Evaluation of Breast Cancer Size Measurement by Computer-Aided Diagnosis (CAD) and a Radiologist on Breast MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Breast MRI Technique
2.3. CAD Analysis
2.4. Size Evaluation of Breast Cancer
2.5. Histopathologic and Imaging Data Collection
2.6. Statistical Analysis
3. Results
3.1. Correlation between CAD-Generated Tumor Size or Radiologist-Measured Size and Histopathologic Size
3.2. Correlation of Accurate and Inaccurate Groups for Size Measurement by MR CAD and Radiologist
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.G.; Han, W.; Lee, J.W.; Ko, E.; Kim, E.K.; Yu, J.H.; Kang, S.Y.; Moon, W.K.; Cho, N.; Park, I.A.; et al. Age and HER2 expression status affect MRI accuracy in predicting residual tumor extent after neo-adjuvant systemic treatment. Ann. Oncol. 2009, 20, 636–641. [Google Scholar] [CrossRef]
- Davis, P.L.; Staiger, M.J.; Harris, K.B.; Ganott, M.A.; Klementaviciene, J.; McCarty, K.S., Jr.; Tobon, H. Breast cancer measurements with magnetic resonance imaging, ultrasonography, and mammography. Breast Cancer Res. Treat. 1996, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Moon, W.K.; Cho, N.; Ko, E.S.; Yang, S.K.; Park, J.S.; Kim, S.M.; Park, I.-A.; Cha, J.H.; Lee, E.H. MRI of the breast for the detection and assessment of the size of ductal carcinoma in situ. Korean J. Radiol. 2007, 8, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Van der Velden, A.P.S.; Boetes, C.; Bult, P.; Wobbes, T. The value of magnetic resonance imaging in diagnosis and size assessment of in situ and small invasive breast carcinoma. Am. J. Surg. 2006, 192, 172–178. [Google Scholar] [CrossRef]
- Yoo, E.Y.; Nam, S.Y.; Choi, H.-Y.; Hong, M.J. Agreement between MRI and pathologic analyses for determination of tumor size and correlation with immunohistochemical factors of invasive breast carcinoma. Acta Radiol. 2018, 59, 50–57. [Google Scholar] [CrossRef]
- Mennella, S.; Garlaschi, A.; Paparo, F.; Perillo, M.; Celenza, M.; Massa, T.; Rollandi, G.A.; Garlaschi, G. Magnetic resonance imaging of breast cancer: Factors affecting the accuracy of preoperative lesion sizing. Acta Radiol. 2015, 56, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Sanderink, W.B.G.; Caballo, M.; Strobbe, L.J.A.; Built, P.; Vreuls, W.; Venderink, D.J.; Sechopoulos, I.; Karssemeijer, N.; Mann, R.M. Reliability of MRI tumor size measurements for minimal invasive treatment selection in small breast cancers. Eur. J. Surg. Oncol. 2020, 46, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Meeuwis, C.; van de Ven, S.M.; Stapper, G.; Fernandez Gallardo, A.M.; van den Bosch, M.A.A.J.; Mali, W.P.T.M.; Veldhuis, W.B. Computer-aided detection (CAD) for breast MRI: Evaluation of efficacy at 3.0T. Eur. Radiol. 2010, 20, 522–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehman, C.D.; Peacock, S.; DeMartini, W.B.; Chen, X. A new automated software system to evaluate breast MR examinations: Improved specificity without decreased sensitivity. AJR Am. J. Roentgenol. 2006, 187, 51–56. [Google Scholar] [CrossRef]
- Beresford, M.J.; Padhani, A.R.; Taylor, N.J.; Ah-See, M.-L.; Stirling, J.J.; Makris, A.; d’Arcy, J.A.; Collins, D.J. Inter-and intraobserver variability in the evaluation of dynamic breast cancer MRI. J. Magn. Reson. Imaging 2006, 24, 1316–1325. [Google Scholar] [CrossRef]
- Lai, H.W.; Chen, D.R.; Wu, Y.C.; Chen, C.J.; Lee, C.W.; Kuo, S.J.; Chen, S.T.; Wu, H.K. Comparison of the diagnostic accuracy of magnetic resonance imaging with sonography in the prediction of breast cancer tumor size: A concordance analysis with histopathologically determined tumor size. Ann. Surg. Oncol. 2015, 222, 3816–3823. [Google Scholar] [CrossRef] [PubMed]
- Lyou, C.Y.; Cho, N.; Kim, S.M.; Jang, M.; Park, J.S.; Baek, S.Y.; Moon, W.K. Computer-aided evaluation of breast MRI for the residual tumor extent and response monitoring in breast cancer patients receiving neoadjuvant chemotherapy. Korean J. Radiol. 2011, 12, 34–43. [Google Scholar] [CrossRef]
- DeMartini, W.B.; Lehman, C.D.; Peacock, S.; Russell, M.T. Computer-aided detection applied to breast MRI: Assessment of CAD-generated enhancement and tumor sizes in breast cancers before and after neoadjuvant chemotherapy. Acad. Radiol. 2005, 12, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Song, S.E.; Seo, B.K.; Cho, K.R.; Woo, O.H.; Son, G.S.; Kim, C.; Cho, S.B.; Kwon, S.S. Computer-aided detection (CAD) system for breast MRI in assessment of local tumor extent, nodal status, and multifocality of invasive breast cancers: Preliminary study. Cancer Imaging 2015, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Levrini, G.; Sghedoni, R.; Mori, C.; Botti, A.; Vacondio, R.; Nitrosi, A.; Iori, M.; Nicoli, F. Size assessment of breast lesions by means of a computer-aided detection (CAD) system for magnetic resonance mammography. Radiol. Med. 2011, 116, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- ACR BI-RADS Atlas, Breast Imaging Reporting and Data System; ACR BI-RADS® Magnetic Resonance Imaging; American College of Radiology: Reston, VA, USA, 2013.
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Gibbs, J.E.; Lu, Y.; Esserman, L.J.; Tripathy, D.; Wolverton, D.S.; Rugo, H.S.; Hwang, E.S.; Ewing, C.A.; Hylton, N.M. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. Am. J. Roentgenol. 2005, 184, 1774–1781. [Google Scholar] [CrossRef]
- Takeda, K.; Kanao, S.; Okada, T.; Kataoka, M.; Ueno, T.; Toi, M.; Ishiguro, H.; Mikami, Y.; Togashi, K. Assessment of CAD-generated tumor volumes measured using MRI in breast cancers before and after neoadjuvant chemotherapy. Eur. J. Radiol. 2012, 81, 2627–2631. [Google Scholar] [CrossRef]
- Rominger, M.B.; Fournell, D.; Nadar, B.T.; Behrens, S.N.M.; Figiel, J.H.; Keil, B.; Heverhagen, J.T. Accuracy of MRI volume measurements of breast lesions: Comparison between automated, semiautomated, and manual assessment. Eur. Radiol. 2009, 19, 1097–1107. [Google Scholar] [CrossRef]
- Leddy, R.; Irshad, A.; Metcalfe, A.; Mabalam, P.; Abid, A.; Ackerman, S.; Lewis, M. Comparative accuracy of preoperative tumor size measurement on mammography, sonography, and MRI: Is the accuracy affected by breast density or cancer subtype? J. Clin. Ultrasound 2016, 44, 17–25. [Google Scholar] [CrossRef]
- Gruber, I.V.; Rueckert, M.; Kagan, K.O.; Staebler, A.; Siegmann, K.C.; Hartkopf, A.; Wallwiener, D.; Hahn, M. Measurement of tumour size with mammography, sonography, and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Velden, A.P.S.; Boetes, C.; Bult, P.; Wobbes, T. Magnetic resonance imaging in size assessment of invasive breast carcinoma with an extensive intraductal component. BMC Med. Imaging 2009, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinn, H.P.; Anton, H.W.; Magener, A.; von Fournier, D.; Bastert, G.; Otto, H.F. Extensive and predominant in situ component in breast carcinoma: Their influence on treatment results after breast-conserving therapy. Eur. J. Cancer 1998, 34, 646–653. [Google Scholar] [CrossRef]
- Ikeda, O.; Nishimura, R.; Miyayama, H.; Yasunaga, T.; Ozaki, Y.; Tsuji, A.; Yamashita, Y. Magnetic resonance evaluation of the presence of an extensive intraductal component in breast cancer. Acta Radiol. 2004, 45, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, M.; Schelfout, K.; Kersschot, E.; Colpaert, C.; Verslegers, I.; Biltjes, I.; Tjalma, W.A.; De Schepper, A.; Weyler, J.; Parizel, P.M. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur. J. Radiol. 2017, 62, 273–282. [Google Scholar] [CrossRef]
- Kumar, A.S.; Chen, D.F.; Au, A.; Chen, Y.-Y.; Leung, J.; Garwood, E.R.; Gibbs, J.; Hylton, N.; Esserman, L.J. Biologic significance of false-positive magnetic resonance imaging enhancement in the setting of ductal carcinoma in situ. Am. J. Surg. 2006, 192, 520–524. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.R.; Lago-Toro, C.E.; Hughes, J.H.; Careaga, E.; Brown, A.T.; Chernick, M.; Barrio, A.V.; Frazier, T.G. Is there a role for MRI in the preoperative assessment of patients with DCIS? Ann. Surg. Oncol. 2010, 17, 2395–2400. [Google Scholar] [CrossRef]
- Marcotte-Bloch, C.; Balu-Maestro, C.; Chamorey, E.; Ettore, F.; Raoust, I.; Flipo, B.; Chapellier, C. MRI for the size assessment of pure ductal carcinoma in situ (DCIS): A prospective study of 33 patients. Eur. J. Radiol. 2011, 77, 462–467. [Google Scholar] [CrossRef]
- Gilles, R.; Zafrani, B.; Guinebretière, J.; Meunier, M.; Lucidarme, O.; Tardivon, A.A.; Rochard, F.; Vanel, D.; Neuenschwander, S.; Arriagada, R. Ductal carcinoma in situ: MR imaging-histopathologic correlation. Radiology 1995, 196, 415–419. [Google Scholar] [CrossRef]
- Baek, J.E.; Kim, S.H.; Lee, A.W. Background parenchymal enhancement in breast MRIs of breast cancer patients: Impact on tumor size estimation. Eur. J. Radiol. 2014, 83, 1356–1362. [Google Scholar] [CrossRef]
- Onesti, J.K.; Mangus, B.E.; Helmer, S.D.; Osland, J.S. Breast cancer tumor size: Correlation between magnetic resonance imaging and pathology measurements. Am. J. Surg. 2008, 196, 844–848. [Google Scholar] [CrossRef]
- Rastelli, F.; Crispino, S. Factors predictive of response to hormone therapy in breast cancer. Tumori 2008, 94, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Kyndi, M.; Sørensen, F.B.; Knudsen, H.; Overgaard, M.; Nielsen, H.M.; Overgaard, J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J. Clin. Oncol. 2008, 26, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Morales, L.A.; Seguí, M.A.; Andreu, X.; Dalmau, E.; Sáez, A.; Pericay, C.; Santos, C.; Montesinos, J.; Gallardo, E.; Arcusa, A.; et al. Analysis of the pathologic response to primary chemotherapy in patients with locally advanced breast cancer grouped according to estrogen receptor, progesterone receptor, and HER2 status. Clin. Breast Cancer 2007, 7, 559–564. [Google Scholar] [CrossRef]
- Blackwell, K.L.; Dewhirst, M.W.; Liotcheva, V.; Snyder, S.; Broadwater, G.; Bentley, R.; Lal, A.; Riggins, G.; Anderson, S.; Vredenburgh, J.; et al. HER-2 gene amplification correlates with higher levels of angiogenesis and lower levels of hypoxia in primary breast tumors. Clin. Cancer Res. 2004, 10, 4083–4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMartini, W.B.; Liu, F.; Peacock, S.; Eby, P.R.; Gutierrez, R.L.; Lehman, C.D. Background parenchymal enhancement on breast MRI: Impact on diagnostic performance. AJR Am. J. Roentgenol. 2012, 198, W373–W380. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Watanabe, J. Background enhancement of mammary glandular tissue on breast dynamic MRI: Imaging features and effect on assessment of breast cancer extent. Breast Cancer 2012, 19, 259–265. [Google Scholar] [CrossRef]
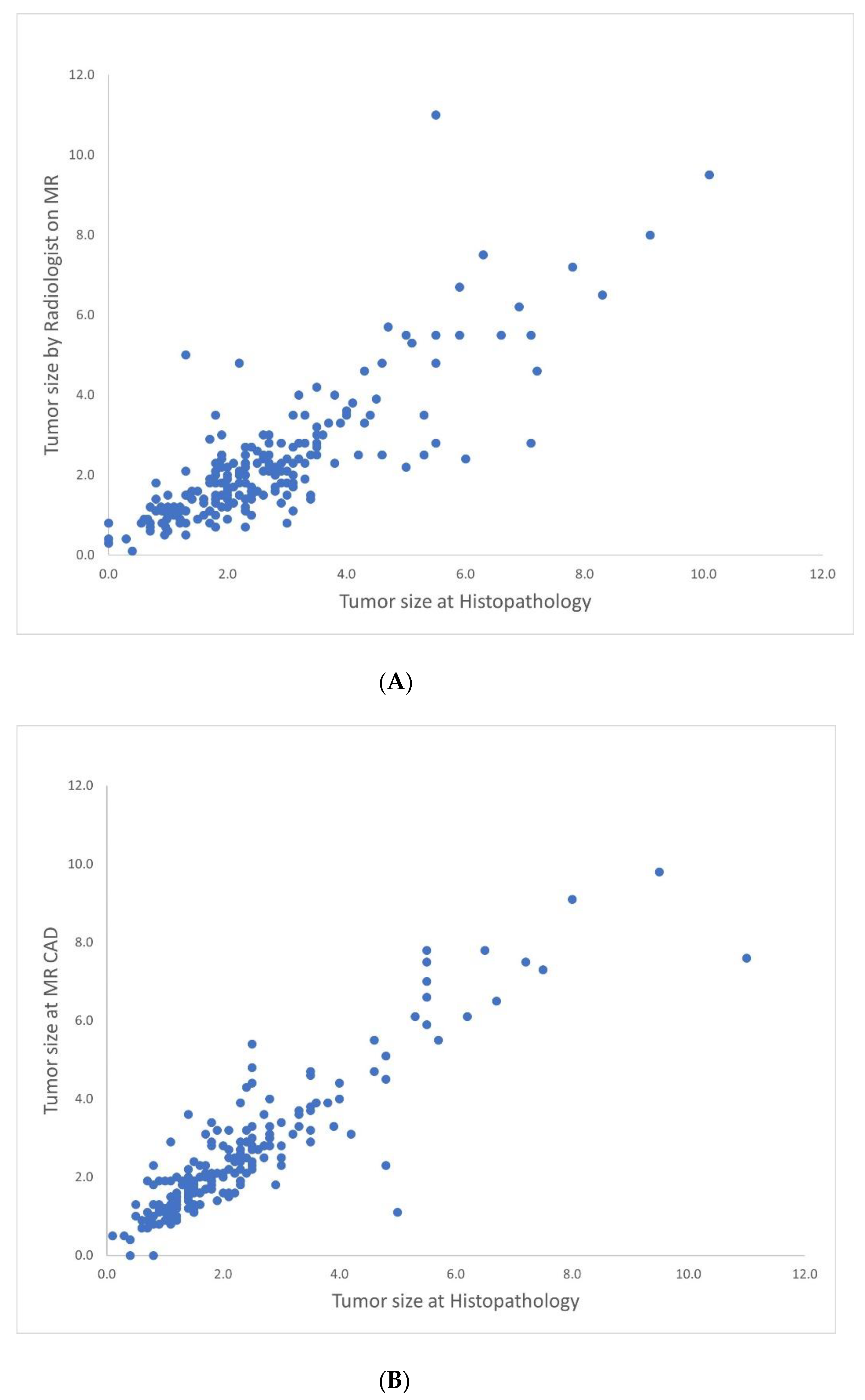




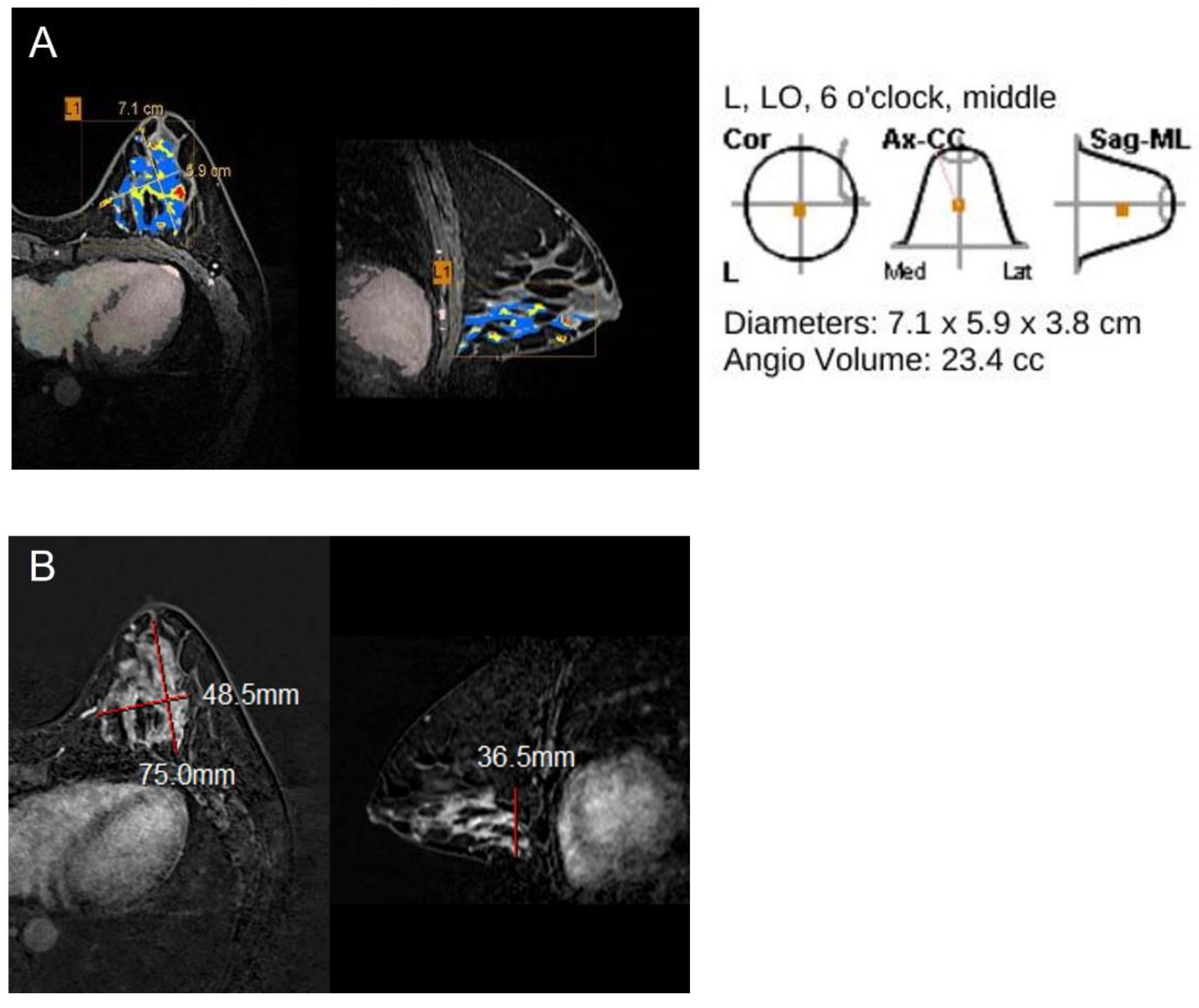
| Variables | N = 208 | CAD-Pathology r (p-Value) | Radiologist-Pathology r (p-Value) | CAD-Radiologist r (p-Value) |
|---|---|---|---|---|
| Overall | 0.823 (<0.001) | 0.898 (<0.001) | 0.925 (<0.001) | |
| Age group | ||||
| <50 years | 62 | 0.775 (<0.001) | 0.893 (<0.001) | 0.925 (<0.001) |
| ≥50 years | 146 | 0.850 (<0.001) | 0.902 (<0.001) | 0.925 (<0.001) |
| Pathologic size | ||||
| 0–2 cm | 111 | 0.605 (<0.001) | 0.649 (<0.001) | 0.812 (<0.001) |
| >2 cm | 97 | 0.733 (<0.001) | 0.854 (<0.001) | 0.896 (<0.001) |
| In situ component | ||||
| (−) | 59 | 0.923 (<0.001) | 0.956 (<0.001) | 0.953 (<0.001) |
| (+) | 148 | 0.794 (<0.001) | 0.883 (<0.001) | 0.916 (<0.001) |
| NA | 1 | |||
| EIC | ||||
| (−) | 134 | 0.807 (<0.001) | 0.898 (<0.001) | 0.905 (<0.001) |
| (+) | 35 | 0.783 (<0.001) | 0.849(<0.001) | 0.933 (<0.001) |
| NA | 39 | |||
| Histologic grade | ||||
| Low | 159 | 0.802 (<0.001) | 0.885 (<0.001) | 0.936 (<0.001) |
| High | 47 | 0.888 (<0.001) | 0.938 (<0.001) | 0.898 (<0.001) |
| NA | 2 | |||
| ER | ||||
| (−) | 52 | 0.928 (<0.001) | 0.950 (<0.001) | 0.935 (<0.001) |
| (+) | 156 | 0.779 (<0.001) | 0.880 (<0.001) | 0.921 (<0.001) |
| PR | ||||
| (−) | 81 | 0.908 (<0.001) | 0.940 (<0.001) | 0.920 (<0.001) |
| (+) | 127 | 0.776 (<0.001) | 0.878 (<0.001) | 0.929 (<0.001) |
| HER2 | ||||
| (−) | 160 | 0.778 (<0.001) | 0.875 (<0.001) | 0.920 (<0.001) |
| (+) | 48 | 0.897 (<0.001) | 0.941 (<0.001) | 0.927 (<0.001) |
| Molecular subtype | ||||
| Luminal A | 83 | 0.733 (<0.001) | 0.865 (<0.001) | 0.916 (<0.001) |
| Luminal B | 74 | 0.825 (<0.001) | 0.896 (<0.001) | 0.921 (<0.001) |
| HER2 | 25 | 0.926 (<0.001) | 0.941 (<0.001) | 0.921 (<0.001) |
| Triple-negative | 26 | 0.928 (<0.001) | 0.960 (<0.001) | 0.955 (<0.001) |
| Ki-67 | ||||
| Low | 93 | 0.744 (<0.001) | 0.863 (<0.001) | 0.921 (<0.001) |
| High | 115 | 0.878 (<0.001) | 0.923 (<0.001) | 0.925 (<0.001) |
| MR finding | ||||
| Mass | 164 | 0.782 (<0.001) | 0.864 (<0.001) | 0.903 (<0.001) |
| NME | 42 | 0.786 (<0.001) | 0.882 (<0.001) | 0.908 (<0.001) |
| NA | 2 | |||
| MR BPE | ||||
| Minimal | 124 | 0.798 (<0.001) | 0.857 (<0.001) | 0.920 (<0.001) |
| Mild | 61 | 0.836 (<0.001) | 0.932 (<0.001) | 0.934 (<0.001) |
| Moderate | 13 | 0.890 (<0.001) | 0.950 (<0.001) | 0.927 (<0.001) |
| Marked | 10 | 0.844 (0.002) | 0.912 (<0.001) | 0.966 (<0.001) |
| MR BPE | ||||
| Minimal + Mild | 185 | 0.818 (<0.001) | 0.894 (<0.001) | 0.927 (<0.001) |
| Moderate + Marked | 23 | 0.879 (<0.001) | 0.934 (<0.001) | 0.937 (<0.001) |
| CAD-Pathology | Radiologist-Pathology | p-Value * | |
|---|---|---|---|
| Accurate | 114 (54.8%) | 152 (73.1%) | <0.001 |
| Inaccurate | 94 (45.2%) | 56 (26.9%) | |
| Underestimation | 17 (8.2%) | 11 (5.3%) | |
| Overestimation | 77 (37.0%) | 45 (21.6%) |
| Variables | Accurate 114 (54.8%) | Inaccurate 94 (45.2%) | p-Value * | |
|---|---|---|---|---|
| Underestimation 17 (8.2%) | Overestimation 77 (37.0%) | |||
| Mean age (years) | 58.38 ± 11.53 | 54.12 ± 15.81 | 57.26 ± 12.54 | 0.326 |
| Age group | 0.129 | |||
| <50 years | 29 (25.4%) | 8 (47.1%) | 25 (32.5%) | |
| ≥50 years | 85 (74.6%) | 9 (52.9%) | 52 (67.5%) | |
| Mean MR CAD size (cm) | 2.02 ± 1.14 | 2.63 ± 1.91 | 3.61 ± 1.86 | <0.001 |
| Mean pathologic size (cm) | 1.97 ± 1.12 | 4.08 ± 2.57 | 2.40 ± 1.72 | 0.002 |
| Pathologic size | 0.045 | |||
| 0–2 cm | 68 (59.6%) | 3 (17.6%) | 40 (51.9%) | |
| >2 cm | 46 (40.4%) | 14 (82.4%) | 37 (48.1%) | |
| In situ component | 0.258 | |||
| (−) | 36 (31.6%) | 3 (17.6%) | 20 (26.0%) | |
| (+) | 78 (68.4%) | 14 (82.4%) | 57 (74.0%) | |
| EIC | 0.045 | |||
| (−) | 79 (69.3%) | 8 (47.1%) | 47 (61.0%) | |
| (+) | 14 (12.3%) | 6 (36.3%) | 15 (19.5%) | |
| NA | 21 (18.4%) | 3 (17.6%) | 15 (19.5%) | |
| Histologic grade | 0.183 | |||
| Low | 84 (73.7%) | 3 (17.6%) | 61 (79.2%) | |
| High | 30 (26.3%) | 14 (82.4%) | 14 (18.2%) | |
| NA | 2 (2.6%) | |||
| ER | 0.148 | |||
| (−) | 33 (28.9%) | 2 (11.8%) | 17 (22.1%) | |
| (+) | 81 (71.1%) | 15 (88.2%) | 60 (77.9%) | |
| PR | 0.030 | |||
| (−) | 52 (45.6%) | 2 (11.8%) | 27 (35.1%) | |
| (+) | 62 (54.4%) | 15 (88.2%) | 50 (64.9%) | |
| HER2 | 0.274 | |||
| (−) | 91 (79.8%) | 12 (70.6%) | 57 (74.0%) | |
| (+) | 23 (20.2%) | 5 (29.4%) | 20 (26.0%) | |
| Molecular subtype | 0.110 | |||
| Luminal A | 43 (37.7%) | 7 (41.2%) | 33 (42.9%) | |
| Luminal B | 39 (34.2%) | 8 (47.1%) | 27 (35.1%) | |
| HER2 | 12 (10.5%) | 2 (11.8%) | 11 (14.3%) | |
| Triple-negative | 20 (17.5%) | 0 (0%) | 6 (7.8%) | |
| Ki-67 | 0.266 | |||
| Low | 47 (41.2%) | 8 (47.1%) | 38 (49.4%) | |
| High | 67 (58.8%) | 9 (52.9%) | 39 (50.6%) | |
| MR finding | 0.002 | |||
| Mass | 99 (86.8%) | 10 (58.8%) | 55 (71.4%) | |
| NME | 14 (12.3%) | 6 (35.3%) | 22 (28.6%) | |
| NA | 1 (0.9%) | 1 (5.9%) | ||
| MR BPE | 0.10 | |||
| Minimal | 76 (66.7%) | 12 (70.6%) | 36 (46.8%) | |
| Mild | 26 (22.8%) | 4 (23.5%) | 31 (40.3%) | |
| Moderate | 6 (5.3%) | 0 (0%) | 7 (9.1%) | |
| Marked | 6 (5.3%) | 1 (5.9%) | 3 (3.9%) | |
| MR BPE | 0.788 | |||
| Minimal + Mild | 102 (89.5%) | 16 (94.1%) | 67 (87.0%) | |
| Moderate + Marked | 12 (10.5%) | 1 (5.9%) | 10 (13.0%) | |
| Variables | Accurate 152 (73.1%) | Inaccurate 56 (26.9%) | p-Value * | |
|---|---|---|---|---|
| Underestimation 11 (5.3%) | Overestimation 45 (21.6%) | |||
| Mean age (years) | 58.01 ± 12.39 | 55.73 ± 12.39 | 56.76 ± 12.08 | 0.451 |
| Age group | 0.916 | |||
| <50 years | 45 (29.6%) | 3 (27.3%) | 14 (31.1%) | |
| ≥50 years | 107 (70.4%) | 8 (72.7%) | 31 (68.9%) | |
| Mean radiologist size (cm) | 2.20 ± 1.42 | 2.50 ± 1.94 | 3.71 ± 1.92 | <0.001 |
| Mean pathologic size (cm) | 2.11 ± 1.41 | 3.95 ± 2.65 | 2.52 ± 1.73 | 0.022 |
| Pathologic size | 0.065 | |||
| 0–2 cm | 87 (57.2%) | 1 (9.1%) | 23 (51.1%) | |
| >2 cm | 65 (42.8%) | 10 (90.9%) | 22 (48.9%) | |
| In situ component | 0.017 | |||
| (−) | 50 (32.9%) | 2 (18.2%) | 7 (15.6%) | |
| (+) | 102 (67.1%) | 9 (81.8%) | 38 (84.4%) | |
| EIC | 0.008 | |||
| (−) | 109 (71.7%) | 7 (63.6%) | 18 (40.0%) | |
| (+) | 21 (13.8%) | 3 (27.3%) | 11 (24.4%) | |
| NA | 22 (14.5%) | 1 (9.1%) | 16 (35.6%) | |
| Histologic grade | 0.358 | |||
| Low | 119 (78.3%) | 9 (81.8%) | 31 (68.9%) | |
| High | 32 (21.0%) | 2 (18.2%) | 13 (28.9%) | |
| NA | 1 (0.7%) | 1 (2.2%) | ||
| ER | 0.279 | |||
| (−) | 35 (23.0%) | 1 (9.1%) | 16 (35.6%) | |
| (+) | 117 (77.0%) | 10 (90.9%) | 29 (64.4%) | |
| PR | 0.702 | |||
| (−) | 58 (38.2%) | 3 (27.3%) | 20 (44.4%) | |
| (+) | 94 (61.8%) | 8 (72.7%) | 25 (55.6%) | |
| HER2 | 0.003 | |||
| (−) | 125 (82.2%) | 8 (72.7%) | 27 (60.0%) | |
| (+) | 27 (17.8%) | 3 (27.3%) | 18 (40.0%) | |
| Molecular subtype | 0.078 | |||
| Luminal A | 63 (41.4%) | 5 (45.5%) | 15 (33.3%) | |
| Luminal B | 55 (36.2%) | 5 (45.5%) | 14 (31.1%) | |
| HER2 | 13 (8.6%) | 1 (9.1%) | 11 (24.4%) | |
| Triple-negative | 21 (13.8%) | 0 (0%) | 5 (11.1%) | |
| Ki-67 | 0.537 | |||
| Low | 66 (43.4%) | 6 (54.5%) | 21 (46.7%) | |
| High | 86 (56.6%) | 5 (45.5%) | 24 (53.3%) | |
| MR finding | <0.001 | |||
| Mass | 132 (86.8%) | 7 (63.6%) | 25 (55.6%) | |
| NME | 19 (12.5%) | 3 (27.3%) | 20 (44.4%) | |
| NA | 1 (0.7%) | 1 (9.1%) | ||
| MR BPE | 0.519 | |||
| Minimal | 92 (60.5%) | 7 (63.6%) | 25 (55.6%) | |
| Mild | 41 (27.0%) | 3 (27.3%) | 17 (37.8%) | |
| Moderate | 11 (7.2%) | 0 (0%) | 2 (4.4%) | |
| Marked | 8 (5.3%) | 1 (9.1%) | 1 (2.2%) | |
| MR BPE | 0.274 | |||
| Minimal + Mild | 133 (87.5%) | 10 (90.9%) | 42 (93.3%) | |
| Moderate + Marked | 19 (12.5%) | 1 (9.1%) | 3 (6.7%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y. Evaluation of Breast Cancer Size Measurement by Computer-Aided Diagnosis (CAD) and a Radiologist on Breast MRI. J. Clin. Med. 2022, 11, 1172. https://doi.org/10.3390/jcm11051172
Park JY. Evaluation of Breast Cancer Size Measurement by Computer-Aided Diagnosis (CAD) and a Radiologist on Breast MRI. Journal of Clinical Medicine. 2022; 11(5):1172. https://doi.org/10.3390/jcm11051172
Chicago/Turabian StylePark, Ji Yeon. 2022. "Evaluation of Breast Cancer Size Measurement by Computer-Aided Diagnosis (CAD) and a Radiologist on Breast MRI" Journal of Clinical Medicine 11, no. 5: 1172. https://doi.org/10.3390/jcm11051172






