Bleeding Risk Factors after Endoscopic Submucosal Dissection in Early Gastric Cancer and the Necessity of “Second-Look” Endoscopic Examination on the following Day
Abstract
:1. Introduction
2. Materials and Methods
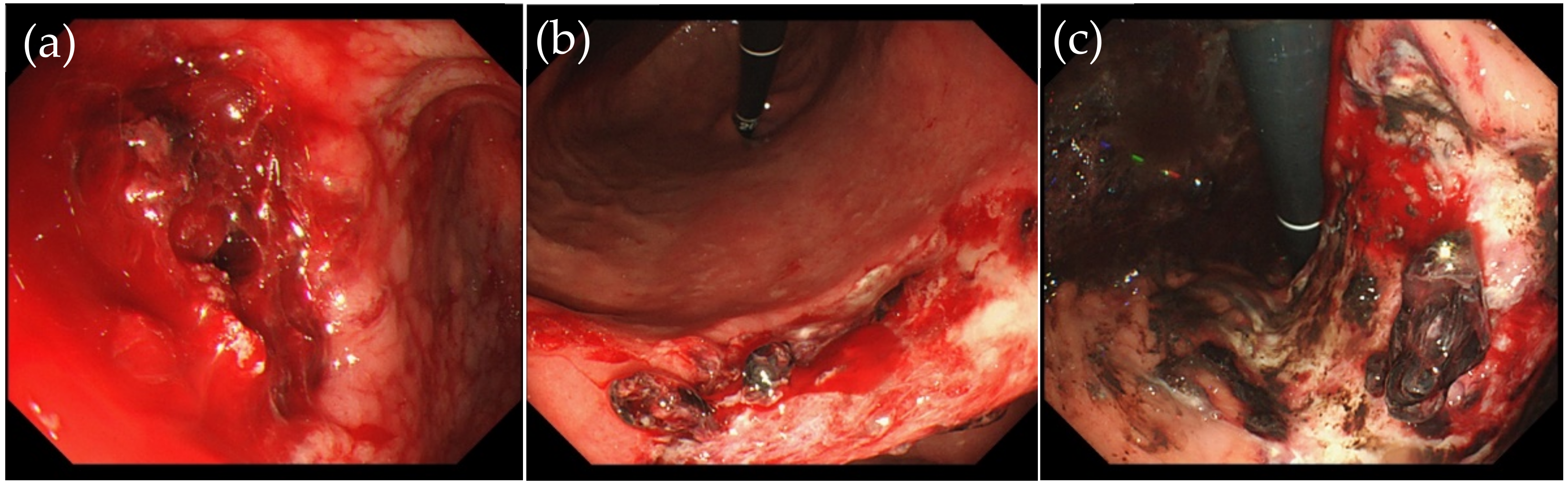
2.1. Definition of Second-Look (2nd-Look) and Active Bleeding
2.2. Risk Factors for Post-ESD Ulcer with Bleeding Risk
2.3. Statistical Analysis
2.4. Treatment Planning in Our Department
2.5. ESD Method in Our Department
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis
4. Discussion
4.1. The Need for Second-Look after ESD
4.2. Percentage of Bleeding Complications in Our Department
4.3. Upper Body Lesions Are Low-Risk Sites
4.4. The Larger the Ulcer, the Higher the Risk of Bleeding
4.5. Antithrombotic Drug Administration can Be a Risk Factor
4.6. High Risk in Antral Lesions
4.7. Risk Factors for Active Bleeding and “Post-Resection Ulcer at Risk of Bleeding”
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endo-scopic mucosal resection for treatment of early gastric cancer. Gut 2001, 48, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyama, T.; Tomori, A.; Hotta, K.; Morita, S.; Kominato, K.; Tanaka, M.; Miyata, Y. Endoscopic submucosal dissection of early esophageal cancer. Clin. Gastroenterol. Hepatol. 2005, 3, 567–570. [Google Scholar] [CrossRef]
- Fujishiro, M.; Kodashima, S.; Goto, O.; Ono, S.; Muraki, Y.; Kakushima, N.; Omata, M. Technical feasibility of endo-scopic submucosal dissection of gastrointestinal epithelial neoplasms with a splash-needle. Surg. Laparosc. Endosc. Percutaneous Tech. 2008, 18, 592–597. [Google Scholar] [CrossRef]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metas-tasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Goto, O.; Fujishiro, M.; Kodashima, S.; Ono, S.; Niimi, K.; Hirano, K.; Yamamichi, N.; Koike, K. A second-look endos-copy after endoscopic submucosal dissection for early gastric epithelial neoplasm may be unnecessary: A retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest. Endosc. 2010, 71, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.Y.; Kim, J.W.; Kim, H.S.; Park, H.J.; Jeon, H.K.; Park, S.Y.; Kim, B.R.; Lang, C.C.; Won, S.H. Second look endos-copy is not associated with better clinical outcomes after gastric endoscopic submucosal dissection: A prospective, randomized clinical trial analyzed on an as-treated basis. Gastrointest. Endosc. 2013, 78, 285–294. [Google Scholar] [CrossRef]
- Goto, O.; Fujishiro, M.; Kodashima, S.; Minatsuki, C.; Niimi, K.; Ono, S.; Yamamichi, N.; Koike, K. Short term healing process of artificial ulcers after gastric endoscopic submucosal dissection. Gut Liver 2011, 5, 293–297. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.K.; Kim, G.H. Second-Look Endoscopy after Endoscopic Submucosal Dissection: Can We Obtain Valuable In-formation? Clin. Endosc. 2016, 49, 212–213. [Google Scholar] [CrossRef] [Green Version]
- Toyokawa, T.; Inaba, T.; Omote, S.; Okamoto, A.; Miyasaka, R.; Watanabe, K.; Izumikawa, K.; Horii, J.; Fujita, I.; Ishi-kawa, S.; et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: Analysis of 1123 lesions. J. Gastroenterol. Hepatol. 2012, 27, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Lee, S.H.; Kim, K.O.; Jang, B.I.; Kim, T.N.; Jeon, S.W.; Kwon, J.G.; Kim, E.Y.; Jung, J.T.; Park, K.S.; et al. Risk factors associated with rebleeding in patients with high risk peptic ulcer bleeding: Focusing on the role of second look endoscopy. Dig. Dis. Sci. 2016, 61, 517–522. [Google Scholar] [CrossRef]
- Oda, I.; Gotoda, T.; Hamanaka, H.; Eguchi, T.; Saito, Y.; Matsuda, T.; Bhandari, P.; Emura, F.; Saito, D.; Ono, H. Endo-scopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig. Endosc. 2005, 17, 54–58. [Google Scholar] [CrossRef]
- Watanabe, K.; Ogata, S.; Kawazoe, S.; Watanabe, K.; Koyama, T.; Kajiwara, T.; Shimoda, Y.; Takase, Y.; Irie, K.; Mi-zuguchi, M.; et al. Clinical outcomes of EMR for gastric tumors: Historical pilot evaluation between endoscopic sub-mucosal dissection and conventional mucosal resection. Gastrointest. Endosc. 2006, 63, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Tanaka, S.; Kaneko, I.; Mouri, R.; Hirata, M.; Kawamura, T.; Yoshihara, M.; Chayama, K. Advantage of endo-scopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest. Endosc. 2006, 64, 877–883. [Google Scholar] [CrossRef]
- Chiu, P.W.; Lam, C.Y.; Lee, S.W.; Kwong, K.H.; Lam, S.H.; Lee, D.T.; Kwok, S.P. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: A prospective randomized trial. Gut 2003, 52, 1403–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niimi, K.; Fujishiro, M.; Goto, O.; Kodashima, S.; Minatsuki, C.; Hirayama, I.; Mochizuki, S.; Ono, S.; Yamamichi, N.; Kakushima, N.; et al. Prospective single-arm trial of two-week rabeprazole treatment for ulcer healing after gastric en-doscopic submucosal dissection. Dig. Endosc. 2012, 24, 110–116. [Google Scholar] [CrossRef] [PubMed]
- El Ouali, S.; Barkun, A.N.; Wyse, J.; Romagnuolo, J.; Sung, J.J.; Gralnek, I.M.; Bardou, M.; Martel, M. Is routine sec-ond-look endoscopy effective after endoscopic hemostasis in acute peptic ulcer bleeding? A meta-analysis. Gastrointest. Endosc. 2012, 76, 283–292. [Google Scholar] [CrossRef]
- Shiba, M.; Higuchi, K.; Kadouchi, K.; Montani, A.; Yamamori, K.; Okazaki, H.; Taguchi, M.; Wada, T.; Itani, A.; Watanabe, T.; et al. Risk factors for bleeding after endoscopic mucosal resection. World J. Gastroenterol. 2005, 11, 7335–7339. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Oda, I.; Gotoda, T.; Yokoi, C.; Matsuda, T.; Saito, Y.; Saito, D.; Ono, H. Routine coagulation of visible ves-sels may prevent delayed bleeding after endoscopic submucosal dissection-an analysis of risk factors. Endoscopy 2008, 40, 179–183. [Google Scholar] [CrossRef]
- Kakushima, N.; Yahagi, N.; Fujishiro, M.; Iguchi, M.; Oka, M.; Kobayashi, K.; Hashimoto, T.; Omote, M. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig. Endosc. 2004, 16, 327–331. [Google Scholar] [CrossRef]
- Kato, T.; Araki, H.; Onogi, F.; Ibuka, T.; Sugiyama, A.; Tomita, E.; Nagaki, M.; Moriwaki, H. Clinical trial: Rebamipide promotes gastric ulcer healing by proton pump inhibitor after endoscopic submucosal dissection—A randomized controlled study. J. Gastroenterol. 2010, 45, 285–290. [Google Scholar] [CrossRef]
- Uedo, N.; Takeuchi, Y.; Yamada, T.; Ishihara, R.; Ogiyama, H.; Yamamoto, S.; Kato, M.; Tatsumi, K.; Masuda, E.; Tamai, C.; et al. Effect of a proton pump Inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after en-doscopic submucosal dissection of early gastric cancer: A prospective randomized controlled trial. Am. J. Gastroenterol. 2007, 102, 1610–1616. [Google Scholar] [CrossRef]
- Fujiwara, S.; Morita, Y.; Toyonaga, T.; Kawakami, F.; Itoh, T.; Yoshida, M.; Kutsumi, H.; Azuma, T. A randomized con-trolled trial of rebamipide plus rabeprazole for the healing of artificial ulcers after endoscopic submucosal dissection. J. Gastroenterol. 2011, 46, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Okada, H.; Kawahara, Y.; Hori, K.; Tanioka, D.; Tsuzuki, T.; Inoue, M.; Yagi, S.; Takenaka, R.; Yamamoto, K. Proton pump Inhibitor dose-related healing rate of artificial ulcers after endoscopic submucosal dissection: A pro-spective randomized controlled trial. Digestion 2011, 84, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, K.; Iwamuro, M.; Inaba, T.; Ishikawa, S.; Kuwaki, K.; Sakakihara, I.; Yamamoto, K.; Takahashi, S.; Tanaka, S.; Wato, M.; et al. Bleeding in patients who underwent scheduled second-look endoscopy 5 days after endoscopic submucosal dissection for gastric lesions. BMC Gastroenterol. 2018, 18, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.S.; Choi, C.W.; Kim, S.J.; Kim, H.W.; Kang, D.H.; Park, S.B.; Ryu, D.G. Risk factors for delayed bleeding by onset time after endoscopic submucosal dissection for early gastric neoplasm. Sci. Rep. 2019, 9, 2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannen, K.; Tsunada, S.; Hara, M.; Yamaguchi, K.; Sakata, Y.; Fujise, T.; Noda, T.; Shimoda, R.; Sakata, H.; Ogata, S.; et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: Analysis of 478 lesions. J. Gastroenterol. 2010, 45, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tano, S.; Horiki, N.; Omata, F.; Tanaka, K.; Hamada, Y.; Katsurahara, M.; Ninomiya, K.; Nishikawa, K.; Nojiri, K.; Yamada, R.; et al. Second and third-look endoscopy for the prevention of post-ESD bleeding. Medicine 2015, 94, e491. [Google Scholar] [CrossRef] [PubMed]
- Koh, R.; Hirasawa, K.; Yahara, S.; Oka, H.; Sugimori, K.; Morimoto, M.; Numata, K.; Kokawa, A.; Sasaki, T.; Nozawa, A.; et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissec-tion for gastric neoplasms. Gastrointest. Endosc. 2013, 78, 476–483. [Google Scholar] [CrossRef]


| Active Bleeding 51 Cases | Ulcer with a Risk of Bleeding 88 Cases | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n = 447 | Absent | Present | n = 396 | Absent | Present | |
| Age | |||||||
| ≤74 | 256 | 231 | 25 | 231 | 179 | 52 | |
| ≥75 | 191 | 165 | 26 | 165 | 129 | 36 | |
| Average 72.6, SD 8.76 | |||||||
| Anesthesia method | |||||||
| Intravenous anesthesia | 261 | 230 | 31 | 230 | 183 | 47 | |
| General anesthesia | 186 | 166 | 20 | 166 | 125 | 41 | |
| Location | |||||||
| Upper | 56 | 54 | 2 | 54 | 49 | 5 | |
| Middle | 55 | 50 | 5 | 50 | 40 | 10 | |
| Lower | 179 | 157 | 22 | 157 | 124 | 33 | |
| Antrum | 157 | 135 | 22 | 135 | 95 | 40 | |
| Location | |||||||
| Anterior wall | 69 | 61 | 8 | 61 | 46 | 15 | |
| Greater Curvature | 86 | 76 | 10 | 76 | 60 | 16 | |
| Posterior wall | 97 | 89 | 8 | 89 | 73 | 16 | |
| Lesser Curvature | 195 | 170 | 25 | 170 | 129 | 41 | |
| Endoscopic findings | |||||||
| Elevated type | 182 | 162 | 20 | 162 | 124 | 38 | |
| Depressed type | 265 | 234 | 31 | 234 | 184 | 50 | |
| Resecting time | |||||||
| ≤60 min | 224 | 201 | 23 | 201 | 152 | 49 | |
| >60 min | 223 | 195 | 28 | 195 | 156 | 39 | |
| Major axis of specimen | |||||||
| ≤30 mm | 193 | 181 | 12 | 181 | 152 | 29 | |
| >30 mm | 254 | 215 | 39 | 215 | 156 | 59 | |
| Depth | |||||||
| Mucosal tumor | 390 | 347 | 43 | 347 | 266 | 81 | |
| Submucosal tumor | 57 | 49 | 8 | 49 | 42 | 7 | |
| Ulcer scars in pathological tissue specimens | |||||||
| Negative | 406 | 360 | 46 | 360 | 279 | 81 | |
| Positive | 41 | 36 | 5 | 36 | 29 | 7 | |
| Histology | |||||||
| Differentiated type | 424 | 377 | 47 | 377 | 292 | 85 | |
| Undifferentiated type | 23 | 19 | 4 | 19 | 16 | 3 | |
| Antithrombotic drugs use ‡ | |||||||
| No | 356 | 321 | 35 | 321 | 254 | 67 | |
| Yes | 91 | 75 | 16 | 75 | 54 | 21 | |
| Number of resections | |||||||
| Singular | 410 | 367 | 43 | 367 | 286 | 81 | |
| Simultaneous multiple | 37 | 29 | 8 | 29 | 22 | 7 | |
| Mode | Effect | |
|---|---|---|
| Marking | Precise APC | 10.0 |
| Circumferential incision | Endo Cut Q | Effect 3 Duration 3 Interval 3 |
| Dissection | Precise SECT | 7.0 |
| Hemostasis | Soft COAG | 6.5 |
| Study Population n = 447 | Univariate Analysis | |||
|---|---|---|---|---|
| Variable | χ2 | HR † (95% CI §) | p-Value | |
| Age | ≤74 ≥75 | 1.586 | Referent 1.456 (0.810–2.622) | 0.2080 |
| Anesthesia method | Intravenous anesthesia General anesthesia | 0.137 | Referent 1.119 (0.620–2.058) | 0.7177 |
| Location | Upper Middle Lower Antrum | 4.984 0.353 0.228 1.582 | 0.259 (0.042–0.868) 0.752 (0.252–1.822) 1.155 (0.635–2.076) 1.467 (0.804–2.643) | 0.0256 0.5527 0.6332 0.2085 |
| Location | Anterior wall Greater Curvature Posterior wall Lesser Curvature | 0.003 0.005 1.310 0.677 | 1.022 (0.428–2.174) 1.027 (0.468–2.067) 0.642 (0.271–1.346) 1.278 (0.710–2.297) | 0.9582 0.9436 0.2524 0.4106 |
| Endoscopic findings | Elevated type Depressed type | 0.054 | Referent 1.073 (0.595–1.975) | 0.8165 |
| Resection time (min) | ≤60 >60 | 0.580 | Referent 1.255 (0.700–2.271) | 0.4464 |
| Major axis of specimen (mm) | ≤30 >30 | 9.623 | Referent 2.736 (1.431–5.601) | 0.0019 |
| Depth | Mucosal tumor Submucosal tumor | 0.423 | Referent 1.318 (0.548–2.834) | 0.5155 |
| Ulcer scars in pathological tissue specimens | Negative Positive | 0.027 | Referent 1.087 (0.360–2.684) | 0.8693 |
| Histology | Differentiated Undifferentiated | 0.760 | Referent 1.689 (0.475–4.726) | 0.3833 |
| Antithrombotic drugs use ‡ | No Yes | 3.920 | Referent 1.957 (1.007–3.700) | 0.0477 |
| Number of resections | Singular Simultaneous multiple | 3.477 | Referent 2.354 (0.954–5.268) | 0.0622 |
| Multivariate Analysis | ||||
| Variable | χ2 | HR† (95% CI§) | p-Value | |
| Location | Middle, Lower, Antrum Upper | 4.616 | Referent 0.265 (0.042–0.905) | 0.0317 |
| Major axis of specimen (mm) | ≤30 >30 | 8.335 | Referent 2.582 (1.343–5.310) | 0.0054 |
| Antithrombotic drugs use ‡ | No Yes | 3.885 | Referent 1.975 (1.004–3.757) | 0.0487 |
| Study Population n = 447 | Univariate Analysis | |||
|---|---|---|---|---|
| Variable | χ2 | HR † (95% CI §) | p-Value | |
| Age | ≤74 ≥75 | 0.289 | Referent 1.117 (0.745–1.672) | 0.5907 |
| Anesthesia method | Intravenous anesthesia General anesthesia | 0.428 | Referent 1.145 (0.763–1.715) | 0.5128 |
| Location | Upper Middle Lower Antrum | 11.931 0.437 0.019 7.828 | 0.280 (0.113–0.598) 0.810 (0.420–1.493) 0.972 (0.643–1.460) 1.805 (1.194–2.729) | 0.0006 0.5086 0.8901 0.0051 |
| Location | Anterior wall Greater Curvature Posterior wall Lesser Curvature | 0.189 0.037 0.671 1.217 | 1.129 (0.645–1.932) 0.951 (0.563–1.571) 0.672 (0.396–1.108) 1.254 (0.838–1.877) | 0.6641 0.8470 0.1207 0.2699 |
| Endoscopic findings | Elevated type Depressed type | 0.085 | Referent 0.941 (0.627–1.417) | 0.7703 |
| Resection time (min) | ≤60 >60 | 0.230 | Referent 0.907 (0.607–1.354) | 0.6318 |
| Major diameter of specimen (mm) | ≤30 >30 | 15.779 | Referent 2.329 (1.528–3.598) | <0.0001 |
| Depth | Mucosal tumor Submucosal tumor | 0.715 | Referent 0.766 (0.398–1.405) | 0.3976 |
| Ulcer scars in pathological tissue specimens | Negative Positive | 0.071 | Referent 0.909 (0.434–1.798) | 0.7897 |
| Histology | Differentiated Undifferentiated | 0.005 | Referent 0.968 (0.365–2.325) | 0.9438 |
| Antithrombotic drugs use ‡ | No Yes | 4.714 | Referent 1.706 (1.054–2.744) | 0.0299 |
| Number of resections | Singular Simultaneous multiple | 1.610 | Referent 1.573 (0.776–3.111) | 0.2045 |
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Variable | χ2 | HR ‡ (95% CI §) | p-Value | |
| Location | Middle, Lower, Antrum Upper | 6.952 | Referent 0.348 (0.137–0.775) | 0.0084 |
| Location | Upper, Middle, Lower, Antrum | 5.177 | Referent 1.662 (1.073–2.581) | 0.0229 |
| Major diameter of specimen (mm) | ≤30 >30 | 14.609 | Referent 2.307 (1.496–3.605) | 0.0001 |
| Antithrombotic drugs use ‡ | No Yes | 5.171 | Referent 1.792 (1.085–2.948) | 0.0230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, R.; Kawaura, K.; Ito, T.; Azukisawa, S.; Kunou, H.; Kamai, J.; Hamada, K.; Mukai, T.; Kitakata, H.; Ishigaki, Y. Bleeding Risk Factors after Endoscopic Submucosal Dissection in Early Gastric Cancer and the Necessity of “Second-Look” Endoscopic Examination on the following Day. J. Clin. Med. 2022, 11, 914. https://doi.org/10.3390/jcm11040914
Kobayashi R, Kawaura K, Ito T, Azukisawa S, Kunou H, Kamai J, Hamada K, Mukai T, Kitakata H, Ishigaki Y. Bleeding Risk Factors after Endoscopic Submucosal Dissection in Early Gastric Cancer and the Necessity of “Second-Look” Endoscopic Examination on the following Day. Journal of Clinical Medicine. 2022; 11(4):914. https://doi.org/10.3390/jcm11040914
Chicago/Turabian StyleKobayashi, Rika, Ken Kawaura, Tohru Ito, Sadafumi Azukisawa, Hiroaki Kunou, Junji Kamai, Kazu Hamada, Tsuyoshi Mukai, Hidekazu Kitakata, and Yasuhito Ishigaki. 2022. "Bleeding Risk Factors after Endoscopic Submucosal Dissection in Early Gastric Cancer and the Necessity of “Second-Look” Endoscopic Examination on the following Day" Journal of Clinical Medicine 11, no. 4: 914. https://doi.org/10.3390/jcm11040914






