Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices
Abstract
:1. Introduction: The Long History of Viscoelastic Hemostatic Assaying in Research and Clinical Medicine
2. Principles of Viscoelastic Hemostatic Assays (VHAs)
2.1. Thromboelastography (TEG® 5000)
2.2. Rotational Thromboelastometry (ROTEM® Delta)
2.3. Chemical Activators and Inhibitors of VHAs and Their Interpretation
2.4. Sonoclot®
2.5. New Clinical Technology for VHAs
2.5.1. TEG® 6s
2.5.2. ROTEM® Sigma
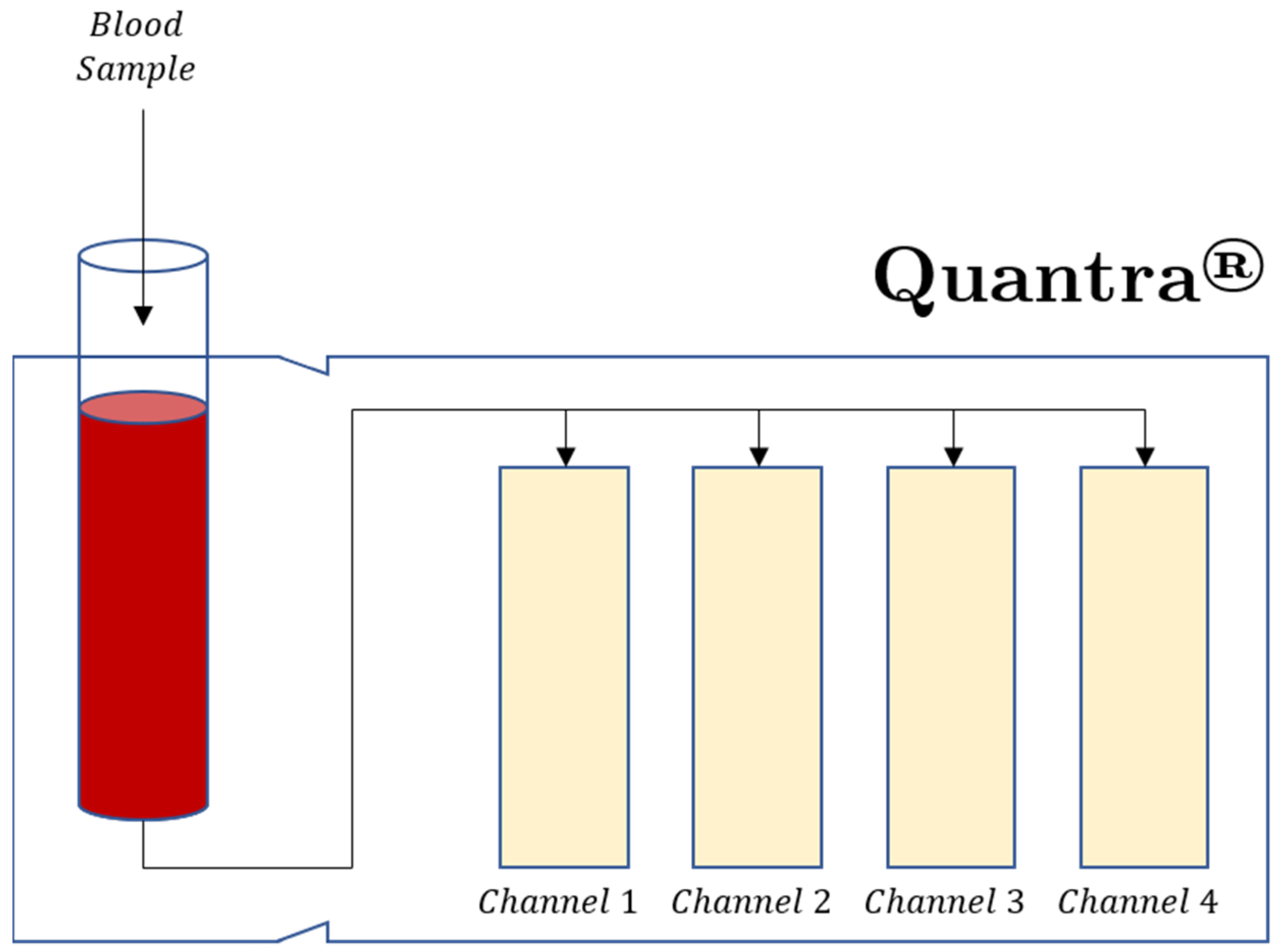
2.5.3. Quantra®
2.5.4. ClotPro®
2.6. Emerging Technologies
2.7. VHA Limitations
2.8. Viscoelastic Hemostatic Assay Guidance of Blood Component Therapy and Pro-Hemostatic Agents
2.8.1. General Principles of VHA-Guided BCT, Hemostatic Adjuncts, and Anticoagulation
2.8.2. Specialized Testing: TEG Platelet Mapping® (TEG/PM®), ROTEM Platelet Analysis®, Isolated Platelet Dysfunction, Multiple Electrode Aggregometry (Multiplate®)
2.8.3. VHAs Reflecting Anticoagulation
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Starzl, T.E. The saga of liver replacement, with particular reference to the reciprocal influence of liver and kidney transplantation (1955–1967). J. Am. Coll. Surg. 2002, 195, 587–610. [Google Scholar] [CrossRef] [Green Version]
- Groth, C.G.; Pechet, L.; Starzl, T.E. Coagulation during and after orthotopic transplantation of the human liver. Arch. Surg. 1969, 98, 31–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.G.; Martin, D.J.; Marquez, J.; Lewis, J.H.; Bontempo, F.A.; Shaw, B.W., Jr.; Starzl, T.E.; Winter, P.M. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth. Analg. 1985, 64, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starzl, T.E.; Marchioro, T.L.; Vonkaulla, K.N.; Hermann, G.; Brittain, R.S.; Waddell, W.R. Homotransplantation of the Liver in Humans. Surg. Gynecol. Obstet. 1963, 117, 659–676. [Google Scholar] [PubMed]
- Shore-Lesserson, L.; Manspeizer, H.E.; DePerio, M.; Francis, S.; Vela-Cantos, F.; Ergin, M.A. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth. Analg. 1999, 88, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Spiess, B.D.; Gillies, B.S.A.; Chandler, W.; Verrier, E. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J. Cardiothorac. Vasc. Anesth. 1995, 9, 168–173. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Minei, K.M.; Scerbo, M.L.; Radwan, Z.A.; Wade, C.E.; Kozar, R.A.; Gill, B.S.; Albarado, R.; McNutt, M.K.; Khan, S.; et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: Experience with 1974 consecutive trauma patients. Ann. Surg. 2012, 256, 476–486. [Google Scholar] [CrossRef]
- Schöchl, H.; Voelckel, W.; Schlimp, C.J. Management of traumatic haemorrhage–the European perspective. Anaesthesia 2015, 70, e102–e137. [Google Scholar] [CrossRef]
- Enriquez, L.J.; Shore-Lesserson, L. Point-of-Care coagulation testing and transfusion algorithms. Br. J. Anaesth. 2009, 103, i14–i22. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.; Fritz, S.; Hake, D.; Son, M.; Greve, S.; Jbara, M.; Chitta, S.; Fritz, B.; Miller, A.; Bader, M.K.; et al. Targeted Thromboelastographic (TEG) Blood Component and Pharmacologic Hemostatic Therapy in Traumatic and Acquired Coagulopathy. Curr. Drug. Targets 2016, 17, 954–970. [Google Scholar] [CrossRef]
- Schöchl, H.; Maegele, M.; Solomon, C.; Görlinger, K.; Voelckel, W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 15. [Google Scholar] [CrossRef] [Green Version]
- Veigas, P.V.; Callum, J.; Rizoli, S.; Nascimento, B.; da Luz, L.T. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, H.; Stanworth, S.; Curry, N.; Woolley, T.; Cooper, C.; Ukoumunne, O.; Zhelev, Z.; Hyde, C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Libr. 2015, 2, CD010438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikkelsø, A.; Wetterslev, J.; Møller, A.M.; Afshari, A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst. Rev. 2016, 2016, CD007871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikkelsø, A.; Wetterslev, J.; Møller, A.M.; Afshari, A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: A systematic review with meta-analysis and trial sequential analysis. Anaesthesia 2017, 72, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Bugaev, N.; Como, J.J.; Golani, G.; Freeman, J.J.; Sawhney, J.S.; Vatsaas, C.J.; Yorkgitis, B.K.; Kreiner, L.A.; Garcia, N.M.; Aziz, H.A. Thromboelastography and Rotational Thromboelastometry in Bleeding Patients with Coagulopathy: Practice Management Guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2020, 89, 999–1017. [Google Scholar] [CrossRef]
- Walsh, M.; Thomas, S.; Kwaan, H.; Aversa, J.; Anderson, S.; Sundararajan, R.; Zimmer, D.; Bunch, C.; Stillson, J.; Draxler, D. Modern methods for monitoring hemorrhagic resuscitation in the United States: Why the delay? J. Trauma Acute Care Surg. 2020, 89, 1018–1022. [Google Scholar] [CrossRef]
- Sahli, S.D.; Rössler, J.; Tscholl, D.W.; Studt, J.-D.; Spahn, D.R.; Kaserer, A. Point-of-care diagnostics in coagulation management. Sensors 2020, 20, 4254. [Google Scholar] [CrossRef]
- Černý, V.; Maegele, M.; Agostini, V.; Fries, D.; Leal-Noval, S.R.; Nardai, G.; Nardi, G.; Östlund, A.; Schöchl, H. Variations and obstacles in the use of coagulation factor concentrates for major trauma bleeding across Europe: Outcomes from a European expert meeting. Eur. J. Trauma Emerg. Surg. 2021, 1–12. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef]
- Johansson, P.I. Goal-Directed hemostatic resuscitation for massively bleeding patients: The Copenhagen concept. Transfus. Apher. Sci. 2010, 43, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.A.; Ogawa, S.; Bolliger, D. A primer for clinical use of rotational thromboelastometry. Point Care 2012, 11, 77–84. [Google Scholar] [CrossRef]
- Görlinger, K.; Almutawah, H.; Almutawaa, F.; Alwabari, M.; Alsultan, Z.; Almajed, J.; Alwabari, M.; Alsultan, M.; Shahwar, D.; Yassen, K.A. The role of rotational thromboelastometry during the COVID-19 pandemic: A narrative review. Korean J. Anesthesiol. 2021, 74, 91. [Google Scholar] [CrossRef] [PubMed]
- Snegovskikh, D.; Souza, D.; Walton, Z.; Dai, F.; Rachler, R.; Garay, A.; Snegovskikh, V.V.; Braveman, F.R.; Norwitz, E.R. Point-of-Care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J. Clin. Anesth. 2018, 44, 50–56. [Google Scholar] [CrossRef] [PubMed]
- McNamara, H.; Mallaiah, S. Managing coagulopathy following PPH. Best Pract. Res. Clin. Obs. Gynaecol. 2019, 61, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Hartmann, J.; Murphy, M.; Dias, J.D. Viscoelastic hemostatic assays: Moving from the laboratory to the site of care—A review of established and emerging technologies. Diagnostics 2020, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, E.; Pieracci, F.M.; Moore, E.E.; Kashuk, J.L. Coagulation abnormalities in the trauma patient: The role of point-of-care thromboelastography. Semin. Thromb. Hemost. 2010, 36, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Kashuk, J.; Moore, E.; Sawyer, M.; Wohlauer, M.; Pezold, M.; Barnett, C.; Biffl, W.; Burlew, C.; Johnson, J.; Sauaia, A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann. Surg. 2010, 252, 434–444. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, S.G.; Luddington, R.J. Critical factors contributing to the thromboelastography trace. Semin. Thromb. Hemost. 2010, 36, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Luddington, R.J. Thrombelastography/thromboelastometry. Clin. Lab. Haematol. 2005, 27, 81–90. [Google Scholar] [CrossRef]
- Schochl, H.; Voelckel, W.; Grassetto, A.; Schlimp, C.J. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J. Trauma Acute Care Surg. 2013, 74, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Einersen, P.M.; Moore, E.E.; Chapman, M.P.; Moore, H.B.; Gonzalez, E.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Rapid-Thrombelastography (r-TEG) thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J. Trauma Acute Care Surg. 2017, 82, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, T.H.; Meyer, M.A.; Meyer, A.S.P.; Gaarder, T.; Naess, P.A.; Stensballe, J.; Ostrowski, S.R.; Johansson, P.I. Thrombelastography early amplitudes in bleeding and coagulopathic trauma patients: Results from a multicenter study. J. Trauma Acute Care Surg. 2018, 84, 334–341. [Google Scholar] [CrossRef]
- Wirtz, M.R.; Baumann, H.M.; Klinkspoor, J.H.; Goslings, J.C.; Juffermans, N.P. Viscoelastic testing in trauma. Semin. Thromb. Hemost. 2017, 43, 375–385. [Google Scholar] [PubMed]
- Curry, N.S.; Davenport, R.; Pavord, S.; Mallett, S.V.; Kitchen, D.; Klein, A.A.; Maybury, H.; Collins, P.W.; Laffan, M. The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br. J. Haematol. 2018, 182, 789–806. [Google Scholar] [CrossRef] [Green Version]
- Gorlinger, K.; Bhardwaj, V.; Kapoor, P.M. Simulation in coagulation testing using rotational thromboelastometry: A fast emerging, reliable point of care technique. Ann. Card. Anaesth. 2016, 19, 516–520. [Google Scholar] [PubMed]
- Stettler, G.R.; Moore, E.E.; Moore, H.B.; Nunns, G.R.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Redefining postinjury fibrinolysis phenotypes using two viscoelastic assays. J. Trauma Acute Care Surg. 2019, 86, 679–685. [Google Scholar] [CrossRef]
- Hartmann, J.; Sikorski, R.A. Thromboelastography (TEG® 5000 and TEG® 6s Hemostasis Analyzers with TEG Manager® Software). In Trauma Induced Coagulopathy, 2nd ed.; Moore, H.B., Moore, E.E., Neal, M.D., Eds.; Spring Nature Switzerland AG: Cham, Switzerland, 2021; pp. 313–331. [Google Scholar]
- Bocci, M.; Maviglia, R.; Consalvo, L.; Grieco, D.; Montini, L.; Mercurio, G.; Nardi, G.; Pisapia, L.; Cutuli, S.; Biasucci, D. Thromboelastography clot strength profiles and effect of systemic anticoagulation in COVID-19 acute respiratory distress syndrome: A prospective, observational study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12466–12479. [Google Scholar]
- Whiting, P.; Al, M.; Westwood, M.; Ramos, I.C.; Ryder, S.; Armstrong, N.; Misso, K.; Ross, J.; Severens, J.; Kleijnen, J. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: A systematic review and cost-effectiveness analysis. Health Technol. Assess 2015, 19, 1–228, v–vi. [Google Scholar] [CrossRef] [Green Version]
- Johansson, P.I.; Stensballe, J.; Oliveri, R.; Wade, C.E.; Ostrowski, S.R.; Holcomb, J.B. How I treat patients with massive hemorrhage. Blood 2014, 124, 3052–3058. [Google Scholar] [CrossRef] [Green Version]
- Stein, P.; Kaserer, A.; Spahn, G.H.; Spahn, D.R. Point-of-Care Coagulation Monitoring in Trauma Patients. Semin. Thromb. Hemost. 2017, 43, 367–374. [Google Scholar] [CrossRef]
- Sankarankutty, A.; Nascimento, B.; da Luz, L.T.; Rizoli, S. TEG® and ROTEM® in trauma: Similar test but different results? World J. Emerg. Surg. 2012, 7, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.; Moore, E.E.; Moore, H.; Thomas, S.; Lune, S.V.; Zimmer, D.; Dynako, J.; Hake, D.; Crowell, Z.; McCauley, R. Use of Viscoelastography in Malignancy-Associated Coagulopathy and Thrombosis: A Review. Semin. Thromb. Hemost. 2019, 45, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H. Use of Viscoelastography in Malignancy-Associated Coagulopathy and Thrombosis: A Review. J. Trauma Acute Care Surg. 2013, 74, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Bauters, A.; Braun, S.L.; Pötzsch, B.; von Pape, K.-W.; Kolde, H.-J.; Lakner, M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul. Fibrinolysis 2005, 16, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Haemonetics Corporation. Introducing TEG 6s—White Paper. Available online: https://pdf.medicalexpo.com/pdf/haemonetics/introducing-teg-6s-white-paper/78504-152619.html (accessed on 4 February 2022).
- Thromboelastography (TEG) Is available 24/7 at UCM. Available online: https://uchicagomedlabs.testcatalog.org/catalogs/367/files/12824 (accessed on 4 February 2022).
- Hartmann, J.; Walsh, M.; Grisoli, A.; Thomas, A.V.; Shariff, F.; McCauley, R.; Lune, S.V.; Zackariya, N.; Patel, S.; Farrell, M.S.; et al. Diagnosis and Treatment of Trauma-Induced Coagulopathy by Viscoelastography. Semin. Thromb. Hemost. 2020, 46, 134–146. [Google Scholar] [CrossRef]
- Cotton, B.A.; Faz, G.; Hatch, Q.M.; Radwan, Z.A.; Podbielski, J.; Wade, C.; Kozar, R.A.; Holcomb, J.B. Rapid Thrombelastography Delivers Real-Time Results That Predict Transfusion Within 1 Hour of Admission. J. Trauma Acute Care Surg. 2011, 71, 407–417. [Google Scholar] [CrossRef]
- Cotton, B.A.; Minei, K.M.; Radwan, Z.A.; Matijevic, N.; Pivalizza, E.; Podbielski, J.; Wade, C.E.; Kozar, R.A.; Holcomb, J.B. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J. Trauma Acute Care Surg. 2012, 72, 1470–1477. [Google Scholar] [CrossRef]
- Görlinger, K.; Dirkmann, D.; Hanke, A.A. Rotational Thromboelastometry (ROTEM®). In Trauma Induced Coagulopathy, 2nd ed.; Moore, H.B., Moore, E.E., Neal, M.D., Eds.; Spring Nature Switzerland AG: Cham, Switzerland, 2021; pp. 279–312. [Google Scholar]
- Simurda, T.; Asselta, R.; Zolkova, J.; Brunclikova, M.; Dobrotova, M.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Congenital Afibrinogenemia and Hypofibrinogenemia: Laboratory and Genetic Testing in Rare Bleeding Disorders with Life-Threatening Clinical Manifestations and Challenging Management. Diagnostics 2021, 11, 2140. [Google Scholar] [CrossRef]
- Niles, S.E.; McLaughlin, D.F.; Perkins, J.G.; Wade, C.E.; Li, Y.; Spinella, P.C.; Holcomb, J.B. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J. Trauma 2008, 64, 1459–1463; discussion 1463–1465. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.S.; Meyer, M.A.; Sorensen, A.M.; Rasmussen, L.S.; Hansen, M.B.; Holcomb, J.B.; Cotton, B.A.; Wade, C.E.; Ostrowski, S.R.; Johansson, P.I. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J. Trauma Acute Care Surg. 2014, 76, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.J.; Lyons, G. Thromboelastography should be available in every labour ward. Int. J. Obstet. Anesth. 2005, 14, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, Á.A.P.; Kim, T.-Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Görlinger, K.; Iqbal, J.; Dirkmann, D.; Tanaka, K.A. Whole Blood Assay: Thromboelastometry. In Management of Bleeding Patients; Teruya, J., Ed.; Spring Nature Switzerland AG: Cham, Switzerland, 2021; pp. 45–87. [Google Scholar]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ganter, M.T.; Hofer, C.K. Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth. Analg. 2008, 106, 1366–1375. [Google Scholar] [CrossRef] [Green Version]
- Haemonetics Corporation. TEG 6s Hemostasis System. U.S. Patent No. 02184, 2019. [Google Scholar]
- Gurbel, P.A.; Bliden, K.P.; Tantry, U.S.; Monroe, A.L.; Muresan, A.A.; Brunner, N.E.; Lopez-Espina, C.G.; Delmenico, P.R.; Cohen, E.; Raviv, G.; et al. First report of the point-of-care TEG: A technical validation study of the TEG-6S system. Platelets 2016, 27, 642–649. [Google Scholar] [CrossRef]
- Dias, J.D.; Haney, E.I.; Mathew, B.A.; Lopez-Espina, C.G.; Orr, A.W.; Popovsky, M.A. New-Generation Thromboelastography: Comprehensive Evaluation of Citrated and Heparinized Blood Sample Storage Effect on Clot-Forming Variables. Arch. Pathol. Lab. Med. 2017, 141, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Leyser, H. Devices and Methods for Measuring Viscoelastic Changes of a Sample. Patent No. WO 2018/137766 Al, 2018. Available online: https://patentimages.storage.googleapis.com/08/d3/f3/f31384ed000c19/WO2018137766A1.pdf (accessed on 6 January 2022).
- Lloyd-Donald, P.; Churilov, L.; Zia, F.; Bellomo, R.; Hart, G.; McCall, P.; Mårtensson, J.; Glassford, N.; Weinberg, L. Assessment of agreement and interchangeability between the TEG5000 and TEG6S thromboelastography haemostasis analysers: A prospective validation study. BMC Anesthesiol. 2019, 19, 45. [Google Scholar] [CrossRef]
- Neal, M.D.; Moore, E.E.; Walsh, M.; Thomas, S.; Callcut, R.A.; Kornblith, L.Z.; Schreiber, M.; Ekeh, A.P.; Singer, A.J.; Lottenberg, L. A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J. Trauma Acute Care Surg. 2020, 88, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Hauck, J.; Welsby, I.J.; Groves, D.S.; Naik, B.; Tanaka, K.; Finley, A.; Greenberg, C.; Viola, F.; Winegar, D. Multi-Center Investigation of the Clinical Performance of the Quantra System versus the Rotem Delta in Surgical Patients. In American Society of Anesthesiologist Annual Meeting; 2001; Available online: http://www.asaabstracts.com/strands/asaabstracts/abstract.htm?year=2018&index=8&absnum=4844 (accessed on 6 January 2022).
- Groves, D.S.; Winegar, D.A.; Fernandez, L.G.; Huffmyer, J.L.; Viola, F. Comparison of Coagulation Parameters in Arterial and Venous Blood in Cardiac Surgery Measured Using the Quantra System. J. Cardiothorac. Vasc. Anesth. 2019, 33, 976–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, D.S.; Welsby, I.J.; Naik, B.I.; Tanaka, K.; Hauck, J.N.; Greenberg, C.S.; Winegar, D.A.; Viola, F. Multicenter evaluation of the Quantra QPlus system in adult patients undergoing major surgical procedures. Anesth. Analg. 2020, 130, 899–909. [Google Scholar] [CrossRef]
- Volod, O.; Selby, R.; Gil, M.R.; Kreuziger, L.B.; Lee, A.Y. COVID-19 and Viscoelastic Hemostasis Assays: Frequently Asked Questions. Available online: https://www.hematology.org/covid-19/covid-19-and-ve (accessed on 19 June 2021).
- Hemosonics. Quantra® Hemostasis System Brochure. Available online: https://hemosonics.com/wp-content/uploads/2020/10/HemoSonics-Quantra-Brochure.pdf (accessed on 4 February 2022).
- ClotPro. ClotPro® System New Generation Viscoelastic Diagnostics. Available online: https://www.clot.pro/site/assets/files/1053/product_specification_rev001_enicor_en_2019_06.pdf (accessed on 21 June 2021).
- Carll, T.; Wool, G.D. Basic principles of viscoelastic testing. Transfusion 2020, 60, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, A.; van den Akker, T.; Caram-Deelder, C.; Henriquez, D.D.; Bloemenkamp, K.W.; Eikenboom, J.; van der Bom, J.G.; de Maat, M.P. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand. J. Clin. Lab. Investig. 2019, 79, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Schenk, B.; Görlinger, K.; Treml, B.; Tauber, H.; Fries, D.; Niederwanger, C.; Oswald, E.; Bachler, M. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia 2019, 74, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Zolkova, J.; Snahnicanova, Z.; Loderer, D.; Skornova, I.; Sokol, J.; Hudecek, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Identification of Two Novel Fibrinogen Bβ Chain Mutations in Two Slovak Families with Quantitative Fibrinogen Disorders. Int. J. Mol. Sci. 2017, 19, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.W.; Winegar, D.; Viola, F. The Quantra® System and SEER Sonorheometry. In Trauma Induced Coagulopathy, 2nd ed.; Moore, H.B., Moore, E.E., Neal, M.D., Eds.; Spring Nature Switzerland AG: Cham, Switzerland, 2021; pp. 693–704. [Google Scholar]
- Hochleitner, G.; Sutor, K.; Levett, C.; Leyser, H.; Schlimp, C.J.; Solomon, C. Revisiting Hartert’s 1962 calculation of the physical constants of thrombelastography. Clin. Appl. Thromb. Hemost. 2017, 23, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffmyer, J.L.; Fernandez, L.G.; Haghighian, C.; Terkawi, A.S.; Groves, D.S. Comparison of SEER Sonorheometry With Rotational Thromboelastometry and Laboratory Parameters in Cardiac Surgery. Anesth. Analg. 2016, 123, 1390–1399. [Google Scholar] [CrossRef]
- Naik, B.I.; Durieux, M.E.; Knisely, A.; Sharma, J.; Bui-Huynh, V.C.; Yalamuru, B.; Terkawi, A.S.; Nemergut, E.C. SEER sonorheometry versus rotational thromboelastometry in large volume blood loss spine surgery. Anesth. Analg. 2016, 123, 1380–1389. [Google Scholar] [CrossRef]
- Reynolds, P.S.; Middleton, P.; McCarthy, H.; Spiess, B.D. A Comparison of a New Ultrasound-Based Whole Blood Viscoelastic Test (SEER Sonorheometry) Versus Thromboelastography in Cardiac Surgery. Anesth. Analg. 2016, 123, 1400–1407. [Google Scholar] [CrossRef]
- Baryshnikova, E.; Di Dedda, U.; Ranucci, M. A Comparative Study of SEER Sonorheometry Versus Standard Coagulation Tests, Rotational Thromboelastometry, and Multiple Electrode Aggregometry in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1590–1598. [Google Scholar] [CrossRef]
- Selby, R. “TEG talk”: Expanding clinical roles for thromboelastography and rotational thromboelastometry. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.L.; Rossi, J.M. Point of care whole blood microfluidics for detecting and managing thrombotic and bleeding risks. Lab Chip 2021, 21, 3667–3674. [Google Scholar] [CrossRef]
- Mohammadi Aria, M.; Erten, A.; Yalcin, O. Technology advancements in blood coagulation measurements for point-of-care diagnostic testing. Front. Bioeng. Biotechnol. 2019, 7, 395. [Google Scholar] [CrossRef]
- Nadkarni, S.K. Optical thromboelastography system and method for evaluation of blood coagulation metrics. J. Biophotonics 2015, 8, 372–381. [Google Scholar]
- Nadkarni, S.K. Comprehensive coagulation profiling at the point-of-care using a novel laser-based approach. Semin. Thromb. Hemost. 2019, 45, 264–274. [Google Scholar] [CrossRef]
- Abhishek, R.; Yoshimizu, N. Methods, Devices, and Systems for Measuring Physical Properties of Fluid. Patent No. US 2013/0192349 A1, 2016. Available online: https://patentimages.storage.googleapis.com/40/1b/79/6e41ce3defdfbc/US20130192349A1.pdf (accessed on 6 January 2022).
- Dennis, R.G.; Fischer, T.H.; Dacorta, J.A. Portable Coagulation Monitoring Device and Method of Assessing Coagulation Response. Patent No. WO/2011/075614, 2013. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011075614 (accessed on 6 January 2022).
- Peng, H.T. Hemostatic agents for prehospital hemorrhage control: A narrative review. Mil. Med. Res. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, B.; Bachler, M.; Haberfellner, H.; Niederwanger, C.; Innerhofer, P.; Hell, T.; Kaufmann, M.; Maegele, M.; Martinowitz, U.; Nebl, C.; et al. Efficacy of prehospital administration of fibrinogen concentrate in trauma patients bleeding or presumed to bleed (FIinTIC): A multicentre, double-blind, placebo-controlled, randomised pilot study. Eur. J. Anaesthesiol. 2021, 38, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Prat, N.J.; Meyer, A.D.; Ingalls, N.K.; Trichereau, J.; DuBose, J.J.; Cap, A.P. Rotational thromboelastometry significantly optimizes transfusion practices for damage control resuscitation in combat casualties. J. Trauma Acute Care Surg. 2017, 83, 373. [Google Scholar] [CrossRef]
- Walsh, M.; Shreve, J.; Thomas, S.; Moore, E.; Moore, H.; Hake, D.; Pohlman, T.; Davis, P.; Ploplis, V.; Piscoya, A. Fibrinolysis in trauma:“myth,”“reality,” or “something in between”. Semin. Thromb. Hemost. 2017, 43, 200–212. [Google Scholar]
- Li, R.; Elmongy, H.; Sims, C.; Diamond, S.L. Ex Vivo recapitulation of trauma-induced coagulopathy and assessment of trauma patient platelet function under flow using microfluidic technology. J. Trauma Acute Care Surg. 2016, 80, 440. [Google Scholar] [CrossRef] [Green Version]
- Bochsen, L.; Wiinberg, B.; Kjelgaard-Hansen, M.; Steinbrüchel, D.A.; Johansson, P.I. Evaluation of the TEG® platelet mapping™ assay in blood donors. Thromb. J. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craft, R.M.; Chavez, J.J.; Bresee, S.J.; Wortham, D.C.; Cohen, E.; Carroll, R.C. A novel modification of the Thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J. Lab. Clin. Med. 2004, 143, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; Chaireti, R.; Bruzelius, M.; Holmström, M.; Antovic, J.; Ågren, A. Correlation of thromboelastography and thrombin generation assays in warfarin-treated patients. Thromb. Res. 2019, 178, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Adcock, D.M.; Bates, S.M.; Douxfils, J.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb. Haemost. 2018, 118, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.D.; Lopez-Espina, C.G.; Ippolito, J.; Hsiao, L.H.; Zaman, F.; Muresan, A.A.; Thomas, S.G.; Walsh, M.; Jones, A.J.; Grisoli, A.; et al. Rapid point-of-care detection and classification of direct-acting oral anticoagulants with the TEG 6s: Implications for trauma and acute care surgery. J. Trauma Acute Care Surg. 2019, 87, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Regling, K.; Kakulavarapu, S.; Thomas, R.; Hollon, W.; Chitlur, M.B. Utility of thromboelastography for the diagnosis of von Willebrand disease. Pediatr. Blood Cancer 2019, 66, e27714. [Google Scholar] [CrossRef] [PubMed]
- Topf, H.-G.; Weiss, D.; Lischetzki, G.; Strasser, E.; Rascher, W.; Rauh, M. Evaluation of a modified thromboelastography assay for the screening of von Willebrand disease. Thromb. Haemost. 2011, 105, 1091–1099. [Google Scholar] [CrossRef]
- Toukh, M.; Ozelo, M.; Angelillo-Scherrer, A.; Othman, M. A novel use of thromboelastography in type 2B von Willebrand disease. Int. J. Lab. Hematol. 2013, 35, e11–e14. [Google Scholar] [CrossRef]
- Guzman-Reyes, S.; Osborne, C.; Pivalizza, E.G. Thrombelastography for perioperative monitoring in patients with von Willebrand disease. J. Clin. Anesth. 2012, 24, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; Majeed, A.; Bruzelius, M.; Odeberg, J.; Holmström, M.; Ågren, A. A prospective diagnostic accuracy study evaluating rotational thromboelastometry and thromboelastography in 100 patients with von Willebrand disease. Haemophilia 2017, 23, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.Z.; Riha, K.; Escobar, M.A.; Pivalizza, E.G. Thrombelastograph platelet mapping in a patient with von Willebrand disease who was treated with Humate-P. J. Clin. Anesth. 2011, 23, 600. [Google Scholar] [CrossRef]
- Topf, H.-G.; Strasser, E.R.; Breuer, G.; Rascher, W.; Rauh, M.; Fahlbusch, F.B. Closing the gap–detection of clinically relevant von Willebrand disease in emergency settings through an improved algorithm based on rotational Thromboelastometry. BMC Anesthesiol. 2019, 19, 10. [Google Scholar] [CrossRef]
- Ramiz, S.; Hartmann, J.; Young, G.; Escobar, M.A.; Chitlur, M. Clinical utility of viscoelastic testing (TEG and ROTEM analyzers) in the management of old and new therapies for hemophilia. Am. J. Hematol. 2019, 94, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artang, R.; Anderson, M.; Nielsen, J.D. Fully automated thromboelastograph TEG 6s to measure anticoagulant effects of direct oral anticoagulants in healthy male volunteers. Res. Pract. Thromb. Haemost. 2019, 3, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Adcock, D.M.; Douxfils, J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int. J. Lab. Hematol. 2019, 41, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, J.; Nehaj, F.; Ivankova, J.; Mokan, M.; Zolkova, J.; Lisa, L.; Linekova, L.; Mokan, M.; Stasko, J. Impact of Dabigatran Treatment on Rotation Thromboelastometry. Clin. Appl. Thromb. Hemost. 2021, 27. [Google Scholar] [CrossRef]
- Seyve, L.; Richarme, C.; Polack, B.; Marlu, R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int. J. Lab. Hematol. 2018, 40, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Chapman, M.P.; Gonzalez, E.; Sauaia, A. Goal-Directed hemostatic resuscitation for trauma induced coagulopathy: Maintaining homeostasis. J. Trauma Acute Care Surg. 2018, 84, S35–S40. [Google Scholar] [CrossRef]
- Colman, E.; Yin, E.B.; Laine, G.; Chatterjee, S.; Saatee, S.; Herlihy, J.P.; Reyes, M.A.; Bracey, A.W. Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature. J. Thorac. Dis. 2019, 11, 3325–3335. [Google Scholar] [CrossRef]
- Christie, S.A.; Kornblith, L.Z.; Howard, B.M.; Conroy, A.S.; Kunitake, R.C.; Nelson, M.F.; Hendrickson, C.M.; Calfee, C.S.; Callcut, R.A.; Cohen, M.J. Characterization of distinct coagulopathic phenotypes in injury: Pathway-specific drivers and implications for individualized treatment. J. Trauma Acute Care Surg. 2017, 82, 1055. [Google Scholar] [CrossRef]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann. Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Theusinger, O.; Felix, C.; Spahn, D. Strategies to reduce the use of blood products: A European perspective. Curr. Opin. Anaesthesiol. 2012, 25, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Theusinger, O.M.; Madjdpour, C.; Spahn, D.R. Resuscitation and transfusion management in trauma patients: Emerging concepts. Curr. Opin. Crit. Care 2012, 18, 661–670. [Google Scholar] [CrossRef]
- Hranjec, T.; Estreicher, M.; Rogers, B.; Kohler, L.; Solomon, R.; Hennessy, S.; Cibulas, M.; Hurst, D.; Hegazy, M.; Lee, J. Integral Use of Thromboelastography with Platelet Mapping to Guide Appropriate Treatment, Avoid Complications, and Improve Survival of Patients with Coronavirus Disease 2019–Related Coagulopathy. Crit. Care Explor. 2020, 2, e0287. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.P.; Moore, E.E.; Ramos, C.R.; Ghasabyan, A.; Harr, J.N.; Chin, T.L.; Stringham, J.R.; Sauaia, A.; Silliman, C.C.; Banerjee, A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J. Trauma Acute Care Surg 2013, 75, 961. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, L.; Cohen, M.; Cotton, B.; Schreiber, M.; Moore, E. Tranexamic acid in trauma: How should we use it? J. Trauma Acute Care Surg. 2013, 74, 1575–1586. [Google Scholar] [CrossRef] [Green Version]
- Theusinger, O.; Wanner, G.; Emmert, M.; Billeter, A.; Eismon, J.; Seifert, B.; Simmen, H.-P.; Spahn, D.; Baulig, W. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth. Analg. 2011, 113, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Kashuk, J.L.; Moore, E.E.; Sabel, A.; Barnett, C.; Haenel, J.; Le, T.; Pezold, M.; Lawrence, J.; Biffl, W.L.; Cothren, C.C.; et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery 2009, 146, 764–772; discussion 772–774. [Google Scholar] [CrossRef]
- Nunns, G.R.; Moore, H.B.; Moore, E.E. Goal-Directed Massive Transfusion Management. In Trauma Induced Coagulopathy, 2nd ed.; Moore, H.B., Moore, E.E., Neal, M.D., Eds.; Spring Nature Switzerland AG: Cham, Switzerland, 2021; pp. 487–494. [Google Scholar]
- Solomon, C.; Traintinger, S.; Ziegler, B.; Hanke, A.; Rahe-Meyer, N.; Voelckel, W.; Schochl, H. Platelet function following trauma. A multiple electrode aggregometry study. Thromb. Haemost. 2011, 106, 322–330. [Google Scholar]
- Kornblith, L.Z.; Moore, H.B.; Cohen, M.J. Trauma-induced coagulopathy: The past, present, and future. J. Thromb. Haemost. 2019, 17, 852–862. [Google Scholar] [CrossRef]
- Wohlauer, M.V.; Moore, E.E.; Thomas, S.; Sauaia, A.; Evans, E.; Harr, J.; Silliman, C.C.; Ploplis, V.; Castellino, F.J.; Walsh, M. Early platelet dysfunction: An unrecognized role in the acute coagulopathy of trauma. J. Am. Coll. Surg. 2012, 214, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.; Shreve, J.; Thomas, S. The value of cold storage whole blood platelets in trauma resuscitation is like real estate: A function of ‘location, location, location’. Br. J. Haematol. 2017, 179, 699–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holcomb, J.B.; Wade, C.E.; Michalek, J.E.; Chisholm, G.B.; Zarzabal, L.A.; Schreiber, M.A.; Gonzalez, E.A.; Pomper, G.J.; Perkins, J.G.; Spinella, P.C.; et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann. Surg. 2008, 248, 447–458. [Google Scholar] [CrossRef]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Moore, H.B.; Moore, E.E.; Gonzalez, E. Mortality and ratio of blood products used in patients with severe trauma. JAMA 2015, 313, 2077. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Bunch, C.M.; Thomas, A.V.; Stillson, J.E.; Gillespie, L.; Lin, K.P.; Speybroeck, J.; Kwaan, H.C.; Fulkerson, D.H.; Zamlut, M.; Khan, R.; et al. Thromboelastography-Guided Anticoagulant Therapy for the Double Hazard of Thrombohemorrhagic Events in COVID-19: A Report of 3 Cases. Am. J. Case Rep. 2021, 22, e931080. [Google Scholar] [CrossRef]
- Bareille, M.; Hardy, M.; Douxfils, J.; Roullet, S.; Lasne, D.; Levy, J.H.; Stépanian, A.; Susen, S.; Frère, C.; Lecompte, T. Viscoelastometric Testing to Assess Hemostasis of COVID-19: A Systematic Review. J. Clin. Med. 2021, 10, 1740. [Google Scholar] [CrossRef]
- Gergi, M.; Goodwin, A.; Freeman, K.; Colovos, C.; Volod, O. Viscoelastic hemostasis assays in septic, critically ill coronavirus disease 2019 patients: A practical guide for clinicians. Blood Coagul. Fibrinolysis 2021, 32, 225–228. [Google Scholar] [CrossRef]
- Stillson, J.E.; Bunch, C.M.; Gillespie, L.; Khan, R.; Wierman, M.; Pulvirenti, J.; Phyu, H.; Anderson, S.; Al-Fadhl, M.; Thomas, A.V.; et al. Thromboelastography-Guided Management of Anticoagulated COVID-19 Patients to Prevent Hemorrhage. Semin. Thromb. Hemost. 2021, 47, 442–446. [Google Scholar] [CrossRef]
- Matthay, Z.A.; Kornblith, L.Z. Platelets. In Trauma Induced Coagulopathy, 2nd ed.; Moore, H.B., Moore, E.E., Neal, M.D., Eds.; Spring Nature Switzerland AG: Cham, Switzerland, 2021; pp. 85–100. [Google Scholar]
- Dias, J.D.; Pottgiesser, T.; Hartmann, J.; Duerschmied, D.; Bode, C.; Achneck, H.E. Comparison of three common whole blood platelet function tests for in vitro P2Y12 induced platelet inhibition. J. Thromb. Thromb. 2020, 50, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimmer, C.; Hamouda, K.; Sommer, S.P.; Özkur, M.; Hain, J.; Leyh, R. The predictive value of multiple electrode platelet aggregometry (multiplate) in adult cardiac surgery. Thorac. Cardiovasc. Surg. 2013, 61, 733–743. [Google Scholar] [PubMed]
- Kander, T.; Larsson, A.; Taune, V.; Schött, U.; Tynngård, N. Assessment of haemostasis in disseminated intravascular coagulation by use of point-of-care assays and routine coagulation tests, in critically ill patients; a prospective observational study. PLoS ONE 2016, 11, e0151202. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.; Beilin, Y. Disorders of coagulation in pregnancy. Br. J. Anaesth. 2015, 115 (Suppl. 2), ii75–ii88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, V.; Finger, T.; Bayerl, S.; Rosenthal, C.; Wolf, S.; Liman, T.; Vajkoczy, P. High prevalence of pharmacologically induced platelet dysfunction in the acute setting of brain injury. Acta Neurochir. 2016, 158, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, L.T.; Nascimento, B.; Shankarakutty, A.K.; Rizoli, S.; Adhikari, N.K. Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: Descriptive systematic review. Crit. Care 2014, 18, 518. [Google Scholar] [CrossRef] [Green Version]
- Lam, H.; Katyal, N.; Parker, C.; Natteru, P.; Nattanamai, P.; Newey, C.R.; Kraus, C.K. Thromboelastography with platelet mapping is not an effective measure of platelet inhibition in patients with spontaneous intracerebral hemorrhage on antiplatelet therapy. Cureus 2018, 10, e2515. [Google Scholar] [CrossRef] [Green Version]
- Connelly, C.R.; Yonge, J.D.; McCully, S.P.; Hart, K.D.; Hilliard, T.C.; Lape, D.E.; Watson, J.J.; Rick, B.; Houser, B.; Deloughery, T.G. Assessment of three point-of-care platelet function assays in adult trauma patients. J. Surg. Res. 2017, 212, 260–269. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E. Sensitivity of viscoelastic tests to platelet function. J. Clin. Med. 2020, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Simurda, T.; Casini, A.; Stasko, J.; Hudecek, J.; Skornova, I.; Vilar, R.; Neerman-Arbez, M.; Kubisz, P. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb. Res. 2020, 188, 1–4. [Google Scholar] [CrossRef]
- Volod, O.; Arabia, F.A.; Lam, L.D.; Runge, A.; Cheng, C.; Czer, L.S. Platelet Mapping by Thromboelastography and Whole Blood Aggregometry in Adult Patients Supported by Mechanical Circulatory Support Device on Aspirin Therapy. J. Extra Corpor. Technol. 2020, 52, 13. [Google Scholar] [PubMed]
- Görlinger, K.; Dirkmann, D.; Gandhi, A.; Simioni, P. COVID-19 associated coagulopathy and inflammatory response: What do we know already and what are the knowledge gaps? Anesth. Analg. 2020, 131, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Ergang, A.; Mason, D.; Dias, J.D. The Role of TEG Analysis in Patients with COVID-19-Associated Coagulopathy: A Systematic Review. Diagnostics 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.M.; Khan, R.; Kwaan, H.C.; Neal, M.D. Fibrinolysis Shutdown in COVID-19-Associated Coagulopathy: A Crosstalk among Immunity, Coagulation, and Specialists in Medicine and Surgery. J. Am. Coll. Surg. 2021, 232, 1003–1006. [Google Scholar] [CrossRef]
- Jayarangaiah, A.; Kariyanna, P.T.; Chen, X.; Jayarangaiah, A.; Kumar, A. COVID-19-associated coagulopathy: An exacerbated immunothrombosis response. Clin. Appl. Thromb. Hemost. 2020, 26. [Google Scholar] [CrossRef]
- Baron, D.; Franchini, M.; Goobie, S.; Javidroozi, M.; Klein, A.; Lasocki, S.; Liumbruno, G.; Muñoz, M.; Shander, A.; Spahn, D. Patient blood management during the COVID–19 pandemic: A narrative review. Anaesthesia 2020, 75, 1105–1113. [Google Scholar] [CrossRef]
- Coagulation Systems for Measurement of Viscoelastic Properties: Enforcement Policy during the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (Revised); USA Food & Drug Administration: Rockville, MD, USA, 2021.
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef]
- NIH ACTIV Initiative Launches Adaptive Clinical Trials of Blood-Clotting Treatments for COVID-19. Available online: https://www.nih.gov/news-events/news-releases/nih-activ-initiative-launches-adaptive-clinical-trials-blood-clotting-treatments-covid-19 (accessed on 21 February 2021).
- Bachler, M.; Bösch, J.; Stürzel, D.P.; Hell, T.; Giebl, A.; Ströhle, M.; Klein, S.J.; Schäfer, V.; Lehner, G.F.; Joannidis, M. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesth. 2021, 126, 590–598. [Google Scholar] [CrossRef]
- Bunch, C.M.; Thomas, A.V.; Stillson, J.E.; Gillespie, L.; Khan, R.Z.; Zackariya, N.; Shariff, F.; Al-Fadhl, M.; Mjaess, N.; Miller, P.D.; et al. Preventing Thrombohemorrhagic Complications of Heparinized COVID-19 Patients Using Adjunctive Thromboelastography: A Retrospective Study. J. Clin. Med. 2021, 10, 3097. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Cannegieter, S.C.; Klok, F.A. COVID-19 associated coagulopathy and thromboembolic disease: Commentary on an interim expert guidance. Res. Pract. Thromb. Haemost. 2020, 4, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraissé, M.; Logre, E.; Pajot, O.; Mentec, H.; Plantefève, G.; Contou, D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: A French monocenter retrospective study. Crit. Care 2020, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Full-Dose Blood Thinners Decreased Need for Life Support and Improved Outcome in Hospitalized COVID-19 Patients. Available online: https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients (accessed on 26 January 2021).
- Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; Carrier, M.; Rosenson, R.S.; Reynolds, H.R.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N. Eng. J. Med. 2021, 385, 790–802. [Google Scholar]
- Tsantes, A.E.; Tsantes, A.G.; Kokoris, S.I.; Bonovas, S.; Frantzeskaki, F.; Tsangaris, I.; Kopterides, P. COVID-19 Infection-Related Coagulopathy and Viscoelastic Methods: A Paradigm for Their Clinical Utility in Critical Illness. Diagnostics 2020, 10, 817. [Google Scholar] [CrossRef]
- Słomka, A.; Kowalewski, M.; Żekanowska, E. Hemostasis in Coronavirus Disease 2019—Lesson from Viscoelastic Methods: A Systematic Review. Thromb. Haemost. 2021, 121, 1181–1192. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stissing, T.; Bochsen, L.; Ostrowski, S.R. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand. J. Trauma Resusc. Emerg. Med. 2009, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.; Kaplan, L.J.; Cannon, J.W. Thromboelastography-Guided Resuscitation of the Trauma Patient. JAMA Surg. 2019, 154, 1152–1153. [Google Scholar] [CrossRef]
- Görlinger, K.; Fries, D.; Dirkmann, D.; Weber, C.F.; Hanke, A.A.; Schöchl, H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus. Med. Hemother. 2012, 39, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Cohen, T.; Haas, T.; Cushing, M.M. The strengths and weaknesses of viscoelastic testing compared to traditional coagulation testing. Transfusion 2020, 60, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Meizoso, J.P.; Moore, H.B.; Moore, E.E. Fibrinolysis shutdown in COVID-19: Clinical manifestations, molecular mechanisms, and therapeutic implications. J. Am. Coll. Surg. 2021, 232, 995–1003. [Google Scholar] [CrossRef]
- Kwaan, H.C.; Lindholm, P.F. The Central Role of Fibrinolytic Response in COVID-19-A Hematologist’s Perspective. Int. J. Mol. Sci. 2021, 22, 1283. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.H.; Conway, E.M. Cross talk pathways between coagulation and inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, G.P.; Letson, H.L.; Sharma, R.; Sheppard, F.R.; Cap, A.P. Mechanisms of early trauma-induced coagulopathy: The clot thickens or not? J. Trauma Acute Care Surg. 2015, 79, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.; Maher, P.; Getrajdman, C.; Hamburger, J.; Zhao, S.; Madek, J.; Bhatt, H.; Levin, M.; Görlinger, K. Monitoring of COVID-19-Associated Coagulopathy and Anticoagulation with Thromboelastometry. Transfus. Med. Hemother. 2021, 48, 168–172. [Google Scholar] [CrossRef]
- Creel-Bulos, C.; Sniecinski, R. Fibrinolysis Shutdown and Thrombosis in a COVID-19 ICU. Shock 2021, 55, 845–846. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; Moghadam, K.G.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality among Patients with COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar]
- Chowdhury, J.F.; Moores, L.K.; Connors, J.M. Anticoagulation in hospitalized patients with COVID-19. N. Engl. J. Med. 2020, 383, 1675–1678. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.; Bornikova, L.; Gupta, S. COVID and Coagulation: Bleeding and Thrombotic Manifestations of SARS-CoV2 Infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Musoke, N.; Lo, K.B.; Albano, J.; Peterson, E.; Bhargav, R.; Gul, F.; DeJoy, R., 3rd; Salacup, G.; Pelayo, J.; Tipparaju, P.; et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb. Res. 2020, 196, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Häberle, H.; Schlensak, C.; Bitzer, M.; Malek, N.P.; Handgretinger, R.; Lang, P.; Hörber, S.; Peter, A.; Martus, P.; et al. Severe SARS-CoV-2 Infection Inhibits Fibrinolysis Leading to Changes in Viscoelastic Properties of Blood Clot: A Descriptive Study of Fibrinolysis in COVID-19. Thromb. Haemost. 2021, 121, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Nougier, C.; Benoit, R.; Simon, M.; Desmurs-Clavel, H.; Marcotte, G.; Argaud, L.; David, J.S.; Bonnet, A.; Negrier, C.; Dargaud, Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020, 18, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, A.M.; Braeken, D.C.W.; Ghossein-Doha, C.; van Santen, S.; Sels, J.E.M.; Kuiper, G.; van der Horst, I.C.C.; Ten Cate, H.; van Bussel, B.C.T.; Olie, R.H.; et al. Hemostasis and fibrinolysis in COVID-19 survivors 6 months after intensive care unit discharge. Res. Pract. Thromb. Haemost. 2021, 5, e12579. [Google Scholar] [CrossRef] [PubMed]
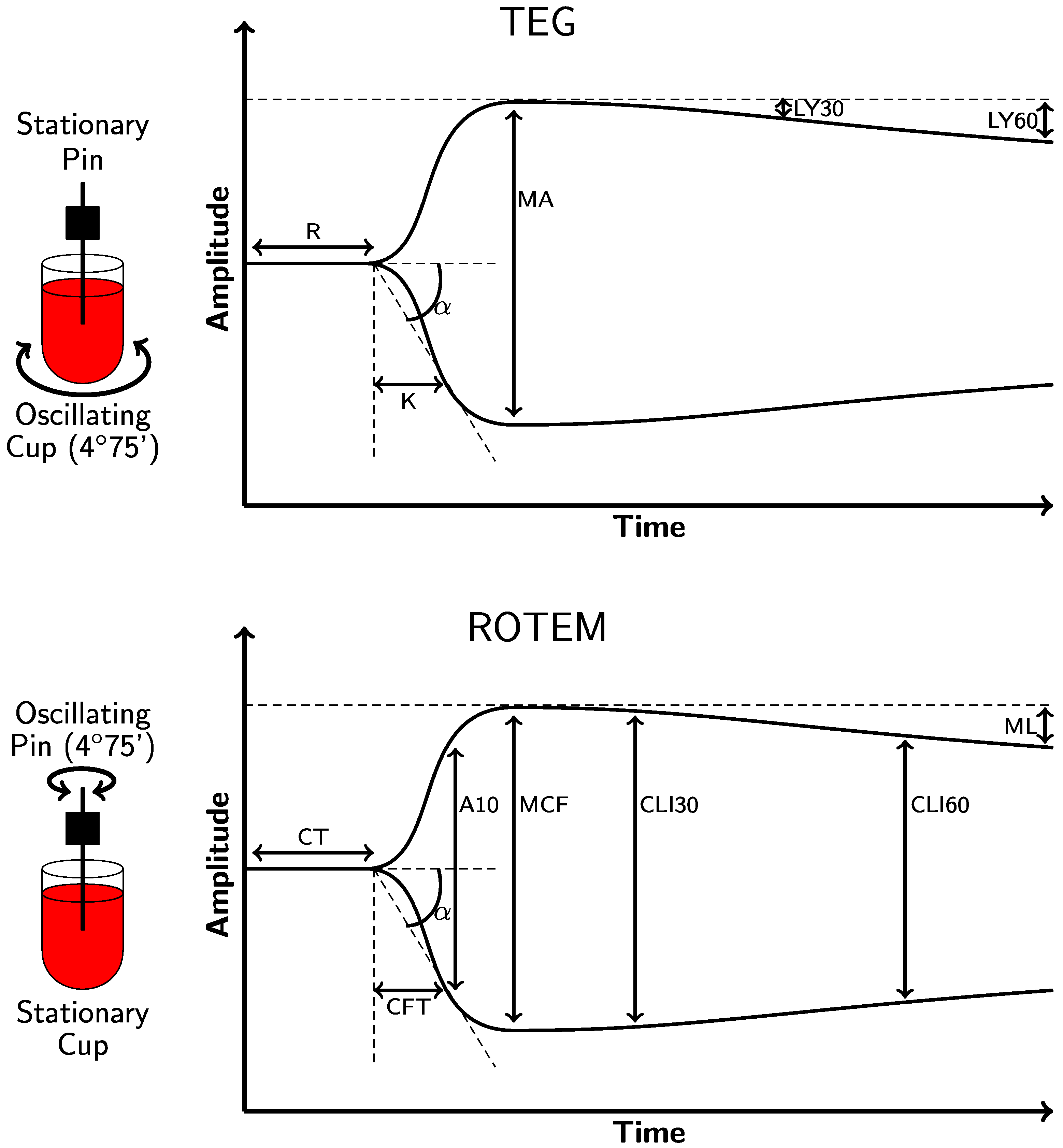

| Parameter | TEG® 5000 Analyzer 1 | ROTEM® Delta Analyzer |
|---|---|---|
| Clot initiation: the time from the test start to amplitude = 2 mm | R (Kaolin)/ACT (rTEG®) Kaolin: 4.6–9.1 min rTEG®: 86–118 s | CT INTEM: 137–246 s EXTEM: 42–74 s |
| Clot formation and clot kinetics: the time taken to achieve a level of clot strength, amplitude = 20 mm | K Kaolin: 0.8–2.1 min rTEG®: 1–2 min | CFT INTEM: 40–100 s EXTEM: 46–148 s |
| Angle of clot formation | α-angle Kaolin: 53–72° rTEG®: 64–80° | α-angle INTEM: 71–82° EXTEM: 63–81° |
| Maximum clot strength | MA Kaolin: 50–70 mm rTEG®: 52–71 mm MAff/CFF: 15–32 mm | MCF INTEM: 52–72 mm EXTEM: 49–71 mm FIBTEM: 9–25 mm |
| Lysis 2 TEG®: rate of clot breakdown 30 min after MA is reached. ROTEM®: the residual % of MCF amplitude when measured 30 min after CT (CLI30); the % reduction MCF amplitude at a given timepoint (ML) | LY30 Kaolin: 0–7.5% rTEG®: 0–7.5% | CLI30: 94–100% ML: <15% |
| VHA | Test | Activator/Inhibitor | Significance |
|---|---|---|---|
| TEG | Kaolin | Kaolin, CaCl2 | Contact activation.; similar information as aPTT; isolates the intrinsic pathway. |
| rTEG® | Kaolin, TF, CaCl2 | Clotting is accelerated by activation of extrinsic TF and intrinsic kaolin pathways; contact activation; roughly analogous to an ACT; information about coagulation kinetics initiated via contact activation alone is lost. | |
| HTEG® | Kaolin, lyophilized heparinase, CaCl2 | Lyophilized heparinase neutralizes UFH; compared to kaolin TEG® to assess the heparin effect. | |
| MAff/CFF | Kaolin, TF, Abciximab, CaCl2 | Abciximab is a GPIIb/IIIa platelet receptor inhibitor that blocks the platelet contribution to clot formation; compared to kaolin TEG® to assess the fibrinogen contribution to clot strength independent of platelets. | |
| Native TEG® | Calcium | Native whole blood sample analyzed following recalcification only; impractical for clinical use given long R. | |
| ROTEM | INTEM | Ellagic acid, CaCl2 | Tests clotting activation through the intrinsic coagulation pathway, FXII, FXI, FIX, FVIII, FX, FV, FII, and fibrinogen; sensitive to the heparin effect; similar information as aPTT. |
| EXTEM | Recombinant TF, CaCl2, polybrene | Polybrene neutralizes UFH; tests clotting activation through the extrinsic coagulation pathway, FVII, FX, FV, FII, and fibrinogen. | |
| HEPTEM | Ellagic acid, CaCl2, lyophilized heparinase | Tests heparin and protamine sulfate effects in patients with high heparin concentration when compared with INTEM. | |
| FIBTEM | Recombinant TF, CaCl2, polybrene, cytochalasin D | Cytochalasin D blocks platelet activation; tests fibrinogen component contribution to clot stability; more sensitive to lysis. | |
| APTEM | Recombinant TF, CaCl2, polybrene, aprotinin/TXA | Tests fibrinolysis when performed together with the EXTEM. | |
| NATEM | CaCl2 | Native whole blood sample analyzed following recalcification only; impractical for clinical use given long CT. |
| Coagulation Event | Main Contributor | TEG® 5000 | TEG® 6s | ROTEM® Delta/Sigma | Quantra® QPlus® QStat® | ClotPro® | Clinical Significance |
|---|---|---|---|---|---|---|---|
| Clot initiation | Coagulation factors | Reaction time (R), minutes | R | Clotting time (CT), seconds | CT | CT | A short R/CT/CTH time indicates a hypercoagulable state. A prolonged R/CT time indicates either hypocoagulability or the presence of an anticoagulant. A short CTH/CKH in the presence of a long CT /CK-R or CTR > 1.1.indicates the presence of heparin anticoagulation. Specifically, heparin and DOAC tests are available with TEG® 6s, ROTEM® sigma, and ClotPro®. 6 |
| Citrated Kaolin (CK) R-time, minutes | CK-R-time, minutes | INTEM 1 CT | CT | IN-test CT | |||
| rTEG® Activated clotting time (ACT), seconds 1 | Citrated Rapid TEG (CRT) ACT, seconds | EXTEM 1 CT | n/a | EX-test CT | |||
| Citrated kaolin-heparinase (CKH) 1 | CKH 1 | HEPTEM 1 CT | Heparinase Clot Time (CTH), seconds | HI-test RVV-test ECA-test NA-test | |||
| n/a | n/a | n/a | Clot time ratio (CTR) | n/a | |||
| Clot kinetics: amplification | Fibrinogen | Kinetic time (K), minutes | K | Clot formation time (CFT), seconds | Fibrinogen contribution to stiffness (FCS), hPA 2 | CFT | Angle reflects fibrin kinetics, including fibrin formation and cross-linking. FCS measures the direct contribution of stiffness generated by fibrinogen. |
| ɑ angle | ɑ angle | ɑ angle | n/a | ɑ angle | |||
| Citrated functional fibrinogen (MAff/CFF) | MAff/CFF 1 | FIBTEM 1 | n/a | FIB-test 1 | |||
| Clot stiffness: propagation | Fibrinogen, Platelets | CK Maximum amplitude (MA), mm | CK MA, mm | Maximum clot firmness (MCF), mm | Clot stiffness (CS), hPA 2 | MCF | MA, MCF, and CS reflect platelet and fibrinogen contributions to the clot stiffness and full platelet potential under maximal stimulation by thrombin. PCS isolates the platelet contribution to clot stiffness. |
| rTEG® MA, mm | CRT MA, mm | EXTEM 3 MCF | Platelet contribution to clot stiffness (PCS), hPA2 | EX-test MCF 3 | |||
| Clot stability: termination | Fibrinolytic enzymes and inhibitors, Factor XIII | Lysis at 30 min after MA, (LY30), % | LY30 | Clot Lysis Index at 30/60 min after CT (CLI30/CLI60), residual % of MCF 4 Maximum Lysis (ML), % of MCF lysed during run | Clot Stability to Lysis (CSL), % 5 | Clot Lysis Index at 30/60 min (CLI30/CLI60), % of MCF 4 | Hyperfibrinolysis is suggested by increased clot lysis that starts within 30 min of clot formation. |
| n/a | n/a | APTEM 1 | n/a | AP-test 3 | |||
| n/a | n/a | n/a | n/a | TPA 3 |
| rTEG® Trigger Value | ROTEM® Trigger Value | Intervention |
|---|---|---|
| ACT > 128 s | EXTEM CT > 80 s | PCC/FFP |
| α-angle < 65° MAff/CFF < 11 mm | EXTEM α-angle < 63° FIBTEM CA10 < 7 mm | fibrinogen/cryoprecipitate |
| MA < 55 mm | MCF < 45 mm | fibrinogen/cryoprecipitate/platelets |
| LY30/60 > 7.5% 1 citrated TEG/r-TEG | EXTEM CLI30/60 < 82% ML > 15% 1 | TXA/aminocaproic acid |
| VHA | Assay | Reagents | Clinical Significance |
|---|---|---|---|
| TEG/PM® | Kaolin TEG MA | Kaolin | MACK parameter is a proxy for the maximum potential function of the platelets. Thrombin overrides inhibition via anti-platelet agents, thus kaolin-activated TEG® samples will not exhibit their effects. |
| Activator TEG MA | Heparin, reptilase, FXIIIa | MAActivator represents the isolated fibrin contribution to clot strength. Performed alongside K-TEG®. | |
| TEG MAAA | Heparin, reptilase, FXIIIa, AA | Measures AA contribution of platelet activity to clot strength. Performed alongside K-TEG®. | |
| TEG MAADP | Heparin, reptilase, FXIIIa, ADP | Measures the ADP/GPIIb/IIIa pathways’ contribution of platelet activity to clot strength. Performed alongside K-TEG®. | |
| ROTEM Platelet Analysis® | ARATEM | AA | Determines GPIIb/IIIa and COX-1 receptor inhibition. |
| ADPTEM | ADP | Determines GPIIb/IIIa and ADP (P2Y12) receptor inhibition. | |
| TRAPTEM | TRAP-6 | Determines GPIIb/IIIa and thrombin (PAR-1) receptor inhibition. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volod, O.; Bunch, C.M.; Zackariya, N.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Neal, M.D.; Al-Fadhl, M.D.; Patel, S.S.; Wiarda, G.; et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J. Clin. Med. 2022, 11, 860. https://doi.org/10.3390/jcm11030860
Volod O, Bunch CM, Zackariya N, Moore EE, Moore HB, Kwaan HC, Neal MD, Al-Fadhl MD, Patel SS, Wiarda G, et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. Journal of Clinical Medicine. 2022; 11(3):860. https://doi.org/10.3390/jcm11030860
Chicago/Turabian StyleVolod, Oksana, Connor M. Bunch, Nuha Zackariya, Ernest E. Moore, Hunter B. Moore, Hau C. Kwaan, Matthew D. Neal, Mahmoud D. Al-Fadhl, Shivani S. Patel, Grant Wiarda, and et al. 2022. "Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices" Journal of Clinical Medicine 11, no. 3: 860. https://doi.org/10.3390/jcm11030860
APA StyleVolod, O., Bunch, C. M., Zackariya, N., Moore, E. E., Moore, H. B., Kwaan, H. C., Neal, M. D., Al-Fadhl, M. D., Patel, S. S., Wiarda, G., Al-Fadhl, H. D., McCoy, M. L., Thomas, A. V., Thomas, S. G., Gillespie, L., Khan, R. Z., Zamlut, M., Kamphues, P., Fries, D., & Walsh, M. M. (2022). Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. Journal of Clinical Medicine, 11(3), 860. https://doi.org/10.3390/jcm11030860









