Angiotensin-Converting Enzyme 2 (ACE2), Transmembrane Peptidase Serine 2 (TMPRSS2), and Furin Expression Increases in the Lungs of Patients with Idiopathic Pulmonary Fibrosis (IPF) and Lymphangioleiomyomatosis (LAM): Implications for SARS-CoV-2 (COVID-19) Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Demographics
2.2. Immunohistochemistry (IHC) Staining
2.3. Quantification of Staining
2.4. Statistical Analysis
3. Results
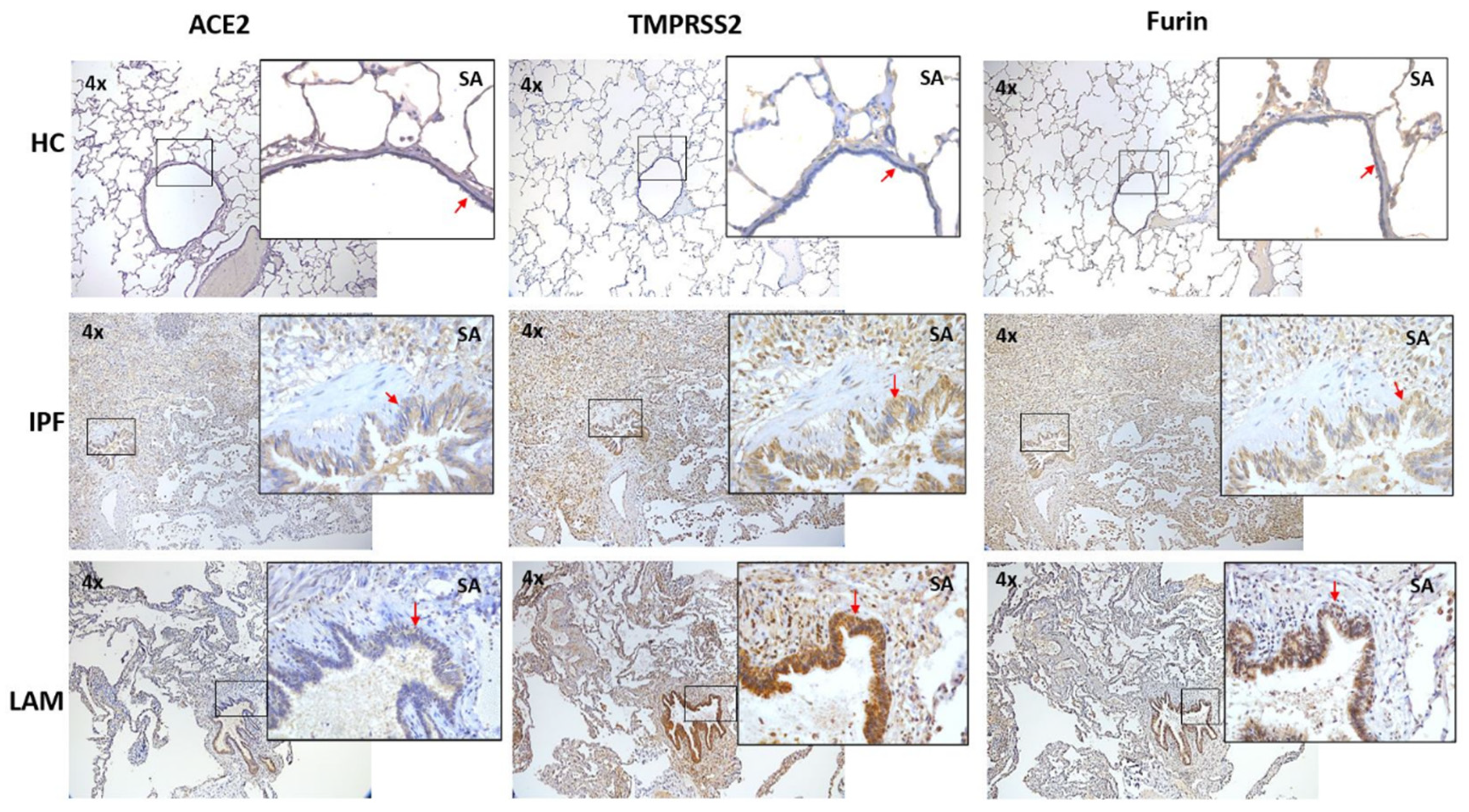
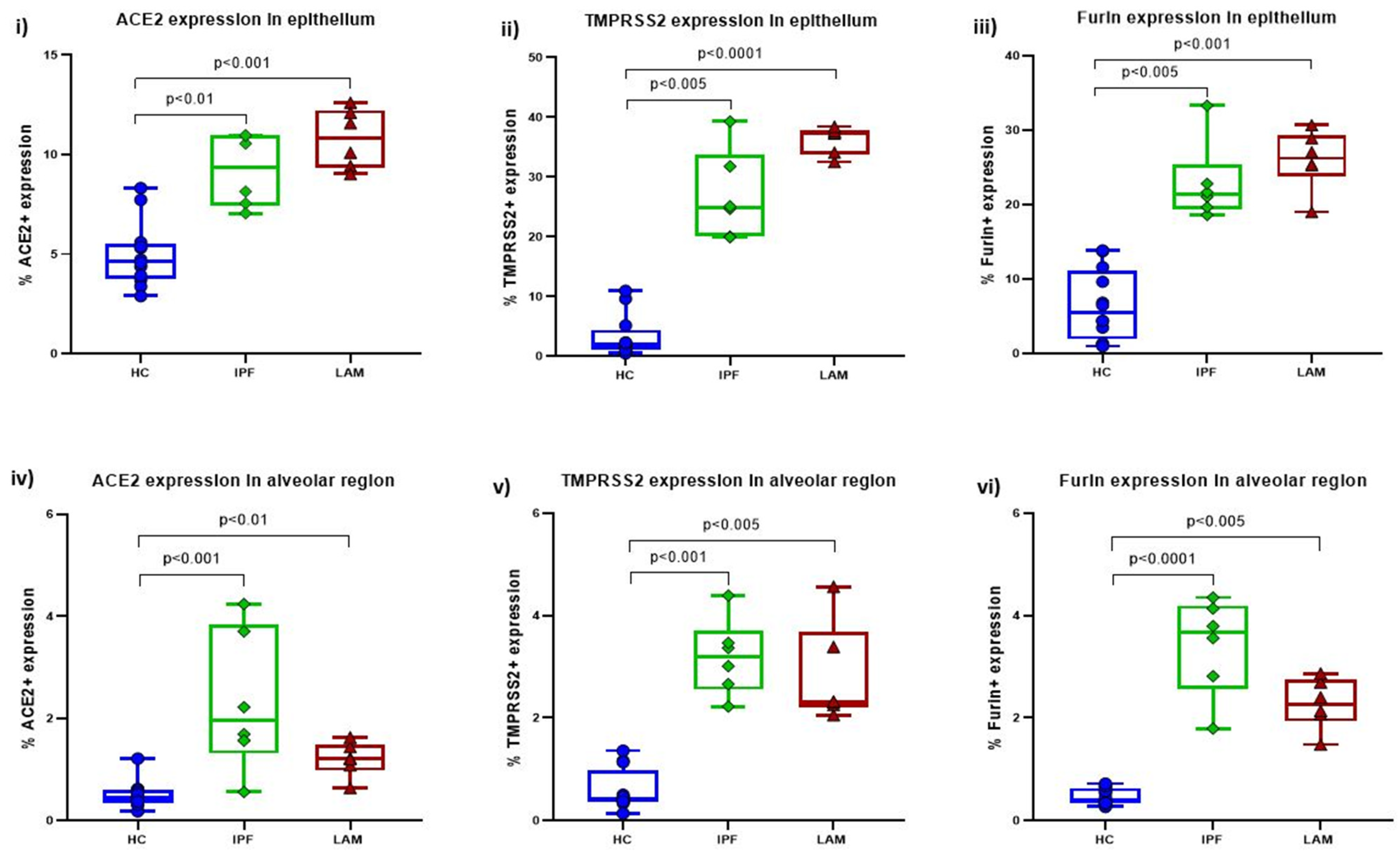
3.1. Increase in ACE2, TMPRSS2 and Furin in IPF and LAM Lungs
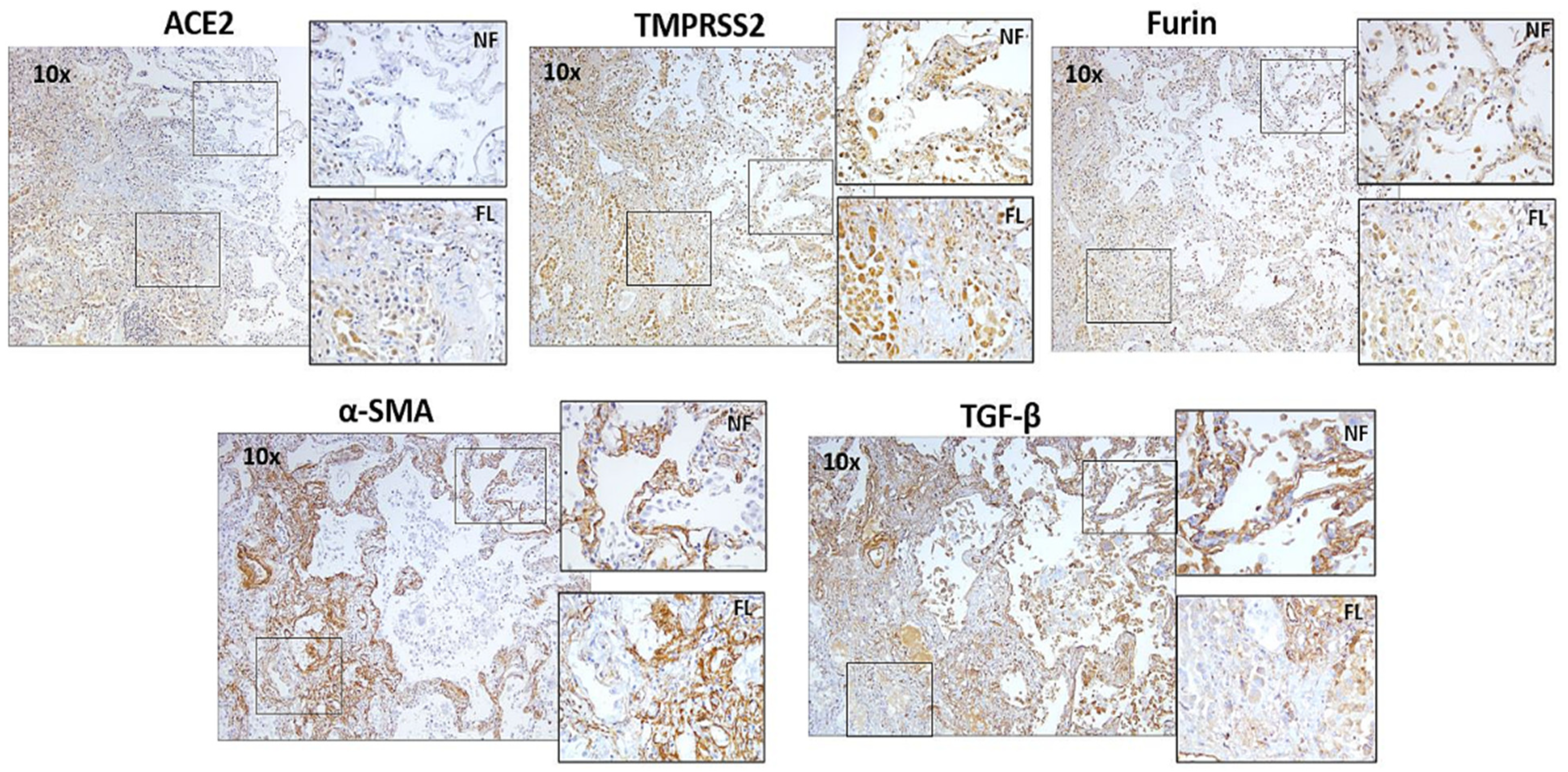
3.2. Descriptive Analysis of IPF and LAM Lung Tissue for α-SMA and TGF-β1
3.3. Correlation of ACE2 Expression and Smoking History in IPF Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronviras (COVID-19) Dashboard World Health Organization. 2021. Available online: https://covid19.who.int/ (accessed on 20 April 2021).
- Wark, P.A.; Pathinayake, P.S.; Eapen, M.S.; Sohal, S.S. Asthma, COPD and SARS-CoV-2 infection (COVID-19): Potential mechanistic insights. Eur. Respir. J. 2021, 58, 2100920. [Google Scholar] [CrossRef]
- Asrani, P.; Eapen, M.S.; Chia, C.; Haug, G.; Weber, H.C.; Hassan, M.I.; Sohal, S.S. Diagnostic approaches in COVID-19: Clinical updates. Expert Rev. Respir. Med. 2021, 15, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Patanavanich, R.; Glantz, S.A. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob. Res. 2020, 22, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Brake, S.J.; Barnsley, K.; Lu, W.; McAlinden, K.D.; Eapen, M.S.; Sohal, S.S. Smoking Upregulates Angiotensin-Converting Enzyme-2 Receptor: A Potential Adhesion Site for Novel Coronavirus SARS-CoV-2 (Covid-19). J. Clin. Med. 2020, 9, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [Green Version]
- Eapen, M.S.; Lu, W.; Hackett, T.L.; Singhera, G.K.; Thompson, I.E.; McAlinden, K.D.; Hardikar, A.; Weber, H.C.; Haug, G.; Wark, P.A.; et al. Dysregulation of endocytic machinery and ACE2 in small airways of smokers and COPD patients can augment their susceptibility to SARS-CoV-2 (COVID-19) infections. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L158–L163. [Google Scholar] [CrossRef]
- Eapen, M.S.; Lu, W.; Gaikwad, A.V.; Bhattarai, P.; Chia, C.; Hardikar, A.; Haug, G.; Sohal, S.S. Endothelial to mesenchymal transition: A precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur. Respir. J. 2020, 56, 2003167. [Google Scholar] [CrossRef]
- Eapen, M.S.; Gaikwad, A.V.; Thompson, I.E.; Lu, W.; Myers, S.; Sharma, P.; Sohal, S.S. The effectiveness of immunosuppressive cyclosporin in attenuating the progression of interstitial lung diseases. J. Thorac. Dis. 2019, 11, S1139–S1142. [Google Scholar] [CrossRef]
- Glassberg, M.K. Overview of idiopathic pulmonary fibrosis, evidence-based guidelines, and recent developments in the treatment landscape. Am. J. Manag. Care 2019, 25, S195–S203. [Google Scholar]
- Nalysnyk, L.; Cid-Ruzafa, J.; Rotella, P.; Esser, D. Incidence and prevalence of idiopathic pulmonary fibrosis: Review of the literature. Eur. Respir. Rev. 2012, 21, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Sgalla, G.; Iovene, B.; Calvello, M.; Ori, M.; Varone, F.; Richeldi, L. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respir. Res. 2018, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Rajasurya, V.; Gunasekaran, K.; Damarla, V.; Kolluru, A. A Fatal Case of Coronavirus Disease 2019 (COVID-19) in a Patient with Idiopathic Pulmonary Fibrosis. Cureus 2020, 12, e8432. [Google Scholar] [CrossRef] [PubMed]
- McCormack, F.X.; Travis, W.D.; Colby, T.V.; Henske, E.P.; Moss, J. Lymphangioleiomyomatosis: Calling it what it is: A low-grade, destructive, metastasizing neoplasm. Am. J. Respir. Crit. Care Med. 2012, 186, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Taveira-DaSilva, A.M.; Moss, J. Lymphangioleiomyomatosis. Clin. Chest Med. 2016, 37, 389–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathirareuangchai, S.; Shimizu, D.; Vierkoetter, K.R. Pulmonary Lymphangioleiomyomatosis: A Case Report and Literature Review. Hawaii J. Health Soc. Welf. 2020, 79, 224–229. [Google Scholar]
- McCormack, F.X.; Gupta, N.; Finlay, G.R.; Young, L.R.; Taveira-DaSilva, A.M.; Glasgow, C.G.; Steagall, W.K.; Johnson, S.R.; Sahn, S.A.; Ryu, J.H.; et al. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2016, 194, 748–761. [Google Scholar] [CrossRef] [Green Version]
- Ferreira Francisco, F.A.; Soares Souza, A.; Zanetti, G.; Marchiori, E. Multiple cystic lung disease. Eur. Respir. Rev. 2015, 24, 552–564. [Google Scholar] [CrossRef] [Green Version]
- Ansotegui Barrera, E.; Mancheno Franch, N.; Vera-Sempere, F.; Padilla Alarcon, J. Lymphangioleiomyomatosis. Arch. Bronconeumol. 2011, 47, 85–93. [Google Scholar] [CrossRef]
- Matsui, K.; Riemenschneider, W.K.; Hilbert, S.L.; Yu, Z.X.; Takeda, K.; Travis, W.D.; Moss, J.; Ferrans, V.J. Hyperplasia of type II pneumocytes in pulmonary lymphangioleiomyomatosis. Arch. Pathol. Lab. Med. 2000, 124, 1642–1648. [Google Scholar] [CrossRef]
- Verleden, S.E.; Vanstapel, A.; De Sadeleer, L.; Weynand, B.; Boone, M.; Verbeken, E.; Piloni, D.; Van Raemdonck, D.; Ackermann, M.; Jonigk, D.D.; et al. Quantitative analysis of airway obstruction in lymphangioleiomyomatosis. Eur. Respir. J. 2020, 56, 1901965. [Google Scholar] [CrossRef] [Green Version]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Noda, Y.; Shiroyama, T.; Amiya, S.; Adachi, Y.; Enomoto, T.; Hara, R.; Niitsu, T.; Miyake, K.; Hirata, H.; Takeda, Y.; et al. COVID-19 in a patient with sporadic lymphangioleiomyomatosis awaiting lung transplantation. Respir. Med. Case Rep. 2021, 34, 101505. [Google Scholar] [CrossRef] [PubMed]
- Asrani, P.; Hasan, G.M.; Sohal, S.S.; Hassan, M.I. Molecular Basis of Pathogenesis of Coronaviruses: A Comparative Genomics Approach to Planetary Health to Prevent Zoonotic Outbreaks in the 21st Century. Omics 2020, 24, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef] [PubMed]
- Drak Alsibai, K. Expression of angiotensin-converting enzyme 2 and proteases in COVID-19 patients: A potential role of cellular FURIN in the pathogenesis of SARS-CoV-2. Med. Hypotheses 2020, 143, 109893. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, H.; Hunter, A.; Murray, R.; Lim, W.S.; McKeever, T. Cigarette smoking and the occurrence of influenza—Systematic review. J. Infect. 2019, 79, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.S.; Hansbro, P.M.; Shukla, S.D.; Eapen, M.S.; Walters, E.H. Potential Mechanisms of Microbial Pathogens in Idiopathic Interstitial Lung Disease. Chest 2017, 152, 899–900. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, H.; Yang, B.; Lee, S.K.; Park, T.S.; Park, D.W.; Moon, J.Y.; Kim, T.H.; Sohn, J.W.; Yoon, H.J.; et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir. J. 2021, 58, 2004125. [Google Scholar] [CrossRef]
- Valenzuela, C.; Waterer, G.; Raghu, G. Interstitial lung disease before and after COVID-19: A double threat? Eur. Respir. J. 2021, 58, 2101956. [Google Scholar] [CrossRef]
- Wark, P.A.B.; Pathinayake, P.S.; Kaiko, G.; Nichol, K.; Ali, A.; Chen, L.; Sutanto, E.N.; Garratt, L.W.; Sohal, S.S.; Lu, W.; et al. ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma. Respirology 2021, 26, 442–451. [Google Scholar] [CrossRef]
- Tang, Y.; Kwiatkowski, D.J.; Henske, E.P. mTORC1 hyperactivation in lymphangioleiomyomatosis leads to ACE2 upregulation in type II pneumocytes: Implications for COVID-19. Eur. Respir. J. 2021, 57, 2002737. [Google Scholar] [CrossRef] [PubMed]
- Asrani, P.; Hussain, A.; Nasreen, K.; AlAjmi, M.F.; Amir, S.; Sohal, S.S.; Hassan, M.I. Guidelines and Safety Considerations in the Laboratory Diagnosis of SARS-CoV-2 Infection: A Prerequisite Study for Health Professionals. Risk Manag. Healthcare Policy 2021, 14, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Kalidhindi, R.S.R.; Borkar, N.A.; Ambhore, N.S.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Sex steroids skew ACE2 expression in human airway: A contributing factor to sex differences in COVID-19? Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L843–L847. [Google Scholar] [CrossRef]
- Haug, G.; Eapen, M.S.; Sohal, S.S. Renin-Angiotensin-Aldosterone System Inhibitors in Covid-19. N. Engl. J. Med. 2020, 382, e92. [Google Scholar]
- Kumari, P.; Singh, A.; Ngasainao, M.R.; Shakeel, I.; Kumar, S.; Lal, S.; Singhal, A.; Sohal, S.S.; Singh, I.K.; Hassan, M.I. Potential diagnostics and therapeutic approaches in COVID-19. Clin. Chim Acta 2020, 510, 488–497. [Google Scholar] [CrossRef]
- Shinde, T.; Hansbro, P.M.; Sohal, S.S.; Dingle, P.; Eri, R.; Stanley, R. Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms 2020, 8, 921. [Google Scholar] [CrossRef]
- Liu, G.; Philp, A.M.; Corte, T.; Travis, M.A.; Schilter, H.; Hansbro, N.G.; Burns, C.J.; Eapen, M.S.; Sohal, S.S.; Burgess, J.K.; et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 2021, 225, 107839. [Google Scholar] [CrossRef]
- Asrani, P.; Eapen, M.S.; Hassan, M.I.; Sohal, S.S. Implications of the second wave of COVID-19 in India. Lancet Respir. Med. 2021, 9, e93–e94. [Google Scholar] [CrossRef]
- McAlinden, K.D.; Lu, W.; Ferdowsi, P.V.; Myers, S.; Markos, J.; Larby, J.; Chia, C.; Weber, H.C.; Haug, G.; Eapen, M.S.; et al. Electronic Cigarette Aerosol Is Cytotoxic and Increases ACE2 Expression on Human Airway Epithelial Cells: Implications for SARS-CoV-2 (COVID-19). J. Clin. Med. 2021, 10, 1028. [Google Scholar] [CrossRef]
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Chia, C.; Haug, G.; Sohal, S.S. COVID-19 and vaping: Risk for increased susceptibility to SARS-CoV-2 infection? Eur. Respir. J. 2020, 56, 2001645. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L585–L595. [Google Scholar] [CrossRef] [PubMed]



| Groups | HC | IPF | LAM |
|---|---|---|---|
| Subject number | 12 | 6 | 6 |
| Gender (F/M) | 6/6 | 3/3 | 6/0 |
| Years of age (median, range) | 42.5, 19–63 | 62, 56–70 | 55.5, 45–65 |
| Smoking status (Never-smoker/Ex-smoker) | 12/0 | 0/6 | 5/1 |
| FEV1/FVC% Post BD (mean ± SD) | NA | 89.32 ± 3.86 | NA |
| DLCO% (mean ± SD) | NA | 25.33 ± 12.3 | NA |
| Comorbidity | NA | NA | Asthma, COPD, pneumonia, fibromyalgia, emphysema, arthritis |
| Treatment | NA | NA | Sirolimus, Armour Thyroid, Oxybutynin, Rapmine, Advair, Lyrica, Pramipexole |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Eapen, M.S.; Singhera, G.K.; Markos, J.; Haug, G.; Chia, C.; Larby, J.; Brake, S.J.; Westall, G.P.; Jaffar, J.; et al. Angiotensin-Converting Enzyme 2 (ACE2), Transmembrane Peptidase Serine 2 (TMPRSS2), and Furin Expression Increases in the Lungs of Patients with Idiopathic Pulmonary Fibrosis (IPF) and Lymphangioleiomyomatosis (LAM): Implications for SARS-CoV-2 (COVID-19) Infections. J. Clin. Med. 2022, 11, 777. https://doi.org/10.3390/jcm11030777
Lu W, Eapen MS, Singhera GK, Markos J, Haug G, Chia C, Larby J, Brake SJ, Westall GP, Jaffar J, et al. Angiotensin-Converting Enzyme 2 (ACE2), Transmembrane Peptidase Serine 2 (TMPRSS2), and Furin Expression Increases in the Lungs of Patients with Idiopathic Pulmonary Fibrosis (IPF) and Lymphangioleiomyomatosis (LAM): Implications for SARS-CoV-2 (COVID-19) Infections. Journal of Clinical Medicine. 2022; 11(3):777. https://doi.org/10.3390/jcm11030777
Chicago/Turabian StyleLu, Wenying, Mathew Suji Eapen, Gurpreet Kaur Singhera, James Markos, Greg Haug, Collin Chia, Josie Larby, Samuel James Brake, Glen P. Westall, Jade Jaffar, and et al. 2022. "Angiotensin-Converting Enzyme 2 (ACE2), Transmembrane Peptidase Serine 2 (TMPRSS2), and Furin Expression Increases in the Lungs of Patients with Idiopathic Pulmonary Fibrosis (IPF) and Lymphangioleiomyomatosis (LAM): Implications for SARS-CoV-2 (COVID-19) Infections" Journal of Clinical Medicine 11, no. 3: 777. https://doi.org/10.3390/jcm11030777
APA StyleLu, W., Eapen, M. S., Singhera, G. K., Markos, J., Haug, G., Chia, C., Larby, J., Brake, S. J., Westall, G. P., Jaffar, J., Kalidhindi, R. S. R., Fonseka, N. D., Sathish, V., Hackett, T. L., & Sohal, S. S. (2022). Angiotensin-Converting Enzyme 2 (ACE2), Transmembrane Peptidase Serine 2 (TMPRSS2), and Furin Expression Increases in the Lungs of Patients with Idiopathic Pulmonary Fibrosis (IPF) and Lymphangioleiomyomatosis (LAM): Implications for SARS-CoV-2 (COVID-19) Infections. Journal of Clinical Medicine, 11(3), 777. https://doi.org/10.3390/jcm11030777







