Rapid Genetic Diagnosis for Okinawan Patients with Enlarged Vestibular Aqueduct Using Single-Stranded Tag Hybridization Chromatographic Printed-Array Strip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Primer Design
2.3. PCR Amplification from gDNA
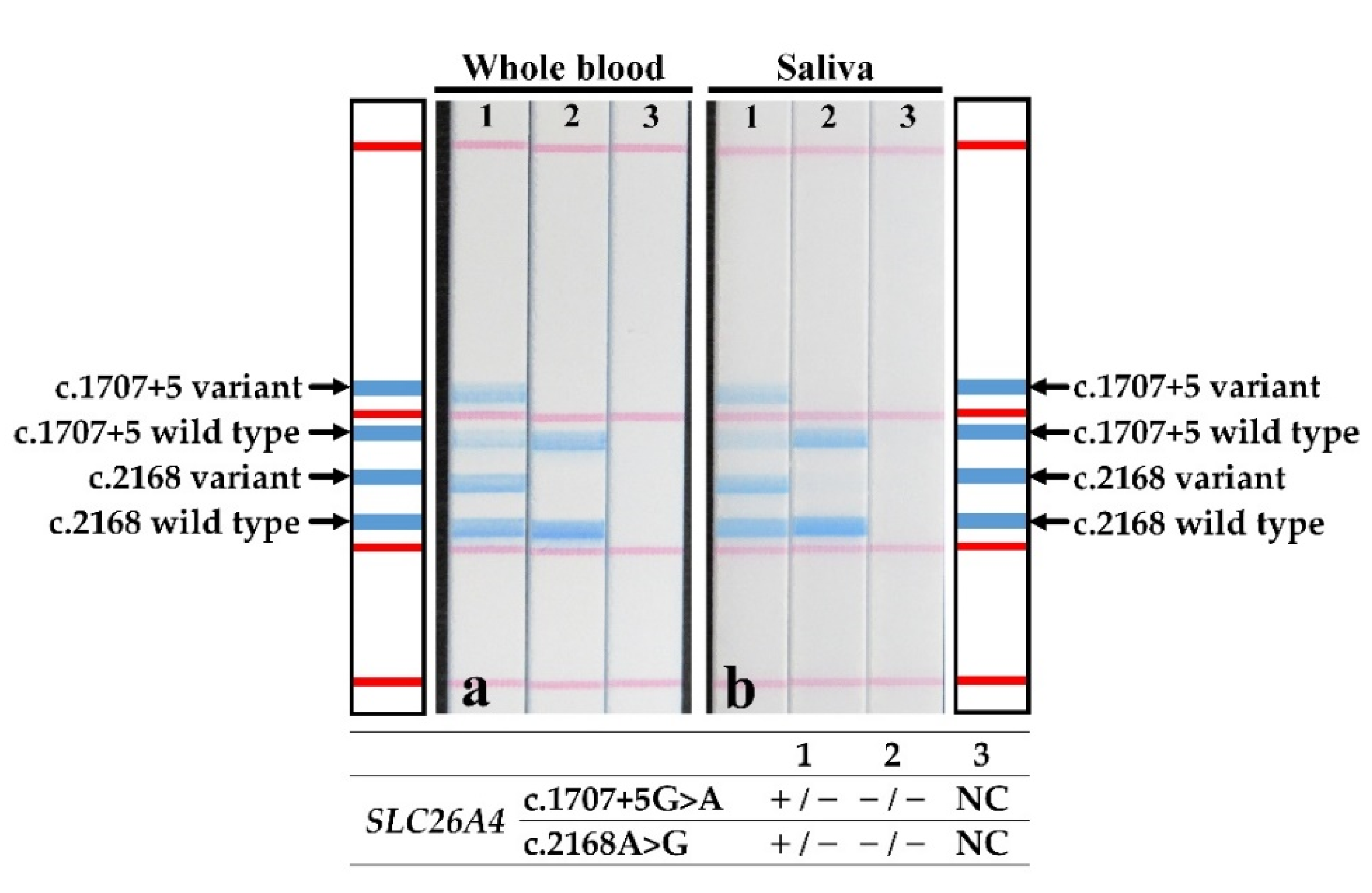
2.4. Direct PCR Amplification from Whole Blood and Saliva Samples
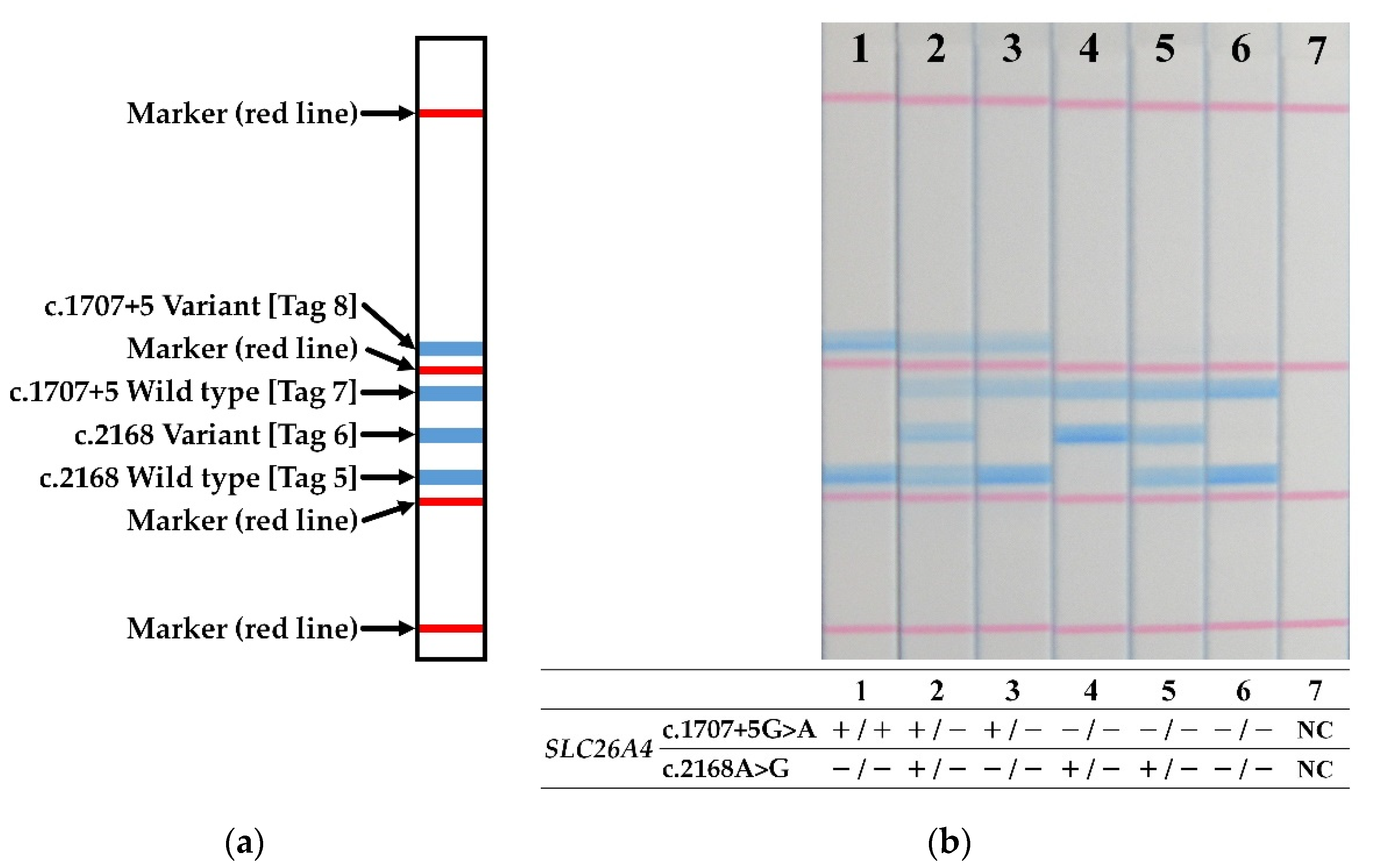
2.5. Dipstick DNA Chromatography
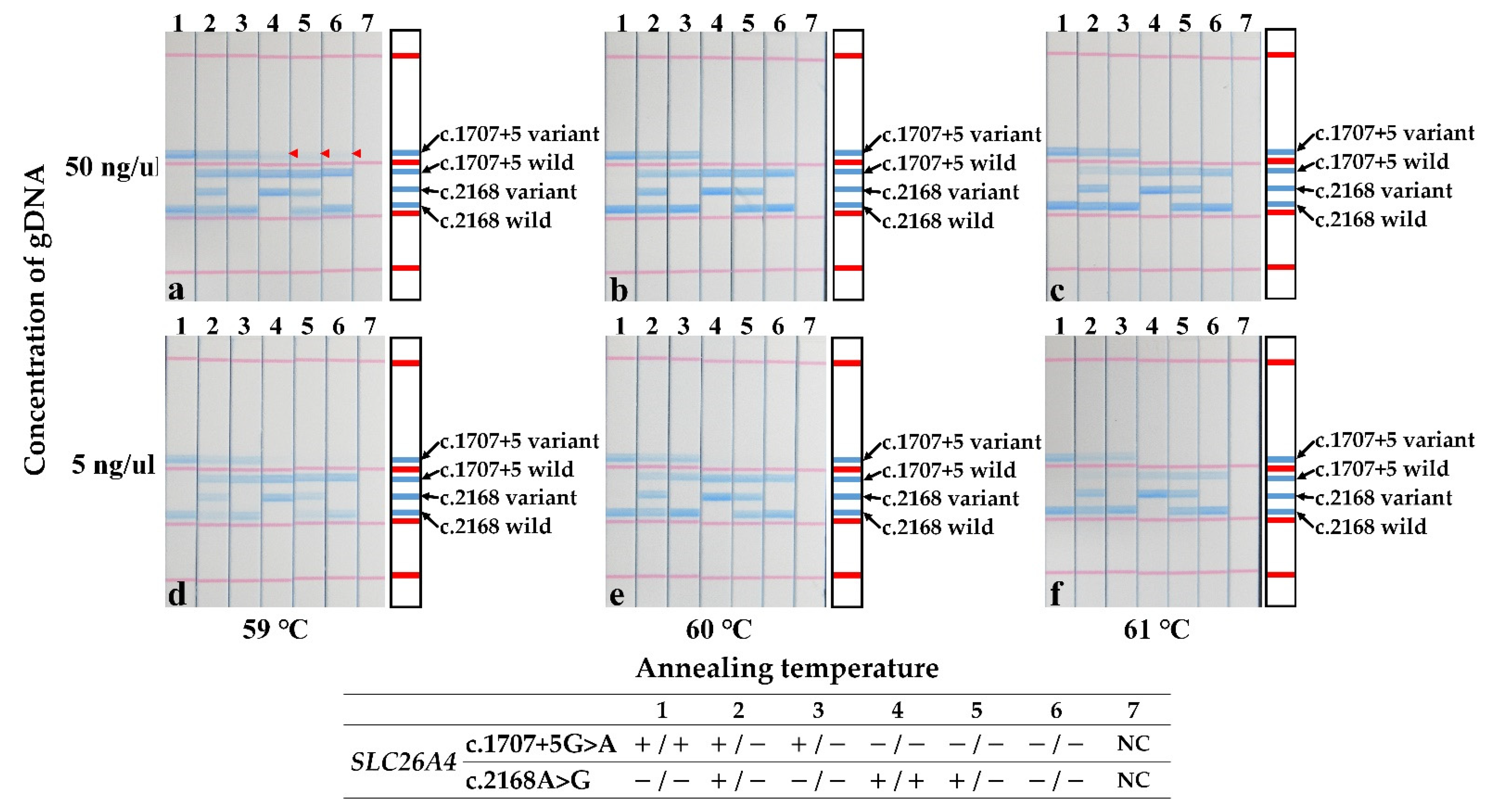
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnsen, T.; Jørgensen, M.B.; Johnsen, S. Mondini cochlea in Pendred’s syndrome. A histological study. Acta Oto-Laryngol. 1986, 102, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, O.; Ito, S.; Hanyu, O.; Yamazaki, M.; Urushiyama, M.; Tani, N.; Shibata, A. Female siblings with Pendred’s syndrome. Intern. Med. 1994, 33, 369–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendred, V. Deaf-mutation and goiter. Lancet 1896, 2, 532. [Google Scholar] [CrossRef] [Green Version]
- Usami, S.; Abe, S.; Weston, M.D.; Shinkawa, H.; Van Camp, G.V.; Kimberling, W.J. Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum. Genet. 1999, 104, 188–192. [Google Scholar] [CrossRef]
- Usami, S.; Nishio, S.Y.; Nagano, M.; Abe, S.; Yamaguchi, T. Deafness Gene Study Consortium Simultaneous screening of multiple mutations by invader assay improves molecular diagnosis of hereditary hearing loss: A multicenter study. PLoS ONE 2012, 7, e31276. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Suzuki, H.; Harada, D.; Namba, A.; Abe, S.; Usami, S. Distribution and frequencies of PDS (SLC26A4) mutations in Pendred syndrome and nonsyndromic hearing loss associated with enlarged vestibular aqueduct: A unique spectrum of mutations in Japanese. Eur. J. Hum. Genet. 2003, 11, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, K.; Nishio, S.Y.; Hattori, M.; Usami, S. Ethnic-specific spectrum of GJB2 and SLC26A4 mutations: Their origin and a literature review. Ann. Otol. Rhinol. Laryngol. 2015, 124, 61S–76S. [Google Scholar] [CrossRef]
- Campbell, C.; Cucci, R.A.; Prasad, S.; Green, G.E.; Edeal, J.B.; Galer, C.E.; Karniski, L.P.; Sheffield, V.C.; Smith, R.J. Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum. Mutat. 2001, 17, 403–411. [Google Scholar] [CrossRef]
- Albert, S.; Blons, H.; Jonard, L.; Feldmann, D.; Chauvin, P.; Loundon, N.; Sergent-Allaoui, A.; Houang, M.; Joannard, A.; Schmerber, S.; et al. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur. J. Hum. Genet. 2006, 14, 773–779. [Google Scholar] [CrossRef]
- Pryor, S.; Madeo, A.C.; Reynolds, J.C.; Sarlis, N.J.; Arnos, K.S.; Nance, W.E.; Yang, Y.; Zalewski, C.K.; Brewer, C.C.; Butman, J.A.; et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): Evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J. Med. Genet. 2005, 42, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.Y.; Stewart, A.K.; Nishimura, K.K.; Cha, W.J.; Seong, M.W.; Park, S.S.; Kim, S.W.; Chun, Y.S.; Chung, J.W.; Park, S.N.; et al. Efficient molecular genetic diagnosis of enlarged vestibular aqueducts in East Asians. Genet. Test. Mol. Biomark. 2009, 13, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, M.; Nishio, S.Y.; Usami, S. Mutation spectrum and genotype-phenotype correlation of hearing loss patients caused by SLC26A4 mutations in the Japanese: A large cohort study. J. Hum. Genet. 2014, 59, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Reyes, S.; Wang, G.; Ouyang, X.; Han, B.; Du, L.L.; Yuan, H.J.; Yan, D.; Dai, P.; Liu, X.Z. Mutation analysis of SLC26A4 in mainland Chinese patients with enlarged vestibular aqueduct. Otolaryngol. Head Neck Surg. 2009, 141, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.J.; Zhao, Y.L.; Rao, S.Q.; Guo, Y.F.; Yuan, H.; Zong, L.; Guan, J.; Xu, B.C.; Wang, D.Y.; Han, M.K.; et al. A distinct spectrum of SLC26A4 mutations in patients with enlarged vestibular aqueduct in China. Clin. Genet. 2007, 72, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Lan, L.; Wang, D.Y.; Han, B.; Qi, Y.; Zhao, Y.; Zong, L.; Li, Q.; Wang, Q.J. Correlation analysis of genotypes, auditory function, and vestibular size in Chinese children with enlarged vestibular aqueduct syndrome. Acta Otolaryngol. 2013, 133, 1242–1249. [Google Scholar] [CrossRef]
- Ganaha, A.; Kaname, T.; Yanagi, K.; Naritomi, K.; Tono, T.; Usami, S.; Suzuki, M. Pathogenic substitution of IVS15 + 5G > A in SLC26A4 in patients of Okinawa Islands with enlarged vestibular aqueduct syndrome or Pendred syndrome. BMC Med. Genet. 2013, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Kabata, Y.; Nakazono, K.; Takahashi, A.; Saito, S.; Hosono, N.; Kubo, M.; Nakamura, Y.; Kamatani, N. Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: Effects on population- based association studies. Am. J. Hum. Genet. 2008, 83, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Japanese Multi Omics Reference Panel. Available online: https://jmorp.megabank.tohoku.ac.jp/202112/ (accessed on 30 December 2021).
- Newton, C.R.; Graham, A.; Heptinstall, L.E.; Powell, S.J.; Summers, C.; Kalsheker, N.; Smith, J.C.; Markham, A.F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989, 17, 2503–2516. [Google Scholar] [CrossRef]
- Livak, K.J.; Marmaro, J.; Todd, J.A. Towards fully automated genome-wide polymorphism screening. Nat. Genet. 1995, 9, 341–342. [Google Scholar] [CrossRef]
- Fujii, K.; Matsubara, Y.; Akanuma, J.; Takahashi, K.; Kure, S.; Suzuki, Y.; Imaizumi, M.; Iinuma, K.; Sakatsume, O.; Rinaldo, P.; et al. Mutation detection by Taqman-allele-specific amplification: Application to molecular diagnosis of glycogen storage disease type Ia and medium-chain acyl-CoA dehydrogenase deficiency. Hum. Mutat. 2000, 15, 189–196. [Google Scholar] [CrossRef]
- Kwiatkowski, R.W.; Lyamichev, V.; de Arruda, M.; Neri, B. Clinical, genetic, and pharmacogenetic applications of the Invader assay. Mol. Diagn. 1999, 4, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Pastinen, T.; Raitio, M.; Lindroos, K.; Tainola, P.; Peltonen, L.; Syvänen, A.C. A system for specific, high-throughput genotyping by allele-specific primer extension on microarrays. Genome Res. 2000, 10, 1031–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumondai, M.; Ito, A.; Hishinuma, E.; Kikuchi, A.; Saito, T.; Takahashi, M.; Tsukada, C.; Saito, S.; Yasuda, J.; Nagasaki, M.; et al. Development and application of a rapid and sensitive genotyping method for pharmacogene variants using the single-stranded tag hybridization chromatographic printed-array strip (STH-PAS). Drug Metab. Pharmacokinet. 2018, 33, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Sato, T.; Niwa, K.; Kawase, M.; Tanner, A.C.; Takahashi, N. Rapid and sensitive PCR-dipstick DNA chromatography for multiplex analysis of the oral microbiota. BioMed Res. Int. 2014, 2014, 180323. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Agatsuma, Y.; Omori, F.; Narahara, K.; Inoue, T.; Kishikawa, Y.; Mizugaki, M. High throughput detection of drug-metabolizing enzyme polymorphisms by allele-specific fluorogenic 5′ nuclease chain reaction assay. Biol. Pharm. Bull. 2000, 23, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- McCann, J.; Ames, B.N. Detection of carcinogens as mutagens in the Salmonella/microsome test: Assay of 300 chemicals: Discussion: Discussion. Proc. Natl. Acad. Sci. USA 1976, 73, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Singer, V.L.; Lawlor, T.E.; Yue, S. Comparison of SYBR Green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the Salmonella/mammalian microsome reverse mutation assay (Ames test). Mutat. Res. 1999, 439, 37–47. [Google Scholar] [CrossRef]
- Roux, K.H. Optimization and troubleshooting in PCR. Cold Spring Harb. Protoc. 2009, 2009, pdb.ip66. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Huang, H.; Zhou, G. Direct polymerase chain reaction (PCR) from human whole blood and filter-paper-dried blood by using a PCR buffer with a higher pH. Anal. Biochem. 2008, 375, 370–372. [Google Scholar] [CrossRef]
- Looi, M.L.; Zakaria, H.; Osman, J.; Jamal, R. Quantity and quality assessment of DNA extracted from saliva and blood. Clin. Lab. 2012, 58, 307–312. [Google Scholar]
- Rah, Y.C.; Kim, A.R.; Koo, J.W.; Lee, J.H.; Oh, S.H.; Choi, B.Y. Audiologic presentation of enlargement of the vestibular aqueduct according to the SLC26A4 genotypes. Laryngoscope 2015, 125, E216–E222. [Google Scholar] [CrossRef] [PubMed]
| NM_000441.2(SLC26A4):c.2168A>G(p.His723Arg) | NM_000441.2(SLC26A4):c.1707+5G>A | |
|---|---|---|
| Position | chr7:107710132 | chr7:107700180 |
| Reference SNP number | rs121908362 | rs192366176 |
| Nucleotide | NC_000007.13:g.107350577A>G | NC_000007.13:g.107340625G>A |
| Functional consequence | Loss of function variant | Sequence variant affecting splicing |
| Genotypes | Patients | Gender (M: F) | Bilateral: Unilateral | Vertigo | Goiter | Threshold (dB) | |
|---|---|---|---|---|---|---|---|
| Right | Left | ||||||
| c.1707+5G>A homozygote | 9 (26.5%) | 3: 6 | 9: 0 | 6 (67%) | 6 (67%) | 96.6 | 100.0 |
| c.2168A>G homozygote | 9 (26.5%) | 4: 5 | 9: 0 | 5 (56%) | 4 (44%) | 98.2 | 91.3 |
| c.1707+5G>A/c.2168A>G | 12 (35.3%) | 4: 8 | 11: 1 | 7 (58%) | 6 (50%) | 95.6 | 101.5 |
| c.2168A>G/c.1579A>C | 1 (2.9%) | 0: 1 | 1: 0 | 1 | 1 | 91.7 | 71.7 |
| c.2168A>G/c.1229C>T | 1 (2.9%) | 1: 0 | 1: 0 | 1 | 0 | 101.7 | 95.0 |
| Undiagnosed | 2 (5.9%) | 0: 2 | 0: 2 | 0 | 0 | 57.0 | 57.0 |
| Total | 34 | 12: 22 | 31: 3 | 20 | 18 | 90.1 | 86.1 |
| Variant | Primer Name | Primer Sequence (5′–3′) | Size (bp) |
|---|---|---|---|
| c.2168A>G | c.2168_Fw_Wt | [Tag 5]-spacer- GGACACATTCTTTTTGACGGTCGA | 219 |
| c.2168_Fw_Vt | [Tag 6]-spacer- GGACACATTCTTTTTGACGGTCTG | ||
| c.2168_Rv | [Biotin]-TGAGGCTCCATGAAGTTATATAGG | ||
| c.1707+5G>A | c.1707_Fw_Wt | [Tag 7]-spacer- AATGTATCAAGTCCACAGTAGG | 361 |
| c.1707_Fw_Vt | [Tag 8]-spacer- AATGTATCAAGTCCACAGTAAAT | ||
| c.1707_Rv | [Biotin]-GCACAAAGCCTGTTAGTCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganaha, A.; Hishinuma, E.; Kaname, T.; Hiratsuka, M.; Kondo, S.; Tono, T. Rapid Genetic Diagnosis for Okinawan Patients with Enlarged Vestibular Aqueduct Using Single-Stranded Tag Hybridization Chromatographic Printed-Array Strip. J. Clin. Med. 2022, 11, 1099. https://doi.org/10.3390/jcm11041099
Ganaha A, Hishinuma E, Kaname T, Hiratsuka M, Kondo S, Tono T. Rapid Genetic Diagnosis for Okinawan Patients with Enlarged Vestibular Aqueduct Using Single-Stranded Tag Hybridization Chromatographic Printed-Array Strip. Journal of Clinical Medicine. 2022; 11(4):1099. https://doi.org/10.3390/jcm11041099
Chicago/Turabian StyleGanaha, Akira, Eiji Hishinuma, Tadashi Kaname, Masahiro Hiratsuka, Shunsuke Kondo, and Tetsuya Tono. 2022. "Rapid Genetic Diagnosis for Okinawan Patients with Enlarged Vestibular Aqueduct Using Single-Stranded Tag Hybridization Chromatographic Printed-Array Strip" Journal of Clinical Medicine 11, no. 4: 1099. https://doi.org/10.3390/jcm11041099







