Role and Impact of Cerebrolysin for Ischemic Stroke Care
Abstract
:1. Introduction
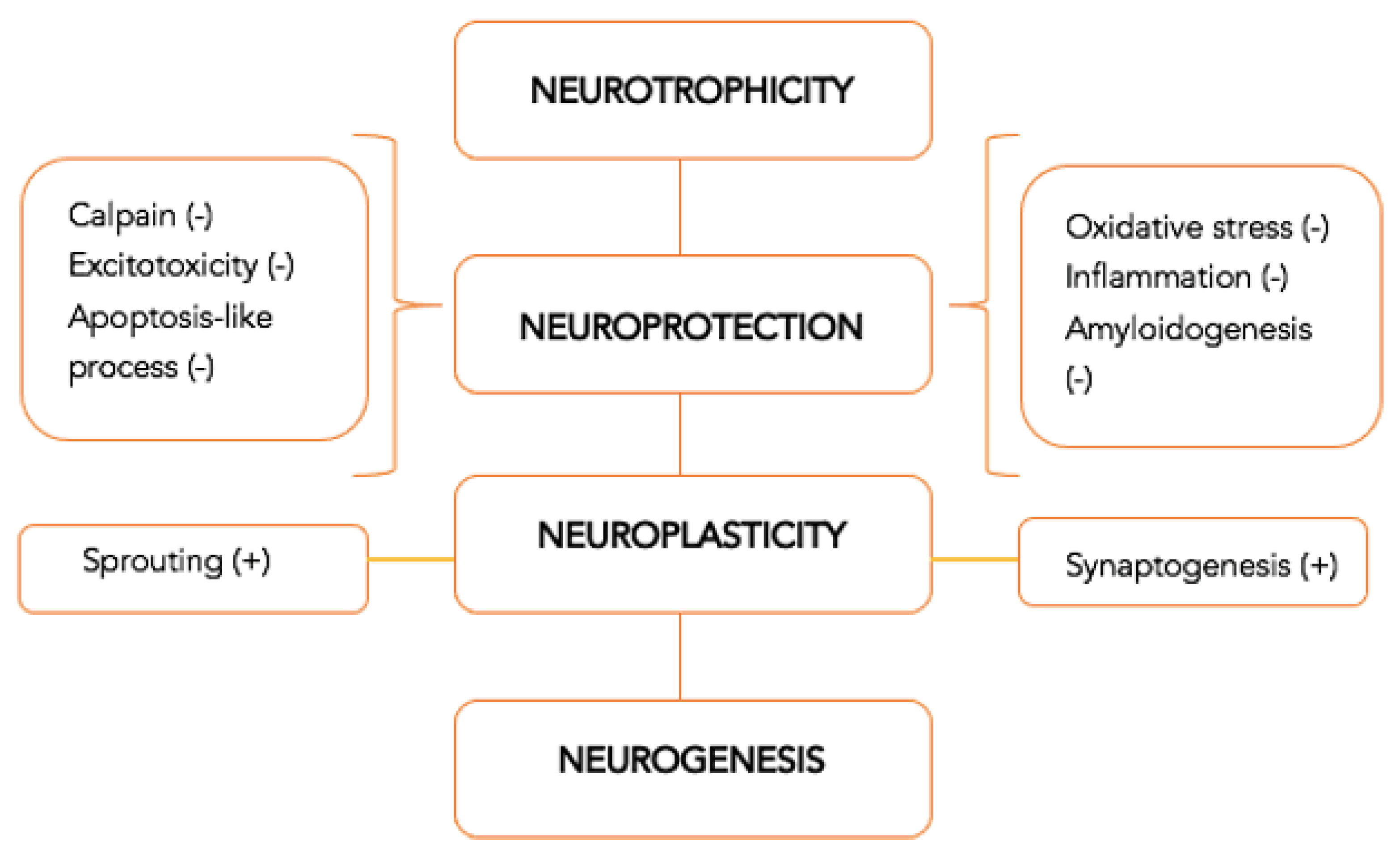
2. Concepts of Neuroprotection and Neuroregeneration
3. Clinical Trials
3.1. Efficacy of Cerebrolysin
3.2. Safety Profile of Cerebrolysin
4. Observational Research—Effectiveness Studies
5. Cost-Effectiveness Research
6. Cerebrolysin Recommendations in Guidelines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chugh, C. Acute Ischemic Stroke: Management Approach. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2019, 23, S140–S146. [Google Scholar] [CrossRef]
- Warburton, E.; Alawneh, J.A.; Clatworthy, P.L.; Morris, R.S. Stroke Management. BMJ Clin. Evid. 2011, 6, 201. [Google Scholar]
- Phipps, M.S.; Cronin, C.A. Management of Acute Ischemic Stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muresanu, D.F.; Heiss, W.-D.; Hoemberg, V.; Bajenaru, O.; Popescu, C.D.; Vester, J.C.; Rahlfs, V.W.; Doppler, E.; Meier, D.; Moessler, H.; et al. Cerebrolysin and Recovery After Stroke (CARS) A randomized, placebo-controlled, double-blind, multicenter trial. Stroke 2016, 47, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Muresanu, D.F.; Buzoianu, A.; Florian, S.I.; von Wild, T. Towards a Roadmap in Brain Protection and Recovery. J. Cell. Mol. Med. 2012, 16, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Li, C.; Zhang, Y.; Lu, M.; Chopp, M.; Zhang, Z.G.; Melcher-Mourgas, M.; Fleckenstein, B. Therapeutic effect of Cerebrolysin on reducing impaired cerebral endothelial cell permeability. Neuroreport 2021, 32, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.; Walsh, A.; Adikari, A.; Goodin, P.; Alahakoon, D.; De Silva, D.; Ong, K.L.; Nilsson, M.; Boyd, L. Finding the Intersection of Neuroplasticity, Stroke Recovery, and Learning: Scope and Contributions to Stroke Rehabilitation. Neural Plast. 2019, 2019, 5232374. [Google Scholar] [CrossRef]
- Muresanu, D.F. Management of Acute Stroke: Neuroprotection. In Stroke; Bornstein, N.M., Ed.; Karger: Basel, Switzerland, 2009; pp. 128–136. ISBN 978-3-8055-9099-0. [Google Scholar]
- Muresanu, D.F.; Strilciuc, S.; Stan, A. Current Drug Treatment of Acute Ischemic Stroke: Challenges and Opportunities. CNS Drugs 2019, 33, 841–847. [Google Scholar] [CrossRef]
- Muresanu, D.F. Neuroprotection and neuroplasticity—A holistic approach and future perspectives. J. Neurol. Sci. 2007, 257, 38–43. [Google Scholar] [CrossRef]
- Muresanu, D.F. Neuroplasticity and Neurorecovery. In Stroke; Bornstein, N.M., Ed.; Karger: Basel, Switzerland, 2009; pp. 37–49. [Google Scholar] [CrossRef]
- Puderbaugh, M.; Emmady, P.D. Neuroplasticity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Brainin, M. Cerebrolysin: A Multi-Target Drug for Recovery after Stroke. Expert Rev. Neurother. 2018, 18, 681–687. [Google Scholar] [CrossRef]
- Brunet, A.; Datta, S.R.; Greenberg, M.E. Transcription-Dependent and -Independent Control of Neuronal Survival by the PI3K–Akt Signaling Pathway. Curr. Opin. Neurobiol. 2001, 11, 297–305. [Google Scholar] [CrossRef]
- Hutter-Paier, B.; Grygar, E.; Frühwirth, M.; Temmel, I.; Windisch, M. Further evidence that Cerebrolysin® protects cortical neurons from neurodegeneration in vitro. J. Neural Transm. Suppl. 1998, 53, 363–372. [Google Scholar] [PubMed]
- Iseda, T.; Nishio, T.; Kawaguchi, S.; Kawasaki, T.; Wakisaka, S. Spontaneous Regeneration of the Corticospinal Tract after Transection in Young Rats: Collagen Type IV Deposition and Astrocytic Scar in the Lesion Site Are Not the Cause but the Effect of Failure of Regeneration. J. Comp. Neurol. 2003, 464, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Díez-Tejedor, E. The Pharmacology of Neurotrophic Treatment with Cerebrolysin: Brain Protection and Repair to Counteract Pathologies of Acute and Chronic Neurological Disorders. Drugs Today 2012, 48, 3–24. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Yuen, E.Y.; Lu, Y.-F.; Matsushita, M.; Matsui, H.; Yan, Z.; Tomizawa, K. Regulation of N-Methyl-D-Aspartate Receptors by Calpain in Cortical Neurons. J. Biol. Chem. 2005, 280, 21588–21593. [Google Scholar] [CrossRef] [Green Version]
- Kotter, M.R.; Setzu, A.; Sim, F.J.; Van Rooijen, N.; Franklin, R.J.M. Macrophage Depletion Impairs Oligodendrocyte Remyelination Following Lysolecithin-Induced Demyelination. Glia 2001, 35, 204–212. [Google Scholar] [CrossRef]
- Windhagen, A.; Newcombe, J.; Dangond, F.; Strand, C.; Woodroofe, M.N.; Cuzner, M.L.; Hafler, D.A. Expression of Costimulatory Molecules B7-1 (CD80), B7-2 (CD86), and Interleukin 12 Cytokine in Multiple Sclerosis Lesions. J. Exp. Med. 1995, 182, 1985–1996. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Arnold, F.J.; Bading, H. A calcium microdomain near NMDA receptors: On switch for ERK-dependent synapse-tonucleus communication. Nat. Neurosci. 2001, 4, 565–566. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging Neuroprotective Strategies for the Treatment of Ischemic Stroke: An Overview of Clinical and Preclinical Studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Kandel, E. Principles of Neural Science, 5th ed.; Appleton and Lange; McGraw Hill: New York, NY, USA, 2006; ISBN 978-0-07-139011-8. [Google Scholar]
- Auriel, E.; Bornstein, N.M. Neuroprotection in acute ischemic stroke-current status. J. Cell. Mol. Med. 2010, 14, 2200–2202. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tung, Y.-C.; Li, B.; Iqbal, K.; Grundke-Iqbal, I. Trophic Factors Counteract Elevated FGF-2-Induced Inhibition of Adult Neurogenesis. Neurobiol. Aging 2007, 28, 1148–1162. [Google Scholar] [CrossRef]
- Zhang, C.; Chopp, M.; Cui, Y.; Wang, L.; Zhang, R.; Zhang, L.; Lu, M.; Szalad, A.; Doppler, E.; Hitzl, M.; et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J. Neurosci. Res. 2010, 88, 3275–3281. [Google Scholar] [CrossRef] [Green Version]
- Wronski, R.; Tompa, P.; Hutter-Paier, B.; Crailsheim, K.; Friedrich, P.; Windisch, M. Inhibitory effect of a brain derived peptide preparation on the intracellular Calcium Ca++- dependent protease, calpain. J. Neural Transm. 2000, 107, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Kondo, T.; Kanazawa, A.; Itou, T.; Mizuno, Y. Protective effect of FPF 1070 (Cerebrolysin) on delayed neuronal death in the gerbil–detection of hydroxyl radicals with salicylic acid. No Shinkei = Brain Nerve 1993, 45, 325–331. [Google Scholar] [PubMed]
- Álvarez, X.A.; Lombardi, V.R.M.; Fernández-Novoa, L.; García, M.; Sampedro, C.; Cagiao, A.; Cacabelos, R.; Windisch, M. Cerebrolysin reduces microglial activation in vivo and in vitro: A potential mechanism of neuroprotection. J. Neural Transm. 2000, 59, 281–292. [Google Scholar]
- Hutter-Paier, B.; Grygar, E.; Windisch, M. Death of cultured telencephalon neurons induced by glutamate is reduced by the peptide derivate Cerebrolysin. J. Neural Transm. 1996, 47, 267–273. [Google Scholar]
- Rockenstein, E.; Adame, A.; Mante, M.; Moessler, H.; Windisch, M.; Masliah, E. The neuroprotective effects of Cerebrolysin trade mark in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance. J. Neural Transm. 2003, 110, 1313–1327. [Google Scholar] [CrossRef]
- Rockenstein, E.; Mante, M.; Adame, A.; Crews, L.; Moessler, H.; Masliah, E. Effects of Cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer’s disease. Acta Neuropathol. 2007, 113, 265–275. [Google Scholar] [CrossRef]
- Jin, Y.; Barnett, A.; Zhang, Y.; Yu, X.; Luo, Y. Poststroke sonic hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke 2017, 48, 1636–1645. [Google Scholar] [CrossRef]
- Zhang, L.; Chopp, M.; Meier, D.H.; Winter, S.; Wang, L.; Szalad, A.; Lu, M.; Wei, M.; Cui, Y.; Zhang, Z.G. Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke 2013, 44, 1965–1972. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.; Alvarez, X.A.; Le, H.-A.; Nguyen, D.-A.; Le, T.; Nguyen, N.; Nguyen, T.; Nguyen, T.; Vo, T.; Tran, T.; et al. Clinical Efficacy of Cerebrolysin and Cerebrolysin plus Nootropics in the Treatment of Patients with Acute Ischemic Stroke in Vietnam. CNS Neurol. Disord.-Drug Targets 2021, 20, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Lee, J.; Shin, Y.-I.; Ko, M.-H.; Kim, D.Y.; Sohn, M.K.; Kim, J.; Kim, Y.-H. Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. J. Pers. Med. 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Park, C.; Kim, D.Y.; Shin, Y.-I.; Ko, M.-H.; Lee, A.; Jang, S.Y.; Kim, Y.-H. Cerebrolysin Combined with Rehabilitation Promotes Motor Recovery in Patients with Severe Motor Impairment after Stroke. BMC Neurol. 2016, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.-X.; Zhang, T.; Zhao, Y.-W.; Geng, Z.; Chen, J.-J.; Chen, H. Efficacy and Safety Comparison of DL-3-n-Butylphthalide and Cerebrolysin: Effects on Neurological and Behavioral Outcomes in Acute Ischemic Stroke. Exp. Ther. Med. 2016, 11, 2015–2020. [Google Scholar] [CrossRef] [Green Version]
- Guekht, A.; Heiss, D.; Gusev, E.; Vester, J.; Doppler, E.; Muresanu, D. Cerebrolysin and Recovery after Stroke (CARS 2): A Randomized, Placebo-Controlled, Double-Blind, Multicenter Clinical Study. J. Neurol. Sci. 2015, 357, e103. [Google Scholar] [CrossRef]
- Rezaei, Y.; Amiri-Nikpour, M.R.; Nazarbaghi, S.; Ahmadi-Salmasi, B.; Mokari, T.; Tahmtan, O. Cerebrolysin Effects on Neurological Outcomes and Cerebral Blood Flow in Acute Ischemic Stroke. Neuropsychiatr. Dis. Treat. 2014, 10, 2299. [Google Scholar] [CrossRef] [Green Version]
- Lang, W.; Stadler, C.H.; Poljakovic, Z.; Fleet, D. A Prospective, Randomized, Placebo-Controlled, Double-Blind Trial about Safety and Efficacy of Combined Treatment with Alteplase (Rt-PA) and Cerebrolysin in Acute Ischaemic Hemispheric Stroke. Int. J. Stroke 2013, 8, 95–104. [Google Scholar] [CrossRef]
- Stan, A.; Birle, C.; Blesneag, A.; Iancu, M. Cerebrolysin and Early Neurorehabilitation in Patients with Acute Ischemic Stroke: A Prospective, Randomized, Placebo-Controlled Clinical Study. J. Med. Life 2017, 10, 216–222. [Google Scholar]
- Heiss, W.-D.; Brainin, M.; Bornstein, N.M.; Tuomilehto, J.; Hong, Z. Cerebrolysin in Patients With Acute Ischemic Stroke in Asia: Results of a Double-Blind, Placebo-Controlled Randomized Trial. Stroke 2012, 43, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Bornstein, N.M.; Guekht, A.; Vester, J.; Heiss, W.-D.; Gusev, E.; Hömberg, V.; Rahlfs, V.W.; Bajenaru, O.; Popescu, B.O.; Muresanu, D. Safety and Efficacy of Cerebrolysin in Early Post-Stroke Recovery: A Meta-Analysis of Nine Randomized Clinical Trials. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2018, 39, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Guekht, A.; Vester, J.; Heiss, W.-D.; Gusev, E.; Hoemberg, V.; Rahlfs, V.W.; Bajenaru, O.; Popescu, B.O.; Doppler, E.; Winter, S.; et al. Safety and Efficacy of Cerebrolysin in Motor Function Recovery after Stroke: A Meta-Analysis of the CARS Trials. Neurol. Sci. 2017, 38, 1761–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strilciuc, S.; Vécsei, L.; Boering, D.; Pražnikar, A.; Kaut, O.; Riederer, P.; Battistin, L. Safety of Cerebrolysin for Neurorecovery after Acute Ischemic Stroke: A Systematic Review and Meta-Analysis of Twelve Randomized-Controlled Trials. Pharmaceuticals 2021, 14, 1297. [Google Scholar] [CrossRef]
- Ziganshina, L.E.; Abakumova, T.; Hoyle, C.H. Cerebrolysin for Acute Ischaemic Stroke. Cochrane Database Syst. Rev. 2020, 7, CD007026. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G. Criteria for Distinguishing Effectiveness From Efficacy Trials in Systematic Reviews; Technical Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. [Google Scholar]
- Martinez, R.M. Efficacy of Cerebrolysin in the Reduction of Spasticity during Stroke Rehabilitation. J. Med. Life 2017, 10, 161–166. [Google Scholar] [PubMed]
- Kim, J.Y.; Kim, H.J.; Choi, H.S.; Park, S.Y.; Kim, D.Y. Effects of Cerebrolysin® in Patients With Minimally Conscious State After Stroke: An Observational Retrospective Clinical Study. Front. Neurol. 2019, 10, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Rochmah, T.N.; Rahmawati, I.T.; Dahlui, M.; Budiarto, W.; Bilqis, N. Economic Burden of Stroke Disease: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 7552. [Google Scholar] [CrossRef]
- Hay, J.W.; Smeeding, J.; Carroll, N.V.; Drummond, M.; Garrison, L.P.; Mansley, E.C.; Mullins, C.D.; Mycka, J.M.; Seal, B.; Shi, L. Good Research Practices for Measuring Drug Costs in Cost Effectiveness Analyses: Issues and Recommendations: The ISPOR Drug Cost Task Force Report—Part I. Value Health 2010, 13, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Mauskopf, J.A.; Sullivan, S.D.; Annemans, L.; Caro, J.; Mullins, C.D.; Nuijten, M.; Orlewska, E.; Watkins, J.; Trueman, P. Principles of Good Practice for Budget Impact Analysis: Report of the ISPOR Task Force on Good Research Practices—Budget Impact Analysis. Value Health 2007, 10, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Kulikov, A.; Abdrashitova, G. Cost-Effectiveness Analysis of Cerebrolysin In The Treatment of Patients With Acute Ischemic Stroke Moderate and Severe Degrees of Severity In The Russian Federation. Value Health 2015, 18, A705. [Google Scholar] [CrossRef] [Green Version]
- Kulikov, A.; Abdrashitova, G. Budget Impact Analysis of Cerebrolysin In The Treatment of Acute Ischemic Stroke of Moderate and Severe Degrees of Severity In The Russian Federation. Value Health 2015, 18, A699. [Google Scholar] [CrossRef] [Green Version]
- Walter, E.; Bauer, M.; Ressl, S. Cost-Effectiveness Of Combined Treatment With Alteplase (Rt-Pa) And Cerebrolysin In Acute Ischemic Hemispheric Stroke In Austria. Value Health 2015, 18, A390. [Google Scholar] [CrossRef]
- European Observatory of Health Systems and Policies Austria HiT. 2018. Available online: https://www.euro.who.int/en/about-us/partners/observatory-old/publications/health-system-reviews-hits/full-list-of-country-hits/austria-hit-2018 (accessed on 12 January 2022).
- Russian Federation Russian Federation: Health System Review. 2011. Available online: https://eurohealthobservatory.who.int/publications/i/russian-federation-health-system-review-2011 (accessed on 12 January 2022).
- Teasell, R.; Hussein, N.; Mirkowski, M.; Vanderlaan, D.; Saikaley, M.; Longval, M.; Iruthayarajah, J. Stroke Rehabilitation Clinician Handbook; Heart and Stroke Foundation: London, ON, Canada, 2020. [Google Scholar]
- Platz, T.; Fheodoroff, K.; Mehrholz, J. S3 Guideline Rehabilitation Therapy for Arm Paresis after Stroke of the DGNR Long Version; Springer: Greifswald, Germany, 2020. [Google Scholar]
- Beghi, E.; Binder, H.; Birle, C.; Bornstein, N.; Diserens, K.; Groppa, S.; Homberg, V.; Lisnic, V.; Pugliatti, M.; Randall, G.; et al. European Academy of Neurology and European Federation of Neurorehabilitation Societies guideline on pharmacological support in early motor rehabilitation after acute ischaemic stroke. Eur. J. Neurol. 2021, 28, 2831–2845. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Nagappan, P.G.; Chen, H.; Wang, D.-Y. Neuroregeneration and Plasticity: A Review of the Physiological Mechanisms for Achieving Functional Recovery Postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef]

| Article | Intervention | Case Numbers | Type | Methods | Primary Endpoint | Secondary Endpoint | Results |
|---|---|---|---|---|---|---|---|
| Tran et al., 2021 | Cerebrolysin + Nootropics | 190-Cerebrolysin 86-Placebo | Non-interventional, controlled, open-label, prospective and multicenter study | Cerebrolysin (10 mL), other nootropics, or a combination of both | Modified Rankin Scale (mRS) | National Institutes of Health Stroke Scale (NIHSS), Montreal Cognitive Assessment (MoCA) | mRS: improvement in Cerebrolysin 81.6%, combination 93.4%/placebo 43% NIHSS: good responders Cerebrolysin 77.5%, combination 92.5%/placebo 47.6% MOCA: scores Cerebrolysin 23.3 ± 4.8, combination: 23.7 ± 4.1/placebo 15.9 ± 7.7 |
| Chang et al., 2021 | Cerebrolysin + Standardized rehabilitation therapy | 59-Cerebrolysin 51-Placebo | Combined data from the both phase IV prospective, multicenter, randomized, double-blind, placebo-controlled trials | Cerebrolysin or placebo with standardized rehabilitation therapy for 21-day treatment course | Fugl–Meyer Assessment | Motor Evoked Potential (MEP) | FMA-upper limb: T1–T2 significant improvement in Cerebrolysingroup MEP T1: positive response Cerebrolysin 33.9%/placebo 27.5% MEP T2: increased both groups, Cerebrolysin 42.4%/placebo 35.3% |
| Stan et al., 2017 | Cerebrolysin | 30-Cerebrolysin 30-Placebo | Prospective, randomized, double-blind, placebo-controlled | 30 mL/day Cerebrolysin or to placebo for 10 consecutive days, starting in the first 24–48 h after stroke | National Institutes of Health Stroke Scale (NIHSS) | Modified Rankin Scale (mRS) | NIHSS higher scores in the Cerebrolysin group day 10: MW = 0.79 day 30: MW = 0.75 mRS day 30: independent patients in Cerebrolysin group: 73.33%/placebo: 44.83% |
| Chang et al., 2016 | Cerebrolysin | 35-Cerebrolysin 35-Placebo | Prospective, multicenter, randomized, double-blind, placebo-controlled, parallel-group study | 30 mL/day Cerebrolysin or to placebo for 21 days | Fugl–Meyer Assessment | National Institutes of Health Stroke Scale (NIHSS) | no significant difference was found between the two groupsTotal FMA: 42 Cerebrolysin, 42.2 placebo NIHSS: 8.4 Cerebrolysin, 7 placebos |
| Xue et al., 2016 | Cerebrolysin vs. DL-3-n-butylphthalide (NBP) | 20-Cerebrolysin 20-Placebo 20-NBP | Randomized, double-blind trial | 10-day intravenous administration of NBP (Cerebrolysin or placebo) | National Institutes of Health Stroke Scale (NIHSS) and Barthel Index (BI) | - | NIHSS day 21: lower scores for Cerebrolysin and NBP group BI day 21: higher scores for Cerebrolysin and NBP group |
| Muresanu et al., 2015 | Cerebrolysin+ Standardized rehabilitation program | 104-Cerebrolysin 104-Placebo | Prospective, randomized, double-blind, placebo-controlled, multicenter, parallel-group study | Cerebrolysin (30 mL/d) or a placebo (saline) once daily for 21 days, beginning at 24 to 72 h after stroke onset + Standardized rehabilitation program for 21 days | Action Research Arm Test | Modified Rankin Scale (mRS) | ARAT day 90: an increase in 92.3% of patients in Cerebrolysin group/84.2% placebo mRS: score of. 0–1 in 42.3% patients in Cerebrolysin group/14.9% placebo |
| Guekht et al., 2015 | Cerebrolysin | 120-Cerebrolysin 120-Placebo | Prospective, randomized, double-blind, placebo-controlled, multicenter, parallel-group study | Cerebrolysin (30 mL/d) or a placebo (saline) | Action Research Arm Test | Gait velocity, fine motor function, global neurological status, disability, quality of life, neglect | No end points showed significant improvement at 90 days for Cerebrolysin group mild baseline levels of impairment showed improvement after 90 days in placebo group |
| Razei et al., 2014 | Cerebrolysin | 23-Cerebrolysin 23-Placebo | Randomized, double-blinded, placebo-controlled trial | Cerebrolysin (30 mL) diluted in normal saline daily or Normal saline alone, adjunct to 100 mg of aspirin daily for 10 days | National Institutes of Health Stroke Scale (NIHSS) | Mean flow velocity and PI of cerebral arteries | NIHSS day 60 and 90: lower values in the Cerebrolysin group mean flow velocity day 30: higher in the placebo group median = 53, Cerebrolysin median = 45PI lower in the Cerebrolysin group 0.85/placebo 1.1 |
| Lang et al. 2013 | Cerebrolysin + Alteplase | 60-Cerebrolysin 59-Placebo | Placebo-controlled, double-blind trial | Cerebrolysin (30 mL) or placebo (1 h after thrombolytic treatment) starting within three-hours after onset of symptoms, given for 10 consecutive days | Modified Rankin Scale (mRS) | National Institutes of Health Stroke Scale (NIHSS), Glasgow Outcome Scale (GOS), Barthel Index (BI) | mRS day 90: no significant improvement in Cerebrolysin group vs. placebo NIHSS, GOS, BI: no significant improvement in Cerebrolysin group vs. placebo |
| Heiss et al., 2012 | Cerebrolysin | 529-Cerebrolysin 541-Placebo | Double-blind, placebo-controlled randomized clinical trial | 30 mL Cerebrolysin daily or placebo (saline solution) given as intravenous infusion for 10 days in addition to aspirin (100 mg daily) | Modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI) | Responder analysis and global test | NIHSS day 90: improved by 6 Cerebrolysin/5 placebo BI: 30 for both groups mRS: 2 for both groups global test MW = 0.50 CI = 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureșanu, D.F.; Livinț Popa, L.; Chira, D.; Dăbală, V.; Hapca, E.; Vlad, I.; Văcăraș, V.; Popescu, B.O.; Cherecheș, R.; Strilciuc, Ș.; et al. Role and Impact of Cerebrolysin for Ischemic Stroke Care. J. Clin. Med. 2022, 11, 1273. https://doi.org/10.3390/jcm11051273
Mureșanu DF, Livinț Popa L, Chira D, Dăbală V, Hapca E, Vlad I, Văcăraș V, Popescu BO, Cherecheș R, Strilciuc Ș, et al. Role and Impact of Cerebrolysin for Ischemic Stroke Care. Journal of Clinical Medicine. 2022; 11(5):1273. https://doi.org/10.3390/jcm11051273
Chicago/Turabian StyleMureșanu, Dafin F., Livia Livinț Popa, Diana Chira, Victor Dăbală, Elian Hapca, Irina Vlad, Vitalie Văcăraș, Bogdan Ovidiu Popescu, Răzvan Cherecheș, Ștefan Strilciuc, and et al. 2022. "Role and Impact of Cerebrolysin for Ischemic Stroke Care" Journal of Clinical Medicine 11, no. 5: 1273. https://doi.org/10.3390/jcm11051273
APA StyleMureșanu, D. F., Livinț Popa, L., Chira, D., Dăbală, V., Hapca, E., Vlad, I., Văcăraș, V., Popescu, B. O., Cherecheș, R., Strilciuc, Ș., & Brainin, M. (2022). Role and Impact of Cerebrolysin for Ischemic Stroke Care. Journal of Clinical Medicine, 11(5), 1273. https://doi.org/10.3390/jcm11051273








