Is NMDA-Receptor-Mediated Oxidative Stress in Mitochondria of Peripheral Tissues the Essential Factor in the Pathogenesis of Hepatic Encephalopathy?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.2.1. Experimental Design
2.2.2. Animals
2.3. Preparative and Analytical Methods
2.3.1. Determination of Ammonia in Plasma
2.3.2. Isolation of Mitochondria Using a Self-Generated Percoll Gradient
2.3.3. Disruption of Mitochondria to Determine Enzyme Activity
2.3.4. Determination of Enzyme Activities in Mitochondria
2.3.5. Determination of Enzyme Activities in Erythrocytes
2.3.6. Preparation of Acid Extracts of Erythrocytes for Determination of Ammonia Concentration
2.3.7. Preparation of Submitochondrial Particles for Determination of Superoxide Radical Production
2.3.8. Measurement of Hydrogen Peroxide Production in Mitochondria
2.3.9. Preparation of Protein-Free Extracts of Mitochondria for Determination of Ammonia Concentration
2.3.10. Statistical Analysis
3. Results
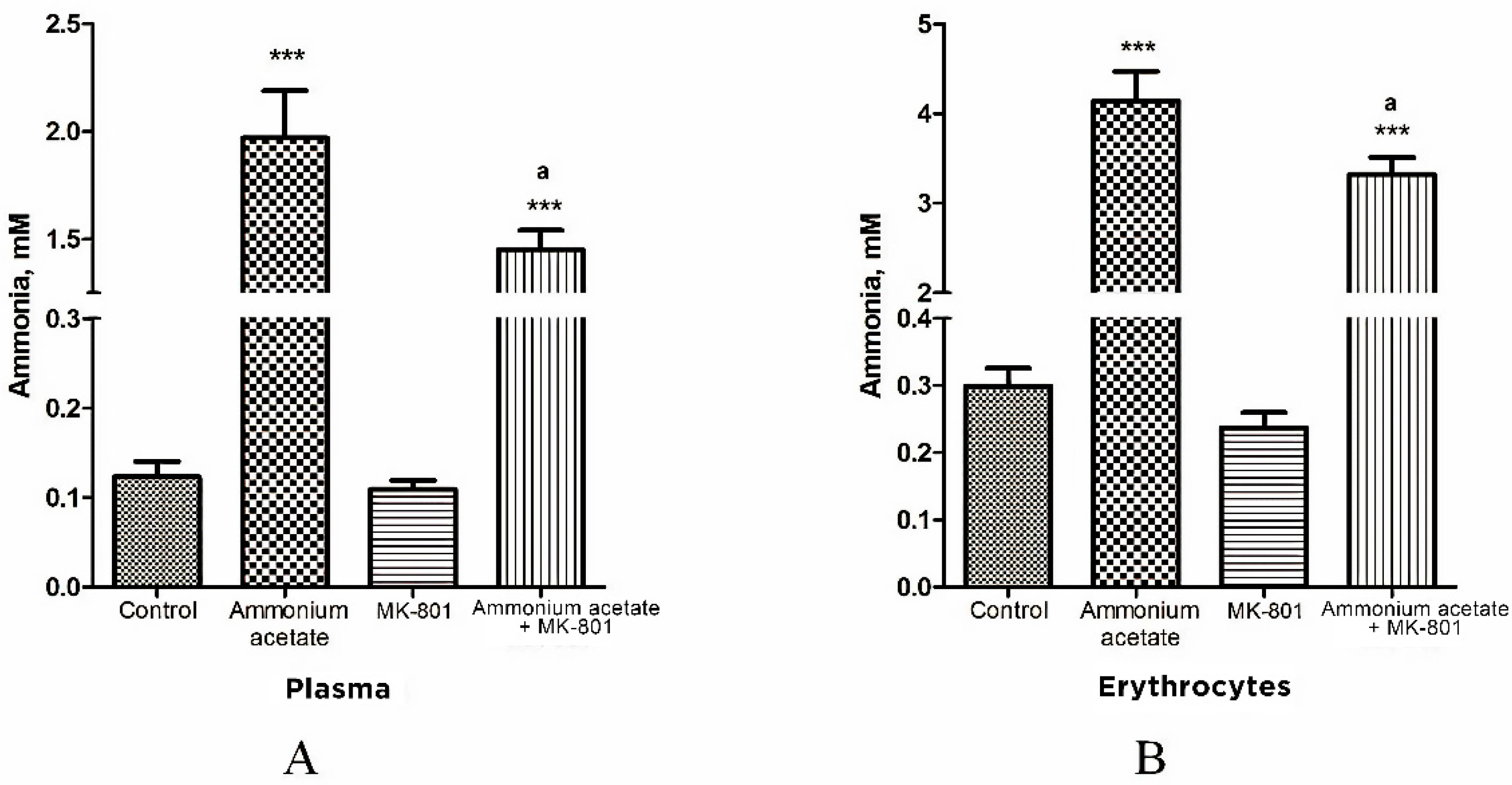
3.1. MK-801 Partially Reduces Ammonia Accumulation in Plasma and Erythrocytes of Hyperammonemic Animals
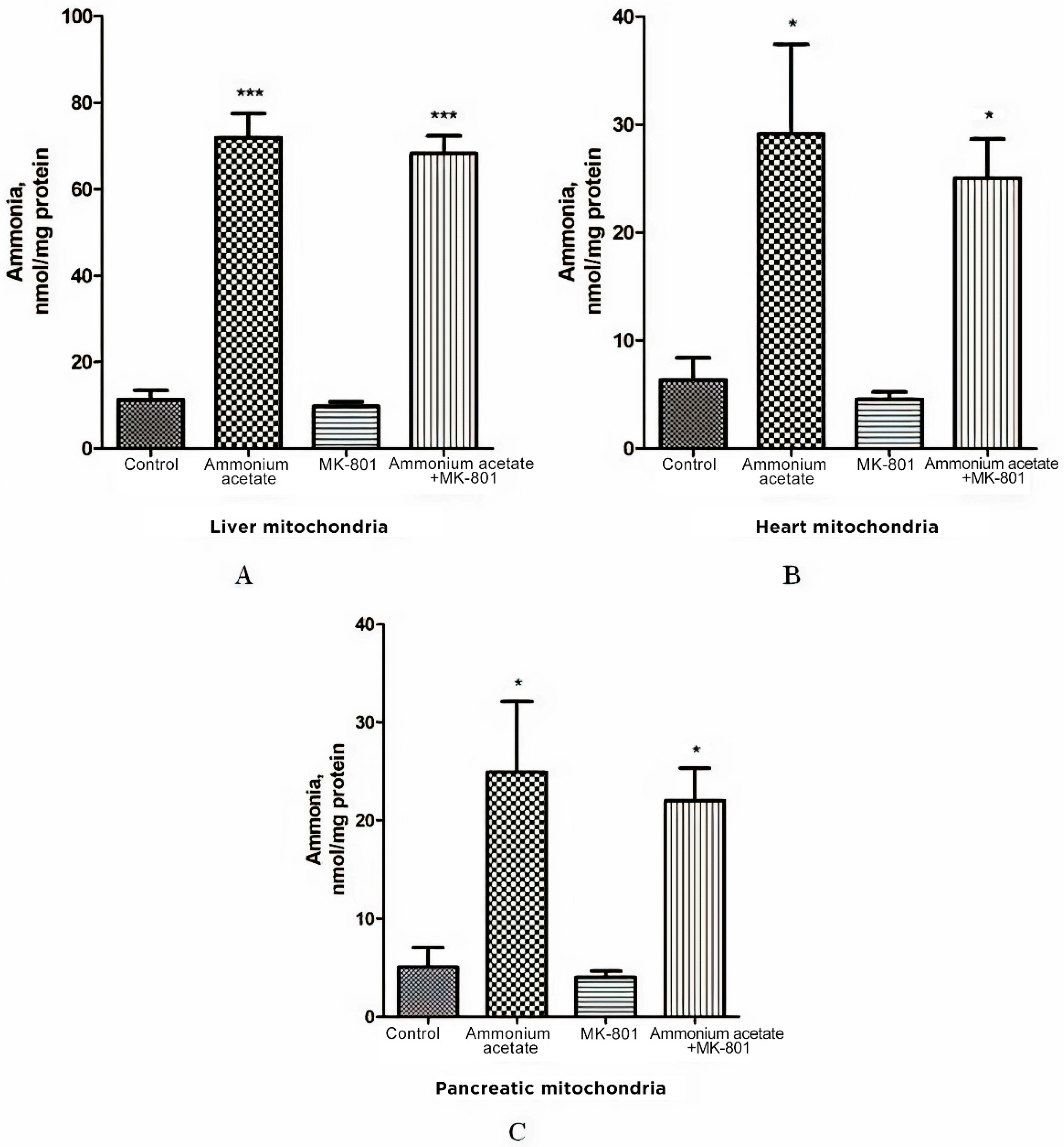
3.2. The Effect of MK-801 on Ammonia Levels in the Liver, Heart, and Pancreas Mitochondria of Hyperammonemic Animals
3.3. The Effect of MK-801 on Ammonium-Dependent Disturbance of the Balance between Oxidant and Antioxidant Systems in Non-Neuronal Tissues
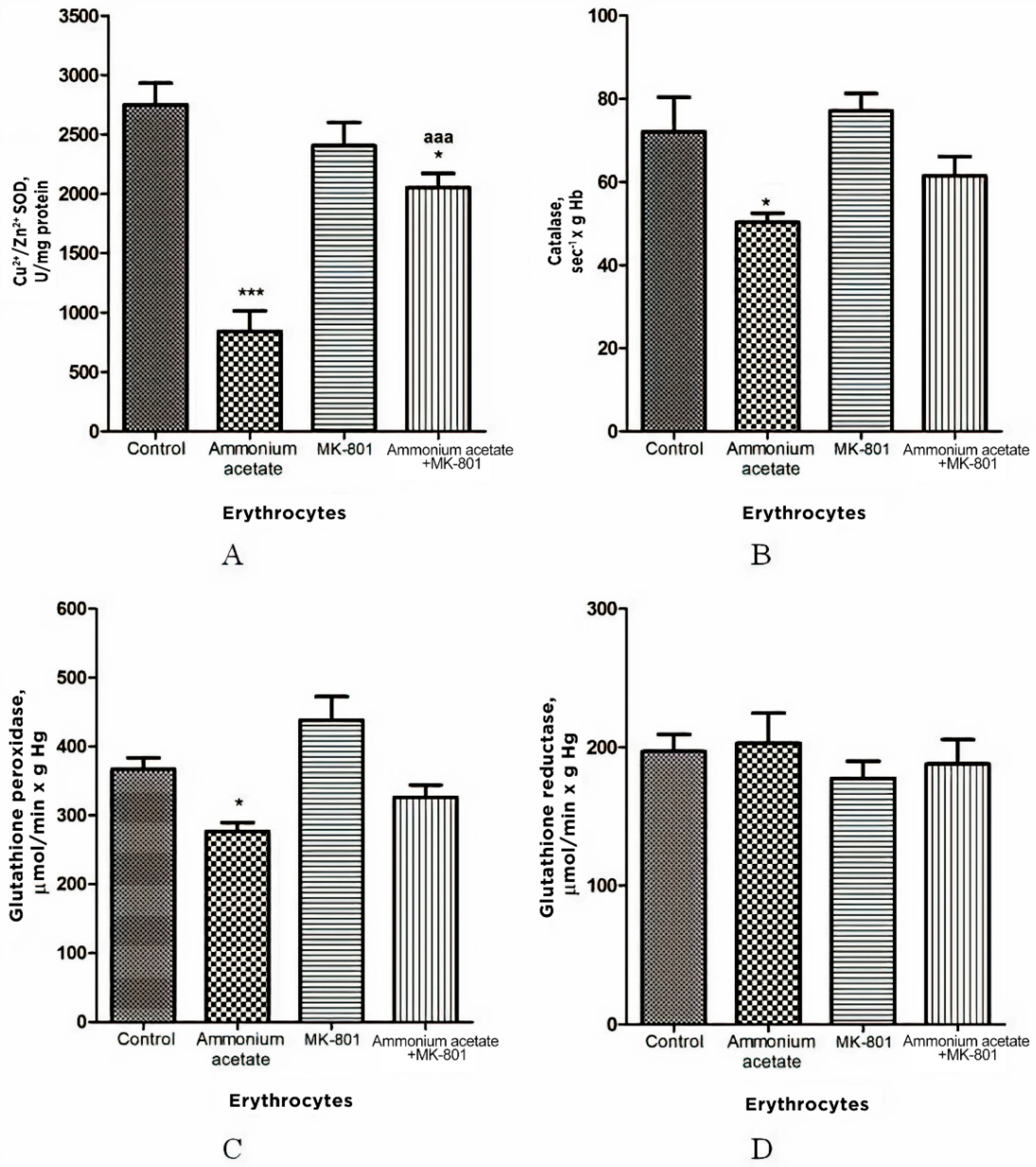
3.3.1. The Effect of MK-801 on the Enzyme Activity of Superoxide Dismutase, Catalase, Glutathione Peroxidase, and Glutathione Reductase in Erythrocytes of Hyperammonemic Rats
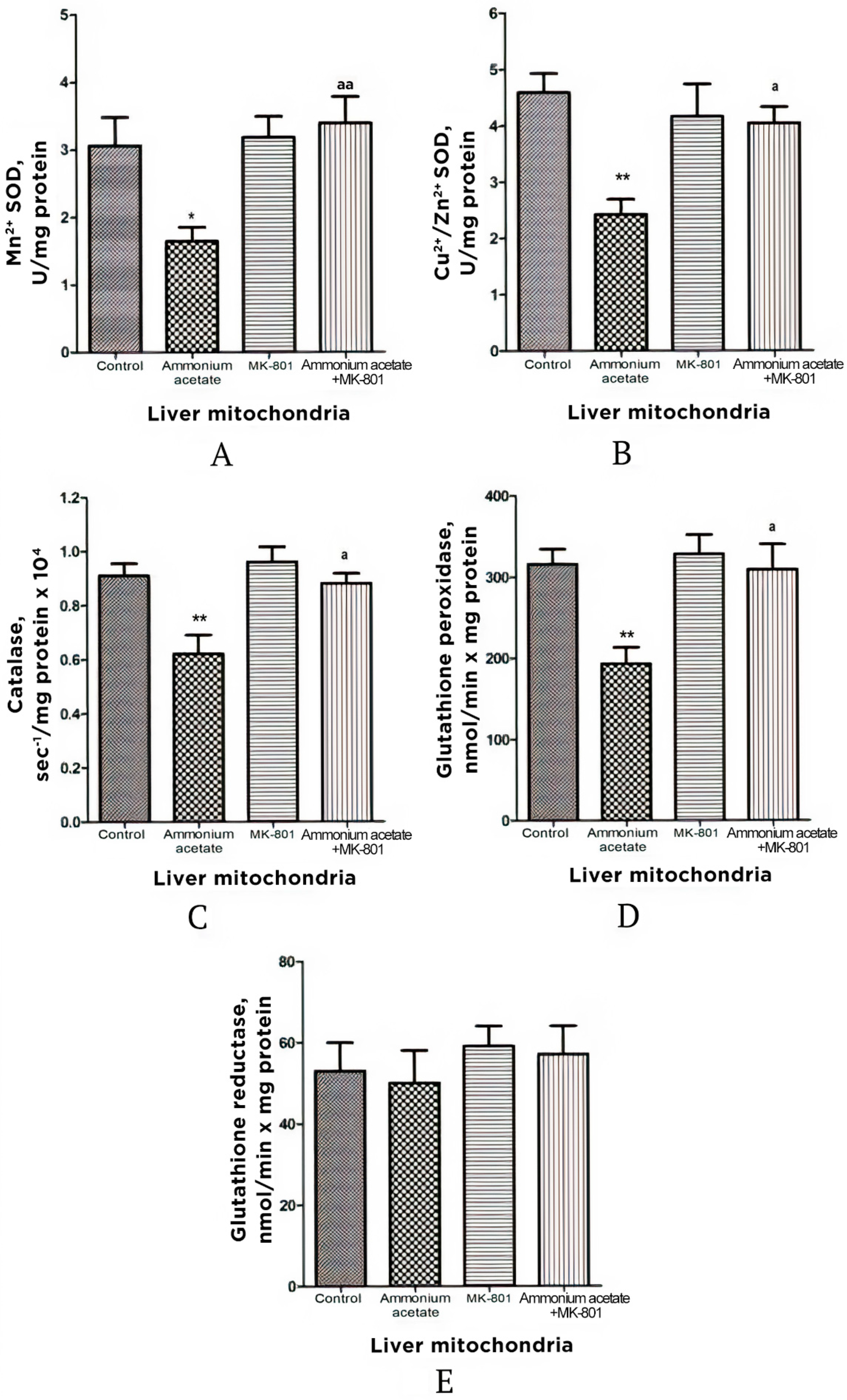
3.3.2. The Effect of MK-801 on the Enzyme Activity of Superoxide Dismutase, Catalase, Glutathione Peroxidase, and Glutathione Reductase in Liver Mitochondria of Hyperammonemic Rats
3.3.3. Effects of Acute Ammonia Intoxication on Superoxide Radical and Hydrogen Peroxide Production by Liver Mitochondria
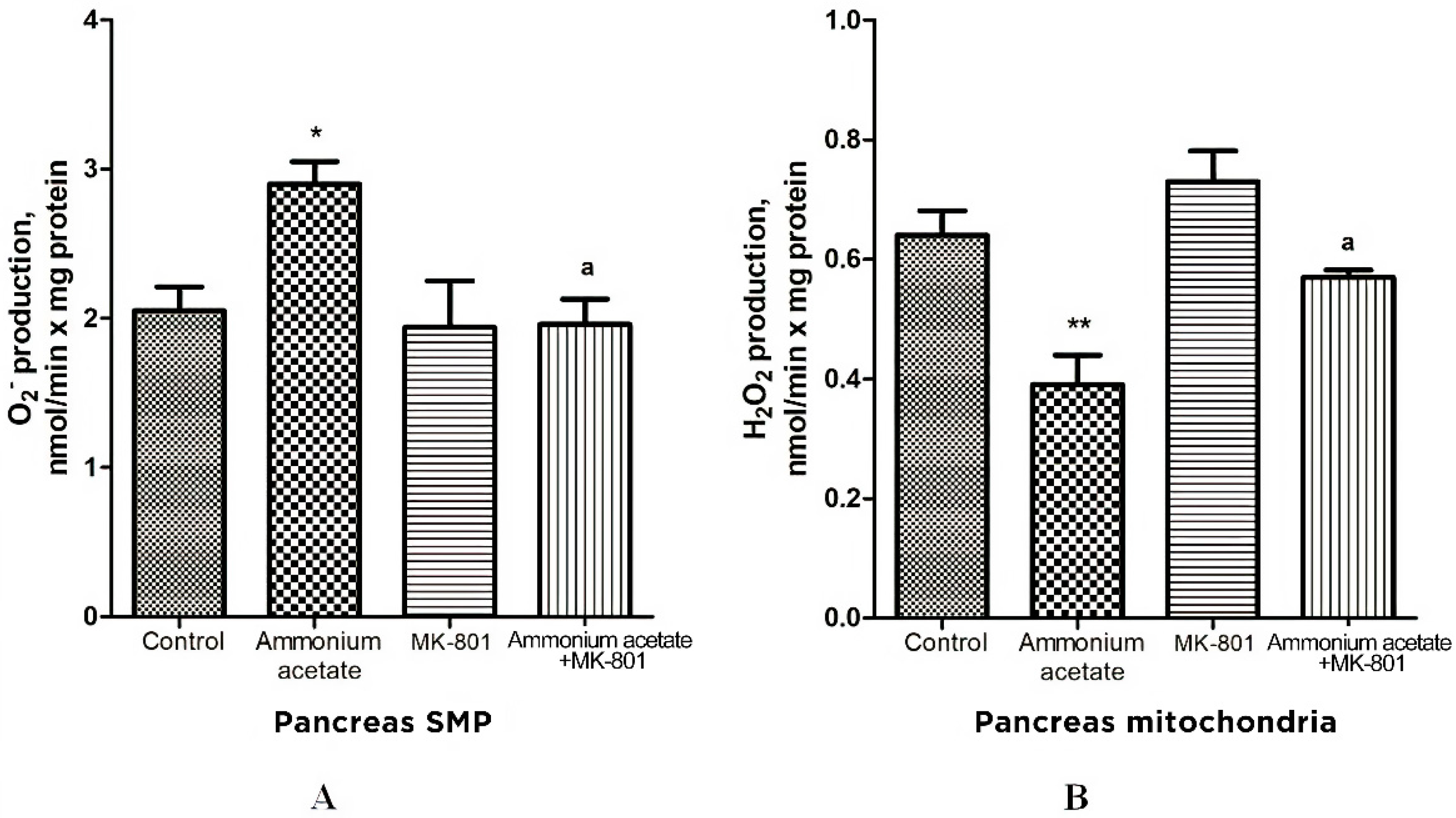
3.3.4. The Effect of MK-801 on Antioxidant Enzyme Activities and Superoxide Radical and Hydrogen Peroxide Production in Pancreas Mitochondria of Hyperammonemic Rats
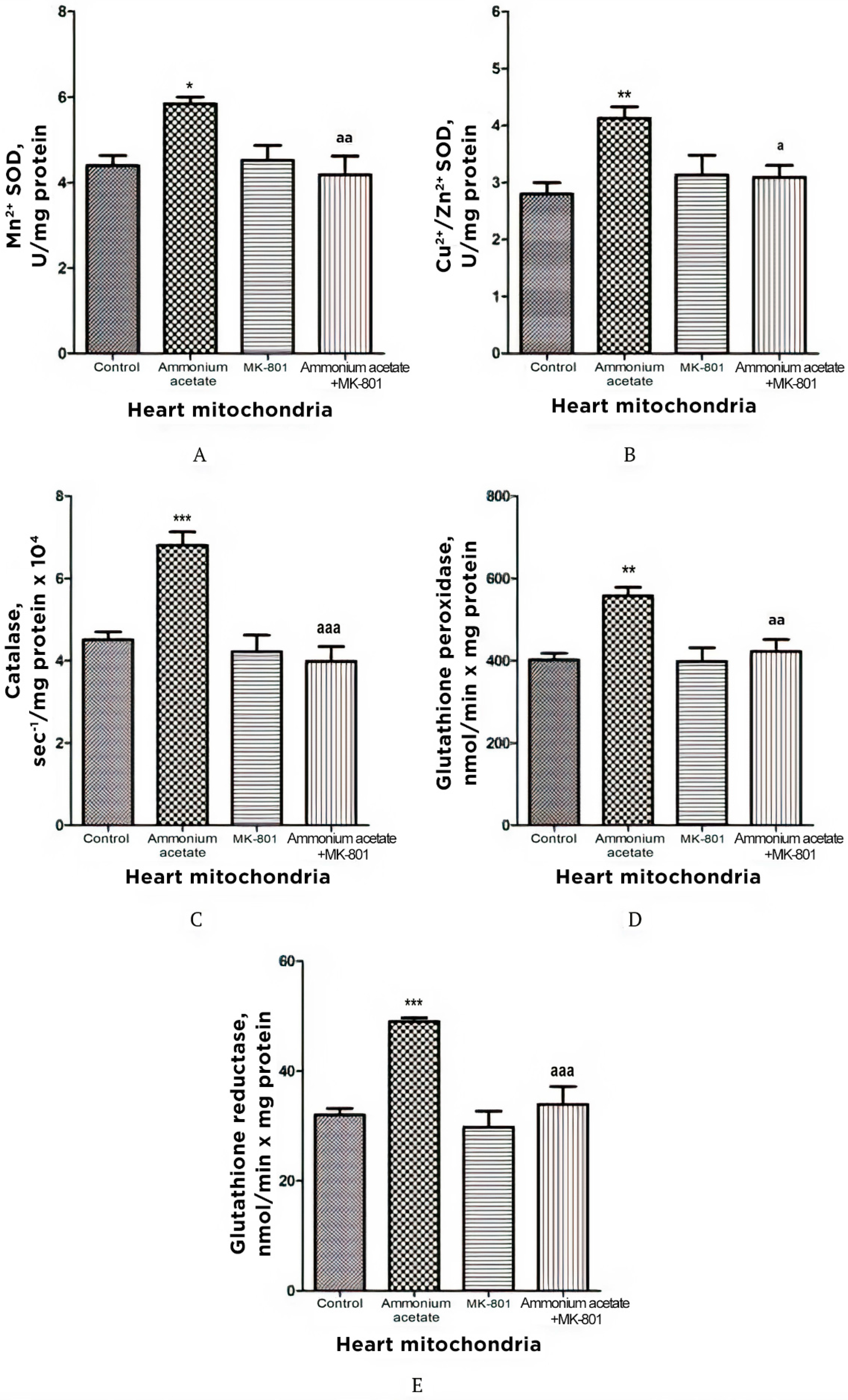
3.3.5. The Effect of MK-801 on Activities of Antioxidant Enzymes, Production of Superoxide Radical and Hydrogen Peroxide in Heart Mitochondria of Hyperammonemic Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butterworth, R.F. Pathogenesis and treatment of portal-systemic encephalopathy: An update. Dig. Dis. Sci. 1992, 37, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, F.T. A Clinical Treatise on Diseases of the Liver by Dr Friedrich Theodor Frerichs; The New Sydenham Society: London, UK, 1860; Volume 1, pp. 193–246. [Google Scholar]
- Butterworth, R.F.; Giguère, J.F.; Michaud, J.; Lavoie, J.; Layrargues, G.P. Ammonia: Key factor in the pathogenesis of hepatic encephalopathy. Neurochem. Pathol. 1987, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, A.H.; Yap, E.W.; Wong, W.H. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J. Cereb. Blood Flow Metab. 1991, 11, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Monfort, P.; Kosenko, E.; Erceg, S.; Canales, J.-J.; Felipo, V. Molecular mechanism of acute ammonia toxicity: Role of NMDA Receptors. Neurochem. Int. 2002, 41, 95–102. [Google Scholar] [CrossRef]
- Rodrigo, R.; Cauli, O.; Boix, J.; ElMlili, N.; Agusti, A.; Felipo, V. Role of NMDA receptors in acute liver failure and ammonia toxicity: Therapeutical implications. Neurochem. Int. 2009, 55, 113–118. [Google Scholar] [CrossRef]
- Cooper, A.J.; Plum, F. Biochemistry and physiology of brain ammonia. Physiol. Rev. 1987, 67, 440–519. [Google Scholar] [CrossRef]
- López-Cervantes, M.; Quintanar-Stephano, A.; Alcauter-Solórzano, S.; Hernández-Pando, R.; Aguilar-Roblero, R.; Gasca-Martínez, D.; Ortíz, J.J.; Vázquez-Martínez, O.; Ximénez-Camilli, C.; Díaz-Muñoz, M. Cerebellar spongiform degeneration is accompanied by metabolic, cellular, and motor disruption in male rats with portacaval anastomosis. J. Neurosci. Res. 2021, 99, 2287–2304. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.G.; Felipo, V.; Miñana, M.D.; Grisolía, S. Chronic hyperammonemia prevents changes in brain energy and ammonia metabolites induced by acute ammonium intoxication. Biochim. Biophys. Acta 1993, 1180, 321–326. [Google Scholar] [CrossRef]
- Kosenko, E.; Felipo, V.; Montoliu, C.; Grisolía, S.; Kaminsky, Y. Effects of acute hyperammonemia in vivo on oxidative metabolism in nonsynaptic rat brain mitochondria. Metab. Brain Dis. 1997, 12, 69–82. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Tikhonova, L.A.; Alilova, G.A.; Montoliu, C.; Barreto, G.E.; Aliev, G.; Kaminsky, Y.G. Portacaval shunting causes differential mitochondrial superoxide production in brain regions. Free Radic. Biol. Med. 2017, 113, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Llansola, M.; Montoliu, C.; Monfort, P.; Rodrigo, R.; Hernandez-Viadel, M.; Erceg, S.; Sánchez-Perez, A.M.; Felipo, V. Glutamine synthetase activity and glutamine content in brain: Modulation by NMDA receptors and nitric oxide. Neurochem. Int. 2003, 43, 493–499. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.; Kaminsky, A.; Valencia, M.; Lee, L.; Hermenegildo, C.; Felipo, V. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic. Res. 1997, 27, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology 2003, 37, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, M.; Albrecht, J. Oxidative and nitrosative stress in ammonia neurotoxicity. Neurochem. Int. 2013, 62, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Kaminsky, Y.; Grau, E.; Miñana, M.D.; Grisolía, S.; Felipo, V. Nitroarginine, an inhibitor of nitric oxide synthetase, attenuates ammonia toxicity and ammonia-induced alterations in brain metabolism. Neurochem. Res. 1995, 20, 451–456. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.; Stavroskaya, I.G.; Felipo, V. Alteration of mitochondrial calcium homeostasis by ammonia-induced activation of NMDA receptors in rat brain in vivo. Brain Res. 2000, 880, 139–146. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.; Grau, E.; Miñana, M.D.; Marcaida, G.; Grisolía, S.; Felipo, V. Brain ATP depletion induced by acute ammonia intoxication in rats is mediated by activation of the nmda receptor and Na+,K(+)-ATPase. J. Neurochem. 1994, 63, 2172–2178. [Google Scholar] [CrossRef]
- Kosenko, E.; Montoliu, C.; Giordano, G.; Kaminsky, Y.; Venediktova, N.; Buryanov, Y.; Felipo, V. Acute ammonia intoxication induces an NMDA receptor-mediated increase in poly(ADP-ribose) polymerase level and NAD metabolism in nuclei of rat brain cells. J. Neurochem. 2004, 89, 1101–1110. [Google Scholar] [CrossRef]
- Kaminsky, Y.; Kosenko, E. Brain purine metabolism and xanthine dehydrogenase/oxidase conversion in hyperammonemia are under control of nmda receptors and nitric oxide. Brain Res. 2009, 1294, 193–201. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminski, Y.; Lopata, O.; Muravyov, N.; Felipo, V. Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic. Biol. Med. 1999, 26, 1369–1374. [Google Scholar] [CrossRef]
- Izumi, Y.; Izumi, M.; Matsukawa, M.; Funatsu, M.; Zorumski, C.F. Ammonia-mediated LTP inhibition: Effects of NMDA receptor antagonists and L-carnitine. Neurobiol. Dis. 2005, 20, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Mueller, R.W.; McGuire, P.F.; Pulido, O.M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol. Pathol. 2000, 28, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Pulido, O.M. Glutamate receptors in peripheral tissues: Current knowledge, future research, and implications for toxicology. Toxicol. Pathol. 2001, 29, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Hogan-Cann, A.D.; Anderson, C.M. Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol. Sci. 2016, 37, 750–767. [Google Scholar] [CrossRef]
- Bozic, M.; Valdivielso, J.M. The potential of targeting NMDA receptors outside the CNS. Expert Opin. Ther. Targets 2015, 19, 399–413. [Google Scholar] [CrossRef]
- Perazzo, J.C.; Tallis, S.; Delfante, A.; Souto, P.A.; Lemberg, A.; Eizayaga, F.X.; Romay, S. Hepatic encephalopathy: An approach to its multiple pathophysiological features. World J. Hepatol. 2012, 4, 50–65. [Google Scholar] [CrossRef]
- Pace, A.; de Weerth, A.; Berna, M.; Hillbricht, K.; Tsokos, M.; Bläker, M.; Pueschel, K.; Lohse, A.W. Pancreas and liver injury are associated in individuals with increased alcohol consumption. Clin. Gastroenterol. Hepatol. 2009, 7, 1241–1246. [Google Scholar] [CrossRef]
- Katakura, Y.; Yotsuyanagi, H.; Hashizume, K.; Okuse, C.; Okuse, N.; Nishikawa, K.; Suzuki, M.; Iino, S.; Itoh, F. Pancreatic involvement in chronic viral hepatitis. World J. Gastroenterol. 2005, 11, 3508–3513. [Google Scholar] [CrossRef]
- Fouad, Y.M.; Yehia, R. Hepato-cardiac disorders. World J. Hepatol. 2014, 6, 41–54. [Google Scholar] [CrossRef]
- Giallourakis, C.C.; Rosenberg, P.M.; Friedman, L.S. The liver in heart failure. Clin. Liver Dis. 2002, 6, 947–967. [Google Scholar] [CrossRef]
- Perseghin, G.; Lattuada, G.; De Cobelli, F.; Esposito, A.; Belloni, E.; Ntali, G.; Ragogna, F.; Canu, T.; Scifo, P.; Del Maschio, A.; et al. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology 2008, 47, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Minemura, M.; Tajiri, K.; Shimizu, Y. Systemic abnormalities in liver disease. World J. Gastroenterol. 2009, 15, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Manrai, M.; Parvathi, V.S.; Kumar, D.; Srivastava, S.; Pathak, B. Association of liver cirrhosis severity with anemia: Does it matter? Ann. Gastroenterol 2020, 33, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Gupte, P.; Nagral, A. Hematological problems and liver disease. Trop. Gastroenterol. 2009, 30, 65–70. [Google Scholar] [PubMed]
- Mehta, A.B.; McIntyre, N. Haematological disorders in liver disease. Forum 1998, 8, 8–25. [Google Scholar]
- Xie, X.; Wang, L.; Yao, M.; Wen, X.; Chen, X.; You, H.; Jia, J.; Zhao, J.; Lu, F. Correlation between red blood cell count and liver function status. Zhonghua Gan Zang Bing Za Zhi 2016, 24, 119–122. [Google Scholar] [CrossRef]
- Owen, J.S.; Brown, D.J.; Harry, D.S.; McIntyre, N.; Beaven, G.H.; Isenberg, H.; Gratzer, W.B. Erythrocyte echinocytosis in liver disease. Role of abnormal plasma high density lipoproteins. J. Clin. Investig. 1985, 76, 2275–2285. [Google Scholar] [CrossRef]
- Senzolo, M.; Burroughs, A.K. Haematological abnormalities in liver disease. In Textbook of Hepatology; Rodés, J., Benhaumou, J.P., Blei, A.T., Reichen, J., Rizzetto, M., Eds.; Blackwell Sci Pub: Oxford, UK, 2007; pp. 1767–1779. [Google Scholar]
- Greenburg, A.G. Pathophysiology of anemia. Am. J. Med. 1996, 101, 7S–11S. [Google Scholar] [CrossRef]
- Johnson, D.; Mayers, I. Multiple organ dysfunction syndrome: A narrative review. Can. J. Anaesth. 2001, 48, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef]
- Sener, A.; Hutton, J.C.; Kawazu, S.; Boschero, A.C.; Somers, G.; Devis, G.; Herchuelz, A.; Malaisse, W.J. the stimulus-secretion coupling of glucose-induced insulin release. Metabolic and functional effects of NH4+ in rat islets. J. Clin. Investig. 1978, 62, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Lafon-Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef]
- Reyes, R.C.; Brennan, A.M.; Shen, Y.; Baldwin, Y.; Swanson, R.A. Activation of neuronal NMDA receptors induces superoxide-mediated oxidative stress in neighboring neurons and astrocytes. J. Neurosci. 2012, 32, 12973–12978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Görg, B.; Qvartskhava, N.; Bidmon, H.-J.; Palomero-Gallagher, N.; Kircheis, G.; Zilles, K.; Häussinger, D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology 2010, 52, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Daniluk, J.; Kandefer-Szerszeń, M. Alcohol-related cirrhosis with pancreatitis. The role of oxidative stress in the progression of the disease. Arch. Immunol. Ther. Exp. 2001, 49, 139–146. [Google Scholar]
- Kovacic, P.; Somanathan, R. Clinical physiology and mechanism of dizocilpine (MK-801): Electron transfer, radicals, redox metabolites and bioactivity. Oxid. Med. Cell. Longev. 2010, 3, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Kosenko, E.; Venediktova, N.; Kaminsky, Y.; Montoliu, C.; Felipo, V. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res. 2003, 981, 193–200. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Yang, Y.X.; Nunes, F.A.; Lewis, J.D.; Tuchman, M.; Tino, G.; Kaiser, L.R.; Palevsky, H.I.; Kotloff, R.M.; Furth, E.E.; et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann. Intern. Med. 2000, 132, 283–287. [Google Scholar] [CrossRef]
- Snyder, M.J.; Bradford, W.D.; Kishnani, P.S.; Hale, L.P. Idiopathic hyperammonemia following an unrelated cord blood transplant for mucopolysaccharidosis, I. Pediatr. Dev. Pathol. 2003, 6, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.A.; Venediktova, N.I.; Kudryavtsev, A.A.; Ataullakhanov, F.I.; Kaminsky, Y.G.; Felipo, V.; Montoliu, C. Encapsulation of glutamine synthetase in mouse erythrocytes: A new procedure for ammonia detoxification. Biochem. Cell Biol. 2008, 86, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.M. Purification of a crude mitochondrial fraction by density-gradient centrifugation. In Current Protocols in Cell Biology; Wiley: New York, NY, USA, 1999; Chapter 3, Unit 3.4. [Google Scholar] [CrossRef]
- Kosenko, E.; Tikhonova, L.; Alilova, G.; Montoliu, C. A Look into liver mitochondrial dysfunction as a hallmark in progression of brain energy crisis and development of neurologic symptoms in hepatic encephalopathy. J. Clin. Med. 2020, 9, 2259. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1984; Volume 3, pp. 273–286. [Google Scholar]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Beutler, E.; Blume, K.G.; Kaplan, J.C.; Löhr, G.W.; Ramot, B.; Valentine, W.N. International committee for standardization in haematology: Recommended methods for red-cell enzyme analysis. Br. J. Haematol. 1977, 35, 331–340. [Google Scholar] [CrossRef]
- Forman, H.J.; Kennedy, J. Dihydroorotate-dependent superoxide production in rat brain and liver. A function of the primary dehydrogenase. Arch. Biochem. Biophys. 1976, 173, 219–224. [Google Scholar] [CrossRef]
- Makhro, A.; Wang, J.; Vogel, J.; Boldyrev, A.A.; Gassmann, M.; Kaestner, L.; Bogdanova, A. Functional NMDA receptors in rat erythrocytes. Am. J. Physiol. Cell Physiol. 2010, 298, C1315–C1325. [Google Scholar] [CrossRef] [Green Version]
- Makhro, A. Functional NMDA Receptors in Red Blood Cells and Heart. Ph.D. Thesis, University of Zurich, Faculty of Science, Zurich, Switzerland, 2014. [Google Scholar]
- Harris, R.T.; Dudley, G.A. Exercise alters the distribution of ammonia and lactate in blood. J. Appl. Physiol. 1989, 66, 313–317. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Tikhonova, L.A.; Kaminsky, Y.G. Ammonia and enzymes of ammonia metabolism in different brain regions in hyperammonemia. Neurochem. J. 2015, 9, 133–140. [Google Scholar] [CrossRef]
- Lemberg, A.; Fernández, M.A. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann. Hepatol. 2009, 8, 95–102. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kareyeva, A.V.; Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial hydrogen peroxide production as determined by the pyridine nucleotide pool and its redox state. Biochim. Biophys. Acta 2012, 1817, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Korge, P.; Calmettes, G.; Weiss, J.N. Reactive oxygen species production in cardiac mitochondria after complex I inhibition: Modulation by substrate-dependent regulation of the NADH/NAD(+) ratio. Free Radic. Biol. Med. 2016, 96, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Das, D.K.; Engelman, R.M.; Kimura, Y. Molecular adaptation of cellular defences following preconditioning of the heart by repeated ischaemia. Cardiovasc. Res. 1993, 27, 578–584. [Google Scholar] [CrossRef]
- Penna, C.; Mancardi, D.; Rastaldo, R.; Pagliaro, P. Cardioprotection: A radical view free radicals in pre and postconditioning. Biochim. Biophys. Acta 2009, 1787, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekar, B.; Colston, J.T.; Freeman, G.L. Induction of proinflammatory cytokine and antioxidant enzyme gene expression following brief myocardial ischaemia. Clin. Exp. Immunol. 1997, 108, 346–351. [Google Scholar] [CrossRef]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: Revisited. Oxid. Med. Cell. Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef] [Green Version]
- Das, D.K.; Maulik, N.; Moraru, I.I. Gene expression in acute myocardial stress. Induction by hypoxia, ischemia, reperfusion, hyperthermia and oxidative stress. J. Mol. Cell. Cardiol. 1995, 27, 181–193. [Google Scholar] [CrossRef]
- Piccirillo, S.; Magi, S.; Castaldo, P.; Preziuso, A.; Lariccia, V.; Amoroso, S. NCX and EAAT transporters in ischemia: At the crossroad between glutamate metabolism and cell survival. Cell Calcium 2020, 86, 102160. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.F. Review article: The burden of hepatic encephalopathy. Aliment. Pharmacol. Ther. 2007, 25 (Suppl. S1), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cauli, O.; Rodrigo, R.; Boix, J.; Piedrafita, B.; Agusti, A.; Felipo, V. Acute liver failure-induced death of rats is delayed or prevented by blocking NMDA receptors in brain. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G503–G511. [Google Scholar] [CrossRef] [Green Version]
- Michalak, A.; Knecht, K.; Butterworth, R.F. Hepatic encephalopathy in acute liver failure: Role of the glutamate system. Adv. Exp. Med. Biol. 1997, 420, 35–43. [Google Scholar] [CrossRef]
- Weissenborn, K. Hepatic encephalopathy: Definition, clinical grading and diagnostic principles. Drugs 2019, 79, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Geetha, A.; Lakshmi Priya, M.D.; Jeyachristy, S.A.; Surendran, R. Level of oxidative stress in the red blood cells of patients with liver cirrhosis. Indian J. Med. Res. 2007, 126, 204–210. [Google Scholar]
- Bjerring, P.N.; Gluud, L.L.; Larsen, F.S. Cerebral blood flow and metabolism in hepatic encephalopathy—A meta-analysis. J. Clin. Exp. Hepatol. 2018, 8, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S. Cardiac abnormalities in liver cirrhosis. West. J. Med. 1989, 151, 530–535. [Google Scholar]
- Davies, K.J.; Goldberg, A.L. Oxygen Radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J. Biol. Chem. 1987, 262, 8220–8226. [Google Scholar] [CrossRef]
- Brewer, G.J.; Eaton, J.W. Erythrocyte metabolism: Interaction with oxygen transport. Science 1971, 171, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Oelshlegel, F.J.; Moore, L.G.; Noble, N.A. In vivo red cell glycolytic control and DPG-ATP levels. Ann. N. Y. Acad. Sci. 1974, 241, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagger, J.E.; Bateman, R.M.; Ellsworth, M.L.; Ellis, C.G. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2833–H2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wijk, R.; van Solinge, W.W. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- Astrup, J.; Rörth, M. Oxygen affinity of hemoglobin and red Cell 2,3-diphosphoglycerate in hepatic cirrhosis. Scand. J. Clin. Lab. Investig. 1973, 31, 311–317. [Google Scholar] [CrossRef]
- Smith, J.R.; Kay, N.E.; Gottlieb, A.J.; Oski, F.A. Abnormal erythrocyte metabolism in hepatic disease. Blood 1975, 46, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Lang, E.; Gatidis, S.; Freise, N.F.; Bock, H.; Kubitz, R.; Lauermann, C.; Orth, H.M.; Klindt, C.; Schuier, M.; Keitel, V.; et al. Conjugated bilirubin triggers anemia by inducing erythrocyte death. Hepatology 2015, 61, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Martinelle, K.; Häggström, L. Mechanisms of ammonia and ammonium ion toxicity in animal cells: Transport across cell membranes. J. Biotechnol. 1993, 30, 339–350. [Google Scholar] [CrossRef]
- Soria, L.R.; Fanelli, E.; Altamura, N.; Svelto, M.; Marinelli, R.A.; Calamita, G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem. Biophys. Res. Commun. 2010, 393, 217–221. [Google Scholar] [CrossRef]
- Soria, L.R.; Marrone, J.; Calamita, G.; Marinelli, R.A. Ammonia detoxification via ureagenesis in rat hepatocytes involves mitochondrial aquaporin-8 channels. Hepatology 2013, 57, 2061–2071. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Y.-X.; Dou, F.-F.; Lü, H.-Z.; Ma, Z.-W.; Lu, P.-H.; Xu, X.-M. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem. Int. 2010, 56, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Alleman, R.J.; Katunga, L.A.; Nelson, M.A.M.; Brown, D.A.; Anderson, E.J. The “Goldilocks Zone” from a redox perspective—Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristow, M. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014, 12, 288–341. [Google Scholar] [CrossRef]
- Degli Esposti, D.; Hamelin, J.; Bosselut, N.; Saffroy, R.; Sebagh, M.; Pommier, A.; Martel, C.; Lemoine, A. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem. Res. Int. 2012, 2012, 387626. [Google Scholar] [CrossRef]
- Schirrmacher, V. Mitochondria at work: New insights into regulation and dysregulation of cellular energy supply and metabolism. Biomedicines 2020, 8, 526. [Google Scholar] [CrossRef]
- Pittman, R.N. Regulation of tissue oxygenation. In Integrated Systems Physiology: From Molecule to Function to Disease; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011. [Google Scholar]
- Moreau, R.; Lee, S.S.; Hadengue, A.; Ozier, Y.; Sicot, C.; Lebrec, D. Relationship between oxygen transport and oxygen uptake in patients with cirrhosis: Effects of vasoactive drugs. Hepatology 1989, 9, 427–432. [Google Scholar] [CrossRef]
- Moreau, R.; Lee, S.S.; Soupison, T.; Roche-Sicot, J.; Sicot, C. Abnormal Tissue oxygenation in patients with cirrhosis and liver failure. J. Hepatol. 1988, 7, 98–105. [Google Scholar] [CrossRef]
- Bihari, D.; Gimson, A.E.; Waterson, M.; Williams, R. Tissue hypoxia during fulminant hepatic failure. Crit. Care Med. 1985, 13, 1034–1039. [Google Scholar] [CrossRef]
- Cain, S.M. Peripheral oxygen uptake and delivery in health and disease. Clin. Chest Med. 1983, 4, 139–148. [Google Scholar] [CrossRef]
- Kosenko, E.; Tikhonova, L.; Alilova, G.; Urios, A.; Montoliu, C. The erythrocytic hypothesis of brain energy crisis in sporadic alzheimer disease: Possible consequences and supporting evidence. J. Clin. Med. 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosenko, E.; Tikhonova, L.; Alilova, G.; Montoliu, C. Is NMDA-Receptor-Mediated Oxidative Stress in Mitochondria of Peripheral Tissues the Essential Factor in the Pathogenesis of Hepatic Encephalopathy? J. Clin. Med. 2022, 11, 827. https://doi.org/10.3390/jcm11030827
Kosenko E, Tikhonova L, Alilova G, Montoliu C. Is NMDA-Receptor-Mediated Oxidative Stress in Mitochondria of Peripheral Tissues the Essential Factor in the Pathogenesis of Hepatic Encephalopathy? Journal of Clinical Medicine. 2022; 11(3):827. https://doi.org/10.3390/jcm11030827
Chicago/Turabian StyleKosenko, Elena, Lyudmila Tikhonova, Gubidat Alilova, and Carmina Montoliu. 2022. "Is NMDA-Receptor-Mediated Oxidative Stress in Mitochondria of Peripheral Tissues the Essential Factor in the Pathogenesis of Hepatic Encephalopathy?" Journal of Clinical Medicine 11, no. 3: 827. https://doi.org/10.3390/jcm11030827
APA StyleKosenko, E., Tikhonova, L., Alilova, G., & Montoliu, C. (2022). Is NMDA-Receptor-Mediated Oxidative Stress in Mitochondria of Peripheral Tissues the Essential Factor in the Pathogenesis of Hepatic Encephalopathy? Journal of Clinical Medicine, 11(3), 827. https://doi.org/10.3390/jcm11030827






