beom, J.; Oh, B.-M.; Choi, K.H.; Kim, W.; Song, Y.J.; You, D.S.; Kim, S.J.; Han, T.R. Effect of Electrical Stimulation of the Suprahyoid Muscles in Brain-Injured Patients with Dysphagia. Dysphagia 2015, 30, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bülow, M.; Speyer, R.; Baijens, L.; Woisard, V.; Ekberg, O. Neuromuscular Electrical Stimulation (NMES) in Stroke Patients with Oral and Pharyngeal Dysfunction. Dysphagia 2008, 23, 302–309. [Google Scholar] [CrossRef] [PubMed]
- El-Tamawy, M.S.; Darwish, M.H.; El-Azizi, H.S.; Abdelalim, A.M.; Taha, S.I. The influence of physical therapy on oropharyngeal dysphagia in acute stroke patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2015, 52, 201–205. [Google Scholar]
- Guillén-Solà, A.; Sartor, M.M.; Soler, N.B.; Duarte, E.; Barrera, M.C.; Marco, E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: A randomized controlled trial. Clin. Rehabilitation 2017, 31, 761–771. [Google Scholar] [CrossRef]
- Heijnen, B.J.; Speyer, R.; Baijens, L.W.J.; Bogaardt, H.C.A. Neuromuscular electrical stimulation versus traditionaltherapy in patients with Parkinson’s Disease and propharyngeal dysphagia: Effects on quality of life. Dysphagia 2012, 27, 336–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.-L.; Liu, T.-Y.; Huang, Y.-C.; Leong, C.-P.; Lin, W.-C.; Pong, Y.-P. Functional Outcome in Acute Stroke Patients with Oropharyngeal Dysphagia after Swallowing Therapy. J. Stroke Cerebrovasc. Dis. 2014, 23, 2547–2553. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.; Park, E.; Min, Y.; Kim, A.; Yang, W.; Oh, H.; Nam, T.; Jung, T. Optimal placement of electrodes for treatment of post-stroke dysphagia by neuromuscular electrical stimulation combined with effortful swallowing. Singap. Med. J. 2020, 61, 487–491. [Google Scholar] [CrossRef]
- Jing, Q.; Yang, X.; Reng, Q. Effect of Neuromuscular Electrical Stimulation in Patients with Post-Stroke Dysphagia. Med. Sci. Technol. 2016, 57, 1–5. [Google Scholar] [CrossRef]
- Langmore, S.E.; McCulloch, T.M.; Krisciunas, G.P.; Lazarus, C.L.; Van Daele, D.J.; Pauloski, B.R.; Rybin, D.; Doros, G. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: A randomized clinical trial. Head Neck 2015, 38, E1221–E1231. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Ri, J.W.; Park, J.G. The Effect of Early Neuromuscular Electrical Stimulation Therapy in Acute/Subacute Ischemic Stroke Patients with Dysphagia. Ann. Rehabil. Med. 2014, 38, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Huang, R.; Yin, J.; Shen, Y.; Shi, J. The value of adding transcutaneous neuromuscular electrical stimulation (VitalStim) to traditional therapy for post-stroke dysphagia: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2014, 51, 200–206. [Google Scholar]
- Maeda, K.; Koga, T.; Akagi, J. Interferential current sensory stimulation, through the neck skin, improves airway defense and oral nutrition intake in patients with dysphagia: A double-blind randomized controlled trial. Clin. Interv. Aging 2017, 12, 1879–1886. [Google Scholar] [CrossRef] [Green Version]
- Meng, P.; Zhang, S.; Wang, Q.; Wang, P.; Han, C.; Gao, J.; Yue, S. The effect of surface neuromuscular electrical stimulation on patients with post-stroke dysphagia. J. Back Musculoskelet. Rehabil. 2018, 31, 363–370. [Google Scholar] [CrossRef]
- Nam, H.S.; Beom, J.; Oh, B.-M.; Han, T.R. Kinematic Effects of Hyolaryngeal Electrical Stimulation Therapy on Hyoid Excursion and Laryngeal Elevation. Dysphagia 2013, 28, 548–556. [Google Scholar] [CrossRef]
- Oh, D.-H.; Park, J.-S.; Kim, H.-J.; Chang, M.-Y.; Hwang, N.-K. The effect of neuromuscular electrical stimulation with different electrode positions on swallowing in stroke patients with oropharyngeal dysphagia: A randomized trial. J. Back Musculoskelet. Rehabil. 2020, 33, 637–644. [Google Scholar] [CrossRef]
- Ortega, O.; Rofes, L.; Martin, A.; Arreola, V.; López, I.; Clavé, P. A Comparative Study Between Two Sensory Stimulation Strategies After Two Weeks Treatment on Older Patients with Oropharyngeal Dysphagia. Dysphagia 2016, 31, 706–716. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, Y.; Oh, J.C.; Lee, H.-J. Effortful Swallowing Training Combined with Electrical Stimulation in Post-Stroke Dysphagia: A Randomized Controlled Study. Dysphagia 2012, 27, 521–527. [Google Scholar] [CrossRef]
- Park, J.-S.; Oh, D.-H.; Hwang, N.-K.; Lee, J.H. Effects of neuromuscular electrical stimulation combined with effortful swallowing on post-stroke oropharyngeal dysphagia: A randomised controlled trial. J. Oral Rehabil. 2016, 43, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Oh, D.H.; Hwang, N.K.; Lee, J.H. Effects of neuromuscular electrical stimulation in patients with Parkinson’s disease and dysphagia: A ran-domized, single-blind, placebo-controlled trial. Neurorehabilitation 2018, 42, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Permsirivanich, W.; Tipchatyotin, S.; Wongchai, M.; Leelamanit, V.; Setthawatcharawanich, S.; Sathirapanya, P.; Boonmeeprakob, A. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimu-lation therapy among stroke Pptients with persistent pharyngeal dysphagia: A randomized controlled study. Med. J. Med. Assoc. Thail. 2009, 92, 259. [Google Scholar]
- Ryu, J.S.; Kang, J.Y.; Park, J.Y.; Nam, S.Y.; Choi, S.H.; Roh, J.L.; Kim, S.Y.; Choi, K.H. The effect of electrical stimulation therapy on dysphagia following treatment for head and neck cancer. Oral Oncol. 2009, 45, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, M.; Ruoppolo, G.; Iosa, M.; Morone, G.; Fusco, A.; Grasso, M.G.; Gallo, A.; Paolucci, S. A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: A pilot randomized controlled trial. Neurorehabilitation 2019, 44, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Park, J.H.; Lee, J.H.; Kim, M.Y. Effects of Neuromuscular Electrical Stimulation on Swallowing Functions in Children with Cerebral Palsy: A Pilot Randomised Controlled Trial. Hong Kong J. Occup. Ther. 2015, 25, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sproson, L.; Pownall, S.; Enderby, P.; Freeman, J. Combined electrical stimulation and exercise for swallow rehabilitation post-stroke: A pilot randomized control trial. Int. J. Lang. Commun. Disord. 2018, 53, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terré, R.; Mearin, F. A randomized controlled study of neuromuscular electrical stimulation in oropharyngeal dysphagia secondary to acquired brain injury. Eur. J. Neurol. 2015, 22, 687-e44. [Google Scholar] [CrossRef]
- Umay, E.K.; Yaylaci, A.; Saylam, G.; Gundogdu, I.; Gurcay, E.; Akcapinar, D.; Kirac, Z. The effect of sensory level electrical stimulation of the masseter muscle in early stroke patients with dysphagia: A randomized controlled study. Neurol. India 2017, 65, 734. [Google Scholar] [CrossRef]
- Umay, E.; Gurcay, E.; Ozturk, E.A.; Akyuz, E.U. Is sensory-level electrical stimulation effective in cerebral palsy children with dysphagia? A randomized controlled clinical trial. Acta Neurol. Belg. 2020, 120, 1097–1105. [Google Scholar] [CrossRef]
- Xia, W.; Zheng, C.; Lei, Q.; Tang, Z.; Hua, Q.; Zhang, Y.; Zhu, S. Treatment of post-stroke dysphagia by vitalstim therapy coupled with conventional swallowing training. J. Huazhong Univ. Sci. Technol. 2011, 31, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yip, J.; Cui, H.; Guan, L.; Zhu, H.; Zhang, W.; Du, H.; Geng, X. Efficacy of neuromuscular electrical stimulation in improving the negative psychological state in patients with cerebral infarction and dysphagia. Neurol. Res. 2018, 40, 473–479. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, T.; Zhang, Z.-B.; Zhu, X.; Fan, W.-G.; Pu, L.-J.; Chu, L.; Yue, S.-W. Effectiveness of Neuromuscular Electrical Stimulation on Patients with Dysphagia with Medullary Infarction. Arch. Phys. Med. Rehabil. 2016, 97, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Scutt, P.; Love, J.; Clavé, P.; Cohen, D.; Dziewas, R.; Hamdy, S. Pharyngeal electrical stimulation for treatment of dysphagia in subacute stroke: A randomised controlled trial. Stroke 2016, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dziewas, R.; Stellato, R.; van der Tweel, I.; Walther, E.; Werner, C.J.; Braun, T.; Citerio, G.; Jandl, M.; Friedrichs, M.; Nötzel, K.; et al. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): A prospective, single-blinded, randomised trial. Lancet Neurol. 2018, 17, 849–859. [Google Scholar] [CrossRef]
- Essa, H.; Vasant, D.H.; Raginis-Zborowska, A.; Payton, A.; Michou, E.; Hamdy, S. The BDNF polymorphism Val66Met may be predictive of swallowing improvement post pharyngeal electrical stimulation in dysphagic stroke patients. Neurogastroenterol. Motil. 2017, 29, e13062. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.; Power, M.; Hamdy, S.; Rothwell, J.; Hobday, D.; Hollander, I.; Tyrell, P.; Hobson, A.; Williams, S.; Thompson, D. Driving Plasticity in Human Adult Motor Cortex Is Associated with Improved Motor Function after Brain Injury. Neuron 2002, 34, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Jayasekeran, V.; Singh, S.; Tyrrell, P.; Michou, E.; Jefferson, S.; Mistry, S.; Gamble, E.; Rothwell, J.; Thompson, D.; Hamdy, S. Adjunctive functional pharyngeal elctrical stimulation reverses swallowing disability after brain lesions. Gastroentrology 2010, 138, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Restivo, D.A.; Casabona, A.; Centonze, D.; Ragona, R.M.; Maimone, D.; Pavone, A. Pharyngeal Electrical Stimulation for Dysphagia Associated with Multiple Sclerosis: A Pilot Study. Brain Stimul. 2013, 6, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Suntrup, S.; Marian, T.; Schröder, J.B.; Suttrup, I.; Muhle, P.; Oelenberg, S.; Hamacher, C.; Minnerup, J.; Warnecke, T.; Dziewas, R. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: A randomized controlled trial. Intensiv. Care Med. 2015, 41, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Michou, E.; O’Leary, N.; Vail, A.; Mistry, S.; Hamdy, S.; Greater Manchester Stroke Research Network. Pharyngeal electrical stimulation in dysphagia poststroke: A prospective, randomized single-blinded interventional study. Neurorehabilit. Neural Repair 2016, 30, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabib, C.; Nascimento, W.; Rofes, L.; Arreola, V.; Tomsen, N.; Mundet, L.; Palomeras, E.; Michou, E.; Clavé, P.; Ortega, O. Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic post-stroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2020, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-B.; Lee, H.-J.; Yoo, J.; Kwon, Y.-G. Effect of Low-Frequency rTMS and NMES on Subacute Unilateral Hemispheric Stroke with Dysphagia. Ann. Rehabil. Med. 2014, 38, 592–602. [Google Scholar] [CrossRef]
- Michou, E.; Mistry, S.; Jefferson, S.; Tyrrell, P.; Hamdy, S. Characterizing the Mechanisms of Central and Peripheral Forms of Neurostimulation in Chronic Dysphagic Stroke Patients. Brain Stimul. 2014, 7, 66–73. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, X.; Lu, R.; Yun, W.; Yun, H.; Zhou, X. Repetitive transcranial magnetic stimulation in combination with neuromuscular electrical stimulation for treatment of post-stroke dysphagia. J. Int. Med. Res. 2019, 47, 662–672. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).