Investigating the Long-Term Effect of an Interdisciplinary Multimodal Rehabilitation Program on Levels of Bioactive Lipids and Telomerase Activity in Blood from Patients with Chronic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients, Clinical Examination, and IPRP
2.2.1. Patients
2.2.2. Clinical Examination
2.2.3. IPRP
2.3. Self-Reports of Pain Intensity, Psychological Distress, Physical Activity, and BMI
2.3.1. Pain Intensity
2.3.2. Hospital Anxiety and Depression Scale
2.3.3. Physical Activity
2.3.4. Body Mass Index
2.4. Biological Measurements
2.4.1. Blood Sampling
2.4.2. Analysis of Bioactive Lipids
2.4.3. TL Measurement
2.4.4. TA Measurement
2.5. Statistical Analysis
3. Results
3.1. Pain Intensity, Psychological Distress, Physical Activity, and BMI
3.2. Biological Measurements
Levels of Bioactive Lipid Mediators
3.3. TL and TA
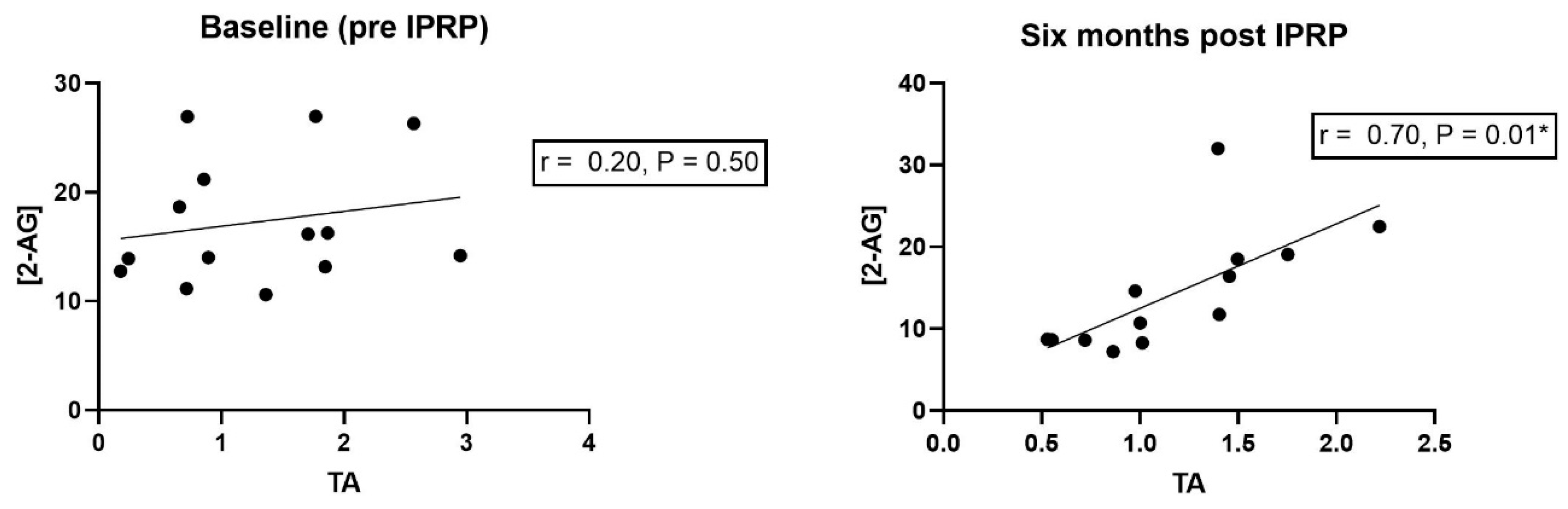
3.4. Correlation Analyses
4. Discussion
- SEA levels were significantly increased at the six-month follow-up, and the changes were inversely correlated with changes in pain intensity;
- AEA and physical activity levels were inversely correlated at baseline and at the follow-up, and moreover, changes in AEA were inversely correlated with changes in physical activity;
- A strong positive correlation existed between levels of 2-AG and TA at the six-month follow-up.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Torrance, N.; Smith, B.H. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [PubMed] [Green Version]
- Bair, M.J.; Wu, J.; Damush, T.M.; Sutherland, J.M.; Kroenke, K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom. Med. 2008, 70, 890–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.; Bromet, E.J.; de Girolamo, G.; de Graaf, R.; et al. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity with Depression-Anxiety Disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.; Ostelo, R.; Guzman, J.; van Tulder, M. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hylands-White, N.; Duarte, R.; Raphael, J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2016, 37, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Ringqvist, A.; Dragioti, E.; Björk, M.; Larsson, B.; Gerdle, B. Moderate and Stable Pain Reductions as a Result of Interdisciplinary Pain Rehabilitation—A Cohort Study from the Swedish Quality Registry for Pain Rehabilitation (SQRP). J. Clin. Med. 2019, 8, 905. [Google Scholar] [CrossRef] [Green Version]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the Immune System in Health and Disease. Endocannabinoids 2015, 231, 185–211. [Google Scholar]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical activity and the endocannabinoid system: An overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [Google Scholar] [CrossRef]
- Verme, J.L.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gaetani, S.; Oveisi, F.; Verme, J.L.; Serrano, A.; De Fonseca, F.R.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 2003, 425, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, A.G.; Kosiakova, H.V.; Onopchenko, O.V.; Panchuk, R.R.; Stoika, R.S.; Hula, N.M. N-Stearoylethanolamine suppresses the pro-inflammatory cytokines production by inhibition of NF-kappaB translocation. Prostaglandins Other Lipid Mediat. 2015, 121, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S. Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef] [PubMed]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Heyman, E.; Gamelin, F.-X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—Possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: Exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J. Exp. Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Belitardo de Oliveira, A.; de Mello, M.T.; Tufik, S.; Peres, M.F.P. Weight loss and improved mood after aerobic exercise training are linked to lower plasma anandamide in healthy people. Physiol. Behav. 2019, 201, 191–197. [Google Scholar] [CrossRef]
- Stensson, N.; Gerdle, B.; Ernberg, M.; Mannerkorpi, K.; Kosek, E.; Ghafouri, B. Increased Anandamide and Decreased Pain and Depression after Exercise in Fibromyalgia. Med. Sci. Sports Exerc. 2020, 52, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Prather, A.A. Stress, Telomeres, and Psychopathology: Toward a Deeper Understanding of a Triad of Early Aging. Annu. Rev. Clin. Psychol. 2018, 14, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Lin, J.; Dhabhar, F.S.; Wolkowitz, O.M.; Puterman, E.; Karan, L.; Blackburn, E.H. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 2010, 24, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, C.M.; Ogata, S.; Reynolds, C.F.; Mischoulon, D.; Chang, G.; Cook, N.R.; E Manson, J.; Crous-Bou, M.; De Vivo, I.; I Okereke, O. Telomere length and its relationships with lifestyle and behavioural factors: Variations by sex and race/ethnicity. Age Ageing 2020, 50, 838–846. [Google Scholar] [CrossRef]
- Deng, W.; Cheung, S.; Tsao, S.; Wang, X.; Tiwari, A. Telomerase activity and its association with psychological stress, mental disorders, lifestyle factors and interventions: A systematic review. Psychoneuroendocrinology 2016, 64, 150–163. [Google Scholar] [CrossRef]
- Stensson, N.; Ghafouri, N.; Ernberg, M.; Mannerkorpi, K.; Kosek, E.; Gerdle, B.; Ghafouri, B. The Relationship of Endocannabinoidome Lipid Mediators with Pain and Psychological Stress in Women with Fibromyalgia: A Case-Control Study. J. Pain 2018, 19, 1318–1328. [Google Scholar] [CrossRef]
- Ghafouri, N.; Ghafouri, B.; Larsson, B.; Stensson, N.; Fowler, C.J.; Gerdle, B. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 2013, 154, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Milton, M.B.; Börsbo, B.; Rovner, G.; Lundgren-Nilsson, A.; Sunnerhagen, K.S.; Gerdle, B. Is Pain Intensity Really That Important to Assess in Chronic Pain Patients? A Study Based on the Swedish Quality Registry for Pain Rehabilitation (SQRP). PLoS ONE 2013, 8, e65483. [Google Scholar]
- Gerdle, B.; Molander, P.; Stenberg, G.; Stålnacke, B.-M.; Enthoven, P. Weak outcome predictors of multimodal rehabilitation at one-year follow-up in patients with chronic pain—a practice based evidence study from two SQRP centres. BMC Musculoskelet. Disord. 2016, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Snaith, R.P.; Zigmond, A.S. The hospital anxiety and depression scale. Br. Med. J. 1986, 292, 344. [Google Scholar] [CrossRef] [Green Version]
- Lisspers, J.; Nygren, A.; Söderman, E. Hospital Anxiety and Depression Scale (HAD): Some psychometric data for a Swedish sample. Acta Psychiatr. Scand. 1997, 96, 281–286. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.J.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online 2011, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xu, D.; Björkholm, M.; Gruber, A. Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin. Chem. 2001, 47, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonare, M.D.; Del Giudice, E.; Stecca, A.; Colavito, D.; Fabris, M.; D’Arrigo, A.; Bernardini, D.; Dam, M.; Leon, A. A Saturated N-Acylethanolamine Other than N-Palmitoyl Ethanolamine with Anti-inflammatory Properties: A Neglected Story. J. Neuroendocrinol. 2008, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef]
- Gasperi, V.; Ceci, R.; Tantimonaco, M.; Talamonti, E.; Battista, N.; Parisi, A.; Florio, R.; Sabatini, S.; Rossi, A.; Maccarrone, M. The Fatty Acid Amide Hydrolase in Lymphocytes from Sedentary and Active Subjects. Med. Sci. Sports Exerc. 2014, 46, 24–32. [Google Scholar] [CrossRef]
- Choi, J.; Fauce, S.R.; Effros, R.B. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 2008, 22, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Ho, W.-S.; Meier, S.E.; Gorzalka, B.B.; Hillard, C.J. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur. J. Pharmacol. 2005, 528, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.; Hill, M.; Bhagat, S.; Karatsoreos, I.; Hillard, C.; McEwen, B. Chronic, noninvasive glucocorticoid administration suppresses limbic endocannabinoid signaling in mice. Neuroscience 2011, 204, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010, 93, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
| Diagnose Code | Denotation | Number of Patients |
|---|---|---|
| M35.7 | Hypermobility syndrome | 1 |
| M53.1 | Cervicobrachial syndrome | 1 |
| M54.4 | Lumbago with sciatica | 3 |
| M54.5 | Low back pain | 2 |
| M54.6 | Pain in thoracic spine | 1 |
| M54.8 | Other dorsalgia | 1 |
| M79.1 | Myalgia | 1 |
| M79.7 | Fibromyalgia | 3 |
| R52.2A | Chronic pain, nociceptive | 2 |
| R52.2B | Chronic pain, neuropathic | 1 |
| R52.2C | Other chronic pain | 1 |
| T91.8A | late discomfort due to Whiplash | 1 |
| Scale | Baseline | Six-Month Follow Up | p-Value |
|---|---|---|---|
| Pain intensity | 6.83 (1.29) | 6.11 (1.74) | 0.05 * |
| HAD-A | 7.67 (5.55) | 5.33 (5.20) | 0.06 |
| HAD-D | 7.00 (3.74) | 5.61 (5.14) | 0.14 |
| PA 1 | 1.79 (1.63) | 2.86 (1.96) | 0.11 |
| PA 2 | 2.64 (1.28) | 2.93 (1.44) | 0.37 |
| Lipid (nM) | Baseline | Six-Month Follow Up | p-Value |
|---|---|---|---|
| AEA | 0.77 (0.48) | 1.08 (0.61) | 0.15 |
| 2-AG | 15.80 (5.96) | 14.60 (6.98) | 0.58 |
| OEA | 5.29 (1.66) | 5.74 (1.50) | 0.36 |
| PEA | 4.67 (1.30) | 4.83 (1.13) | 0.66 |
| SEA | 3.90 (2.59) | 5.10 (2.92) | 0.04 * |
| PA1 Pre | PA1 Post | PA2 Pre | PA2 Post | ∆PA1 | ∆PA2 | |
|---|---|---|---|---|---|---|
| AEA Pre | −0.49 * | −0.10 | ||||
| AEA Post | −0.45 | −0.56 * | ||||
| ∆AEA | −0.50 * | −0.58 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stensson, N.; Gerdle, B.; Rönne-Petersén, L.; Yang, L.L.; Lavebratt, C.; Falkenberg, T.; Ghafouri, B. Investigating the Long-Term Effect of an Interdisciplinary Multimodal Rehabilitation Program on Levels of Bioactive Lipids and Telomerase Activity in Blood from Patients with Chronic Pain. J. Clin. Med. 2022, 11, 1291. https://doi.org/10.3390/jcm11051291
Stensson N, Gerdle B, Rönne-Petersén L, Yang LL, Lavebratt C, Falkenberg T, Ghafouri B. Investigating the Long-Term Effect of an Interdisciplinary Multimodal Rehabilitation Program on Levels of Bioactive Lipids and Telomerase Activity in Blood from Patients with Chronic Pain. Journal of Clinical Medicine. 2022; 11(5):1291. https://doi.org/10.3390/jcm11051291
Chicago/Turabian StyleStensson, Niclas, Björn Gerdle, Linn Rönne-Petersén, Liu L. Yang, Catharina Lavebratt, Torkel Falkenberg, and Bijar Ghafouri. 2022. "Investigating the Long-Term Effect of an Interdisciplinary Multimodal Rehabilitation Program on Levels of Bioactive Lipids and Telomerase Activity in Blood from Patients with Chronic Pain" Journal of Clinical Medicine 11, no. 5: 1291. https://doi.org/10.3390/jcm11051291
APA StyleStensson, N., Gerdle, B., Rönne-Petersén, L., Yang, L. L., Lavebratt, C., Falkenberg, T., & Ghafouri, B. (2022). Investigating the Long-Term Effect of an Interdisciplinary Multimodal Rehabilitation Program on Levels of Bioactive Lipids and Telomerase Activity in Blood from Patients with Chronic Pain. Journal of Clinical Medicine, 11(5), 1291. https://doi.org/10.3390/jcm11051291







