TMAO and Gut Microbial-Derived Metabolites TML and γBB Are Not Associated with Thrombotic Risk in Patients with Venous Thromboembolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Coagulation Parameters
2.2.1. Platelet Function Analysis (PFA)
2.2.2. Platelet Hyperreactivity (PHR) Analysis
2.2.3. Platelet Adhesiveness (PA)
2.2.4. Thrombosis-Associated Parameters
2.2.5. Thrombin Generation Parameters
2.3. Plasma TMAO, γBB, and TML Determination
2.4. Statistical Analysis
3. Results
3.1. Study Cohort Characteristics
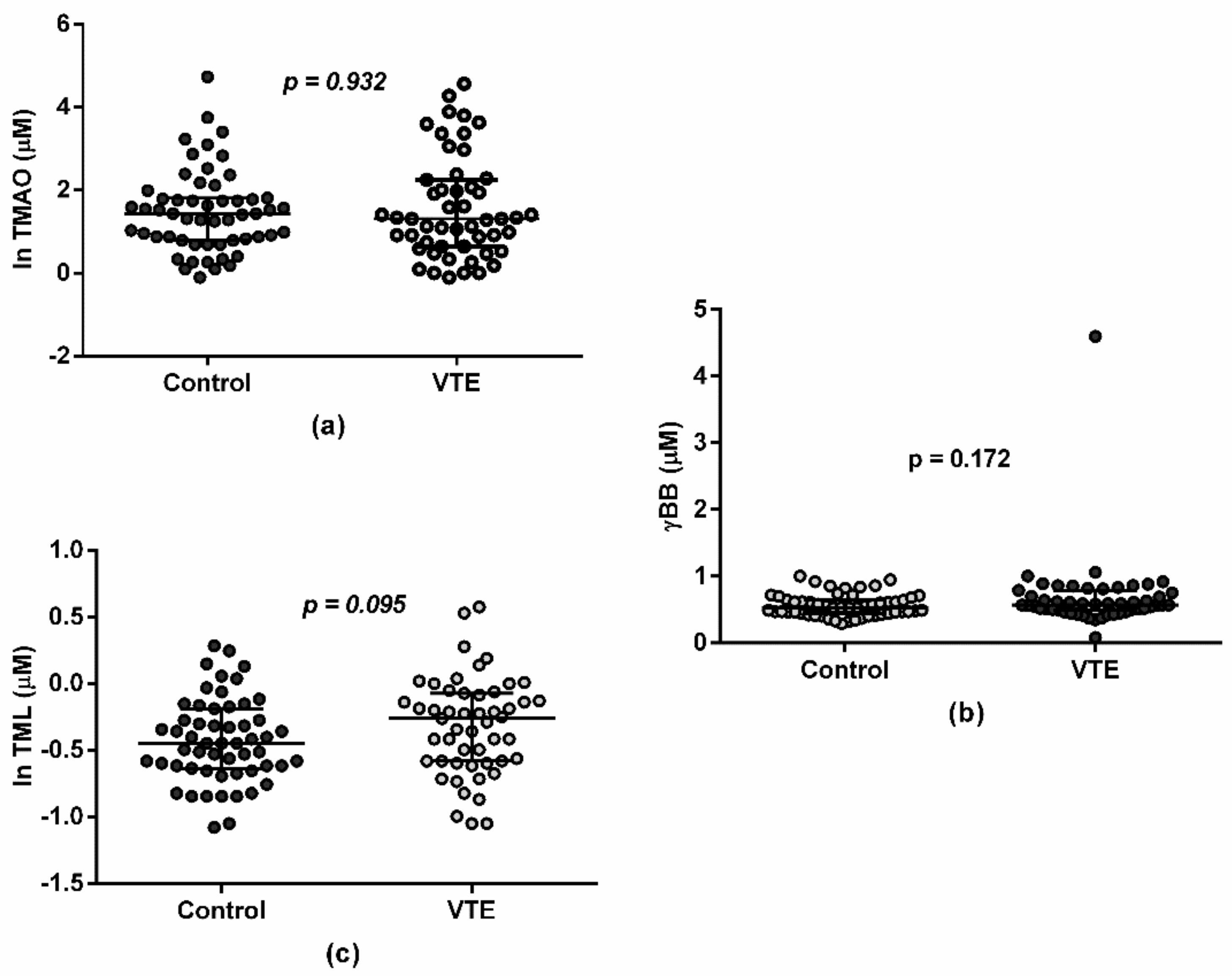
3.2. Gut-Related Metabolites and VTE
3.3. Gut-Related Metabolites and Coagulation Parameters
3.4. Gut-Derived Metabolites over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic Value of Choline and Betaine Depends on Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Skagen, K.; Trøseid, M.; Ueland, T.; Holm, S.; Abbas, A.; Gregersen, I.; Kummen, M.; Bjerkeli, V.; Reier-Nilsen, F.; Russell, D.; et al. The Carnitine-Butyrobetaine-Trimethylamine-N-Oxide Pathway and Its Association with Cardiovascular Mortality in Patients with Carotid Atherosclerosis. Atherosclerosis 2016, 247, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Qiu, X.; Liu, Y.; Yuan, C.; Yang, X. Trimethylamine N-Oxide Promotes Tissue Factor Expression and Activity in Vascular Endothelial Cells: A New Link between Trimethylamine N-Oxide and Atherosclerotic Thrombosis. Thromb. Res. 2019, 177, 110–116. [Google Scholar] [CrossRef]
- Zhu, W.; Zeneng, W.; Wilson Tang, W.H.; Hazen, S.L. Gut Microbe-Generated TMAO from Dietary Choline Is Prothrombotic in Subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, K.; Szewczyk, E.M. Microbial Modulation of Coagulation Disorders in Venous Thromboembolism. J. Inflamm. Res. 2020, 13, 387–400. [Google Scholar] [CrossRef]
- Reiner, M.F.; Müller, D.; Gobbato, S.; Stalder, O.; Limacher, A.; Bonetti, N.R.; Pasterk, L.; Méan, M.; Rodondi, N.; Aujesky, D.; et al. Gut Microbiota-Dependent Trimethylamine-N-Oxide (TMAO) Shows a U-Shaped Association with Mortality but Not with Recurrent Venous Thromboembolism. Thromb. Res. 2019, 174, 40–47. [Google Scholar] [CrossRef]
- Llobet, D.; Vallvé, C.; Tirado, I.; Vilalta, N.; Carrasco, M.; Oliver, A.; Mateo, J.; Fontcuberta, J.; Souto, J.C. Platelet Hyperaggregability and Venous Thrombosis Risk: Results from the RETROVE Project. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2021, 32, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Santiago, M.; Vilalta, N.; Cuevas, B.; Murillo, J.; Llobet, D.; Macho, R.; Pujol-Moix, N.; Carrasco, M.; Mateo, J.; Fontcuberta, J.; et al. Short Closure Time Values in PFA–100® Are Related to Venous Thrombotic Risk. Results from the RETROVE Study. Thromb. Res. 2018, 169, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; García-Osuna, Á.; Junza, A.; Yanes, O.; Puig, N.; Ordóñez-Llanos, J.; Sionis, A.; Sans-Roselló, J.; Alquézar-Arbé, A.; Santos, D.; et al. The Capacity of APOB-Depleted Plasma in Inducing ATP-Binding Cassette A1/G1-Mediated Macrophage Cholesterol Efflux—But Not Gut Microbial-Derived Metabolites—Is Independently Associated with Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Biomedicines 2021, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Zhu, W.; Schugar, R.C.; Meng, Y.; Jia, X.; Miikeda, A.; Wang, Z.; Zieger, M.; Lee, R.; Graham, M.; et al. Genetic Deficiency of Flavin-Containing Monooxygenase 3 (Fmo3) Protects Against Thrombosis but Has Only a Minor Effect on Plasma Lipid Levels-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Buffa, J.A.; Wang, Z.; Warrier, M.; Schugar, R.; Shih, D.M.; Gupta, N.; Gregory, J.C.; Org, E.; Fu, X.; et al. Flavin Monooxygenase 3, the Host Hepatic Enzyme in the Metaorganismal Trimethylamine N-Oxide-Generating Pathway, Modulates Platelet Responsiveness and Thrombosis Risk. J. Thromb. Haemost. 2018, 16, 1857–1872. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.B.; Gu, X.; Buffa, J.A.; Hurd, A.G.; Wang, Z.; Zhu, W.; Gupta, N.; Skye, S.M.; Cody, D.B.; Levison, B.S.; et al. Development of a Gut Microbe-Targeted Nonlethal Therapeutic to Inhibit Thrombosis Potential. Nat. Med. 2018, 24, 1407–1417. [Google Scholar] [CrossRef]
- Van Mens, T.E.; Büller, H.R.; Nieuwdorp, M. Targeted Inhibition of Gut Microbiota Proteins Involved in TMAO Production to Reduce Platelet Aggregation and Arterial Thrombosis: A Blueprint for Drugging the Microbiota in the Treatment of Cardiometabolic Disease? J. Thromb. Haemost. 2019, 17, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Skye, S.M.; Zhu, W.; Romano, K.A.; Guo, C.-J.; Wang, Z.; Jia, X.; Kirsop, J.; Haag, B.; Lang, J.M.; DiDonato, J.A.; et al. Microbial Transplantation With Human Gut Commensals Containing CutC Is Sufficient to Transmit Enhanced Platelet Reactivity and Thrombosis Potential. Circ. Res. 2018, 123, 1164–1176. [Google Scholar] [CrossRef]
- Berger, M.; Kleber, M.E.; Delgado, G.E.; März, W.; Andreas, M.; Hellstern, P.; Marx, N.; Schuett, K.A. Trimethylamine N-Oxide and Adenosine Diphosphate-Induced Platelet Reactivity Are Independent Risk Factors for Cardiovascular and All-Cause Mortality. Circ. Res. 2020, 9, 660–662. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, L.; Zhang, Y.; Wang, F.; Zhao, Z.; Zhou, X. Gut Microbial Metabolite Trimethylamine N-Oxide Is Related to Thrombus Formation in Atrial Fibrillation Patients. Am. J. Med. Sci. 2019, 358, 422–428. [Google Scholar] [CrossRef]
- Haissman, J.M.; Haugaard, A.K.; Ostrowski, S.R.; Berge, R.K.; Hov, J.R.; Trøseid, M.; Nielsen, S.D. Microbiota-Dependent Metabolite and Cardiovascular Disease Marker Trimethylamine-N-Oxide (TMAO) Is Associated with Monocyte Activation but Not Platelet Function in Untreated HIV Infection. BMC Infect. Dis. 2017, 17, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Levison, B.S.; Hazen, J.E.; Donahue, L.; Li, X.-M.; Hazen, S.L. Measurement of Trimethylamine-N-Oxide by Stable Isotope Dilution Liquid Chromatography Tandem Mass Spectrometry. Anal. Biochem. 2014, 455, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEntyre, C.J.; Lever, M.; Chambers, S.T.; George, P.M.; Slow, S.; Elmslie, J.L.; Florkowski, C.M.; Lunt, H.; Krebs, J.D. Variation of Betaine, N,N-Dimethylglycine, Choline, Glycerophosphorylcholine, Taurine and Trimethylamine-N-Oxide in the Plasma and Urine of Overweight People with Type 2 Diabetes over a Two-Year Period. Ann. Clin. Biochem. 2015, 52, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Müller, D. Intra-Individual Variation of Plasma Trimethylamine-N-Oxide (TMAO), Betaine and Choline over 1 Year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Nemet, I.; Wang, Z.; Lai, H.T.M.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Sotoodehnia, N.; Budoff, M.; DiDonato, J.A.; et al. Longitudinal Plasma Measures of Trimethylamine N-Oxide and Risk of Atherosclerotic Cardiovascular Disease Events in Community-Based Older Adults. J. Am. Heart Assoc. 2021, 10, e020646. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Nakahashi, H.; Konishi, M.; Sato, R.; Kawashima, C.; Kikuchi, S.; Akiyama, E.; Iwahashi, N.; Maejima, N.; Okada, K.; et al. Microbiota-Derived Trimethylamine N-Oxide Predicts Cardiovascular Risk After STEMI. Sci. Rep. 2019, 9, 11647. [Google Scholar] [CrossRef] [Green Version]
| Spontaneous | Non-Spontaneous | |||||
|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | Total | |
| Isolated deep vein thrombosis (n, %) | 11 (55.0) | 8 (50.0) | 19 (52.8) | 5 (41.7) | 2 (33.3) | 7 (38.9) |
| No isolated deep vein thrombosis (n, %) | 2 (10.0) | 1 (6.3) | 3 (8.3) | 4 (33.3) | 3 (50.0) | 7 (38.9) |
| Isolated pulmonary Embolism (n, %) | 6 (30.0) | 6 (37.5) | 12 (33.3) | 3 (25.0) | 1 (16.7) | 4 (22.2) |
| Visceral thrombosis (n, %) | - | 1 (6.3) | 1 (2.8) | - | - | - |
| Venous sinus thrombosis (n, %) | 1 (5.0) | - | 1 (2.8) | - | - | - |
| Total | 20 (100) | 16 (100) | 36 (100) | 12 (100) | 6 (100) | 18 (100) |
| Control (n = 57) | VTE (n = 54) | p Value | |
|---|---|---|---|
| Age at baseline (y) | 64 (48–75) | 61.5 (46.8–78) | 0.873 |
| Gender (% males) | 40.4 | 40.7 | 0.967 |
| BMI (Kg/m2) | 26 (24.2–29) | 27.6 (24.2–29.7) | 0.335 |
| Smoking (n, %) | 10 (17.5) | 10 (18.5) | 0.894 |
| Alcohol consumption (n, %) | 28 (49.1) | 26 (48.1) | 0.918 |
| Hypertension (n, %) | 23 (40.4) | 26 (48.1) | 0.408 |
| Dyslipidemia (n, %) | 19 (33.3) | 17 (31.5) | 0.835 |
| Statins (n, %) | 14 (24.6) | 14 (25.9) | 0.869 |
| Diabetes mellitus (n, %) | 7 (12.3) | 2 (3.7) | 0.098 |
| Autoimmune disease (n, %) | 7 (12.3) | 5 (9.3) | 0.608 |
| Arterial thrombosis background (n, %) | - | 2 (3.7) | 0.239 |
| Anti-platelet drugs (n, %) | 2 (3.5) | 8 (14.8) | 0.049 |
| PFA_ADP (s) | 82 (72–96.8) | 59 (54.8–63) | <0.0001 |
| PFA_EPI (s) | 116.5 (97.8–137.8) | 83 (77–88) | <0.0001 |
| Platelet count (×109/L) | 234 (204–270.5) | 234 (203–268.8) | 0.750 |
| eGFR (mL/min/1.73 m2) | 85.4 (66.7–90) | 70.8 (60–90) | 0.012 |
| ALT (IU/L) | 18 (15–24) | 20 (16–35.5) | 0.060 |
| AST (IU/L) | 19 (16–21) | 19 (16–21) | 0.266 |
| LAG (min) | TTP (min) | ETP | PEAK | |
|---|---|---|---|---|
| TMAO | 0.220 (p = 0.024) | 0.285 (p = 0.003) | −0.209 (p = 0.033) | −0.33 (p = 0.001) |
| γBB | 0.207 (p = 0.035) | 0.259 (p = 0.008) | −0.042 (p = 0.673) | −0.212 (p = 0.031) |
| TML | 0.228 (p = 0.02) | 0.205 (p = 0.036) | −0.124 (p = 0.206) | −0.195 (p = 0.046) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canyelles, M.; Plaza, M.; Rotllan, N.; Llobet, D.; Julve, J.; Mojal, S.; Diaz-Ricart, M.; Soria, J.M.; Escolà-Gil, J.C.; Tondo, M.; et al. TMAO and Gut Microbial-Derived Metabolites TML and γBB Are Not Associated with Thrombotic Risk in Patients with Venous Thromboembolism. J. Clin. Med. 2022, 11, 1425. https://doi.org/10.3390/jcm11051425
Canyelles M, Plaza M, Rotllan N, Llobet D, Julve J, Mojal S, Diaz-Ricart M, Soria JM, Escolà-Gil JC, Tondo M, et al. TMAO and Gut Microbial-Derived Metabolites TML and γBB Are Not Associated with Thrombotic Risk in Patients with Venous Thromboembolism. Journal of Clinical Medicine. 2022; 11(5):1425. https://doi.org/10.3390/jcm11051425
Chicago/Turabian StyleCanyelles, Marina, Melania Plaza, Noemí Rotllan, Dolors Llobet, Josep Julve, Sergi Mojal, Maribel Diaz-Ricart, José Manuel Soria, Joan Carles Escolà-Gil, Mireia Tondo, and et al. 2022. "TMAO and Gut Microbial-Derived Metabolites TML and γBB Are Not Associated with Thrombotic Risk in Patients with Venous Thromboembolism" Journal of Clinical Medicine 11, no. 5: 1425. https://doi.org/10.3390/jcm11051425
APA StyleCanyelles, M., Plaza, M., Rotllan, N., Llobet, D., Julve, J., Mojal, S., Diaz-Ricart, M., Soria, J. M., Escolà-Gil, J. C., Tondo, M., Blanco-Vaca, F., & Souto, J. C. (2022). TMAO and Gut Microbial-Derived Metabolites TML and γBB Are Not Associated with Thrombotic Risk in Patients with Venous Thromboembolism. Journal of Clinical Medicine, 11(5), 1425. https://doi.org/10.3390/jcm11051425








