Predisposing Factors and Neurologic Outcomes of Patients with Elevated Serum Amylase and/or Lipase after Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
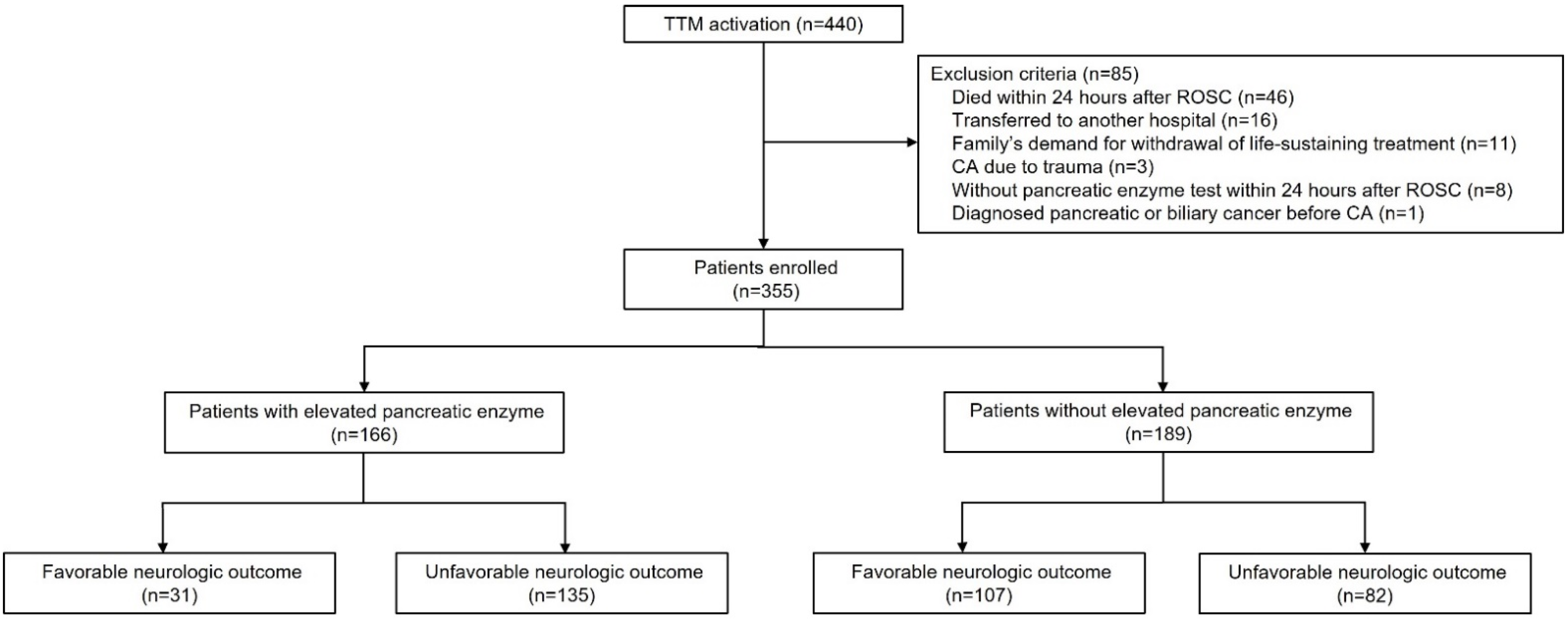
2.1. Study Design and Population
2.2. Data Collection
2.3. Definition of Elevated Serum Pancreatic Enzyme Level
2.4. Outcome Measures
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Characteristics of and Risk Factors for Elevated Pancreatic Enzyme Levels
3.3. Neurologic Outcome and 28-Day Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dumas, F.; White, L.; Stubbs, B.A.; Cariou, A.; Rea, T.D. Long-term prognosis following resuscitation from out of hospital cardiac arrest: Role of percutaneous coronary intervention and therapeutic hypothermia. J. Am. Coll. Cardiol. 2012, 60, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Bougouin, W.; Lamhaut, L.; Marijon, E.; Jost, D.; Dumas, F.; Deye, N.; Beganton, F.; Empana, J.P.; Chazelle, E.; Cariou, A.; et al. Characteristics and prognosis of sudden cardiac death in greater paris: Population-based approach from the paris sudden death expertise center (paris-sdec). Intensive Care Med. 2014, 40, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Lemiale, V.; Dumas, F.; Mongardon, N.; Giovanetti, O.; Charpentier, J.; Chiche, J.D.; Carli, P.; Mira, J.P.; Nolan, J.; Cariou, A. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013, 39, 1972–1980. [Google Scholar] [CrossRef]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Böttiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the international liaison committee on resuscitation; the american heart association emergency cardiovascular care committee; the council on cardiovascular surgery and anesthesia; the council on cardiopulmonary, perioperative, and critical care; the council on clinical cardiology; the council on stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [PubMed]
- Adrie, C.; Adib-Conquy, M.; Laurent, I.; Monchi, M.; Vinsonneau, C.; Fitting, C.; Fraisse, F.; Dinh-Xuan, A.T.; Carli, P.; Spaulding, C.; et al. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation 2002, 106, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Geri, G.; Guillemet, L.; Dumas, F.; Charpentier, J.; Antona, M.; Lemiale, V.; Bougouin, W.; Lamhaut, L.; Mira, J.P.; Vinsonneau, C.; et al. Acute kidney injury after out-of-hospital cardiac arrest: Risk factors and prognosis in a large cohort. Intensive Care Med. 2015, 41, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Yanta, J.; Guyette, F.X.; Doshi, A.A.; Callaway, C.W.; Rittenberger, J.C. Renal dysfunction is common following resuscitation from out-of-hospital cardiac arrest. Resuscitation 2013, 84, 1371–1374. [Google Scholar] [CrossRef]
- Laurent, I.; Monchi, M.; Chiche, J.D.; Joly, L.M.; Spaulding, C.; Bourgeois, B.; Cariou, A.; Rozenberg, A.; Carli, P.; Weber, S.; et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2002, 40, 2110–2116. [Google Scholar] [CrossRef] [Green Version]
- Roberts, B.W.; Kilgannon, J.H.; Chansky, M.E.; Mittal, N.; Wooden, J.; Parrillo, J.E.; Trzeciak, S. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit. Care Med. 2013, 41, 1492–1501. [Google Scholar] [CrossRef]
- Roedl, K.; Spiel, A.O.; Nürnberger, A.; Horvatits, T.; Drolz, A.; Hubner, P.; Warenits, A.M.; Sterz, F.; Herkner, H.; Fuhrmann, V. Hypoxic liver injury after in- and out-of-hospital cardiac arrest: Risk factors and neurological outcome. Resuscitation 2019, 137, 175–182. [Google Scholar] [CrossRef]
- Shu, W.; Wan, J.; Yang, X.; Chen, J.; Yang, Q.; Liu, F.; Xia, L. Heparin-binding protein levels at admission and within 24 h are associated with persistent organ failure in acute pancreatitis. Dig. Dis. Sci. 2021, 66, 3597–3603. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis--2012: Revision of the atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Chmura, A.; Galazka, K.; Kusnierz-Cabala, B.; Warzecha, Z. Administration of warfarin accelerates the recovery in ischemia/reperfusion-induced acute pancreatitis. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Gullo, L.; Cavicchi, L.; Tomassetti, P.; Spagnolo, C.; Freyrie, A.; D’Addato, M. Effects of ischemia on the human pancreas. Gastroenterology 1996, 111, 1033–1038. [Google Scholar] [CrossRef]
- Polderman, K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009, 37, S186–S202. [Google Scholar] [CrossRef]
- Choi, S.P.; Youn, C.S.; Park, K.N.; Wee, J.H.; Park, J.H.; Oh, S.H.; Kim, S.H.; Kim, J.Y. Therapeutic hypothermia in adult cardiac arrest because of drowning. Acta Anaesthesiol. Scand. 2012, 56, 116–123. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; You, J.S.; Lee, H.S.; Park, Y.S.; Park, I.; Chung, S.P. Multimodal approach for neurologic prognostication of out-of-hospital cardiac arrest patients undergoing targeted temperature management. Resuscitation 2019, 134, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, I.; Nadkarni, V.; Bahr, J.; Berg, R.A.; Billi, J.E.; Bossaert, L.; Cassan, P.; Coovadia, A.; D’Este, K.; Finn, J.; et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (american heart association, european resuscitation council, australian resuscitation council, new zealand resuscitation council, heart and stroke foundation of canada, interamerican heart foundation, resuscitation council of southern africa). Resuscitation 2004, 63, 233–249. [Google Scholar]
- Banks, P.A.; Freeman, M.L. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006, 101, 2379–2400. [Google Scholar] [CrossRef]
- National Library of Medicine. Uk guidelines for the management of acute pancreatitis. Gut 2005, 54 (Suppl. 3), iii1–iii9. [Google Scholar]
- Uhl, W.; Warshaw, A.; Imrie, C.; Bassi, C.; McKay, C.J.; Lankisch, P.G.; Carter, R.; Di Magno, E.; Banks, P.A.; Whitcomb, D.C.; et al. Iap guidelines for the surgical management of acute pancreatitis. Pancreatology 2002, 2, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, M.; Delhaye, M.; De Maertelaere, V.; Bali, M.; Winant, C.; Coppens, E.; Jeanmart, J.; Zalcman, M.; Van Gansbeke, D.; Devière, J.; et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 2004, 126, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Bollen, T.L.; van Santvoort, H.C.; Besselink, M.G.; van Es, W.H.; Gooszen, H.G.; van Leeuwen, M.S. Update on acute pancreatitis: Ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR 2007, 28, 371–383. [Google Scholar] [CrossRef]
- Rittenberger, J.C.; Raina, K.; Holm, M.B.; Kim, Y.J.; Callaway, C.W. Association between cerebral performance category, modified rankin scale, and discharge disposition after cardiac arrest. Resuscitation 2011, 82, 1036–1040. [Google Scholar] [CrossRef] [Green Version]
- Adrie, C.; Laurent, I.; Monchi, M.; Cariou, A.; Dhainaou, J.F.; Spaulding, C. Postresuscitation disease after cardiac arrest: A sepsis-like syndrome? Curr. Opin. Crit. Care 2004, 10, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Tsiotos, G.G.; Sarr, M.G. Ischemia/reperfusion-induced pancreatitis. Dig. Surg. 2000, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.H.; Toledano, A.E.; Toth, L.S.; Premus, G.; Dreiling, D.A. Hypovolemic shock, pancreatic blood flow, and pancreatitis. Int. J. Pancreatol. 1988, 3, 283–292. [Google Scholar] [CrossRef]
- Sanfey, H.; Broe, P.J.; Cameron, J.L. Experimental ischemic pancreatitis: Treatment with albumin. Am. J. Surg. 1985, 150, 297–300. [Google Scholar] [CrossRef]
- Lefer, A.M.; Spath, J.A., Jr. Pancreatic hypoperfusion and the production of a myocardial depressant factor in hemorrhagic shock. Ann. Surg. 1974, 179, 868–876. [Google Scholar] [CrossRef]
- Piton, G.; Barbot, O.; Manzon, C.; Moronval, F.; Patry, C.; Navellou, J.C.; Belle, E.; Capellier, G. Acute ischemic pancreatitis following cardiac arrest: A case report. Jop 2010, 11, 456–459. [Google Scholar]
- Popa, C.C.; Badiu, D.C.; Rusu, O.C.; Grigorean, V.T.; Neagu, S.I.; Strugaru, C.R. Mortality prognostic factors in acute pancreatitis. J. Med. Life 2016, 9, 413–418. [Google Scholar] [PubMed]
- Carnovale, A.; Rabitti, P.G.; Manes, G.; Esposito, P.; Pacelli, L.; Uomo, G. Mortality in acute pancreatitis: Is it an early or a late event? Jop 2005, 6, 438–444. [Google Scholar] [PubMed]
- Lee, C.C.; Chung, W.Y.; Shih, Y.H. Elevated amylase and lipase levels in the neurosurgery intensive care unit. J. Chin. Med. Assoc. 2010, 73, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Malinoski, D.J.; Hadjizacharia, P.; Salim, A.; Kim, H.; Dolich, M.O.; Cinat, M.; Barrios, C.; Lekawa, M.E.; Hoyt, D.B. Elevated serum pancreatic enzyme levels after hemorrhagic shock predict organ failure and death. J. Trauma 2009, 67, 445–449. [Google Scholar] [CrossRef]
- Oh, H.C.; Kwon, C.I.; El, H., II; Easler, J.J.; Watkins, J.; Fogel, E.L.; McHenry, L.; Sherman, S.; Zimmerman, M.K.; Lehman, G.A. Low serum pancreatic amylase and lipase values are simple and useful predictors to diagnose chronic pancreatitis. Gut Liver 2017, 11, 878–883. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Zhou, R.; Li, C.; Hu, D.; Xue, W.; Wu, T.; Mohan, C.; Peng, A. Hyperamylasemia as an early predictor of mortality in patients with acute paraquat poisoning. Med. Sci. Monit. 2016, 22, 1342–1348. [Google Scholar] [CrossRef] [Green Version]
- Boon, P.; de Reuck, J.; Achten, E.; de Bleecker, J. Pancreatic encephalopathy. A case report and review of the literature. Clin. Neurol. Neurosurg. 1991, 93, 137–141. [Google Scholar] [CrossRef]
- Farkas, G.; Márton, J.; Nagy, Z.; Mándi, Y.; Takács, T.; Deli, M.A.; Abrahám, C.S. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: A potential role for tumor necrosis factor and interleukin 6. Neurosci. Lett. 1998, 242, 147–150. [Google Scholar] [CrossRef]
- Muniraj, T.; Dang, S.; Pitchumoni, C.S. Pancreatitis or not?--elevated lipase and amylase in icu patients. J. Crit. Care 2015, 30, 1370–1375. [Google Scholar] [CrossRef]
- Da, B.L.; Shulman, I.A.; Joy Lane, C.; Buxbaum, J. Origin, presentation, and clinical course of nonpancreatic hyperlipasemia. Pancreas 2016, 45, 846–849. [Google Scholar] [CrossRef]

| All Patients | Non-Elevated Pancreatic Enzyme Levels | Elevated Pancreatic Enzyme Levels | p-Value | |
|---|---|---|---|---|
| (n = 355) | (n = 189, 53.2%) | (n = 166, 46.8%) | ||
| Patients’ demographic data | ||||
| Age (years) | 63.1 ± 15.6 | 61.5 ± 15.5 | 65.0 ± 15.6 | 0.03 |
| Sex, male | 266 (74.9) | 139 (73.5) | 127 (76.5) | 0.52 |
| Weight (kg) | 64.9 ± 12.4 | 64.7 ± 13.1 | 65.1 ± 11.6 | 0.77 |
| Height (cm) | 166.4 ± 9.0 | 166.7 ± 10.5 | 166.1 ± 7.2 | 0.53 |
| CA information | ||||
| Witnessed | 255 (71.8) | 142 (75.1) | 113 (68.1) | 0.14 |
| Bystander CPR | 248 (69.9) | 139 (73.5) | 109(65.7) | 0.11 |
| Shockable rhythm (VF/VT) | 138 (38.9) | 87 (46.0) | 51 (30.7) | 0.003 |
| Cardiac cause | 225 (63.4) | 127 (67.2) | 98 (59) | 0.11 |
| Time from collapse to ROSC (minutes) | 29.2 ± 20.6 | 25.1 ± 19.3 | 33.9 ± 21.0 | <0.001 |
| Total dose of epinephrine (mg) | 2.5 ± 3.2 | 2.1 ± 3.1 | 3.0 ± 3.3 | 0.01 |
| Comorbidities | ||||
| Hypertension | 135 (38) | 70 (37) | 65 (39.2) | 0.68 |
| Diabetes mellitus | 90 (25.4) | 46 (24.3) | 44 (26.5) | 0.64 |
| Coronary artery disease | 86 (24.2) | 38 (20.1) | 48 (28.9) | 0.053 |
| Cerebrovascular accident | 28 (7.9) | 10 (5.3) | 18 (10.8) | 0.053 |
| Pulmonary disease | 33 (9.3) | 20 (10.6) | 13 (7.8) | 0.37 |
| Renal disease | 46 (13) | 22 (11.6) | 24 (14.5) | 0.43 |
| Liver cirrhosis | 2 (0.6) | 0 | 2 (1.2) | 0.22 |
| Other cancer | 27 (7.7) | 17 (9) | 10 (6) | 0.29 |
| Laboratory test levels (U/L) | ||||
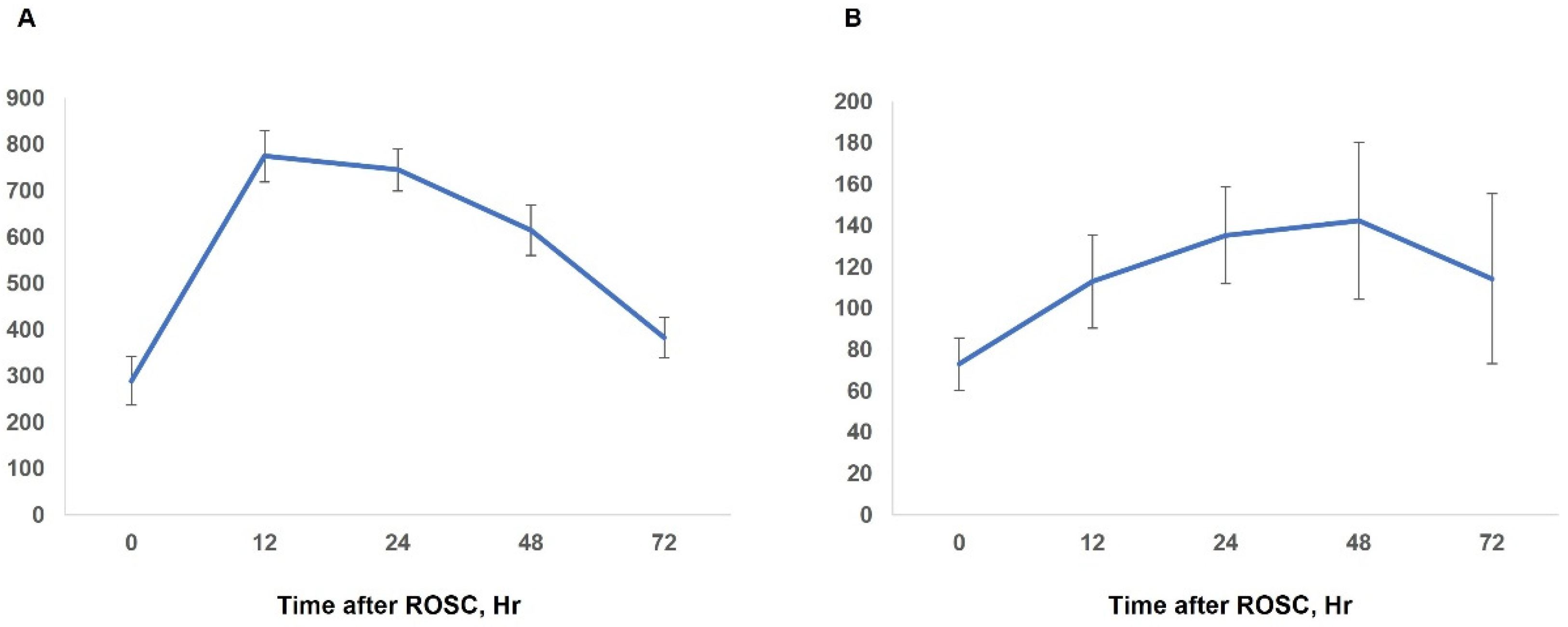
| Amylase at 0 h (n = 232) | 179.9 ± 362.6 | 95.3 ± 49.7 | 289.6 ± 526.5 | <0.001 |
| Amylase at 12 h (n = 333) | 422.1 ± 575.3 | 118.2 ± 71.4 | 775.2 ± 691.9 | <0.001 |
| Amylase at 24 h (n = 326) | 417.3 ± 510.3 | 116.2 ± 77.9 | 745.4 ± 575.9 | <0.001 |
| Maximum amylase | 487.4 ± 628.5 | 141.1 ± 80.2 | 881.7 ± 739.1 | <0.001 |
| Lipase at 0 h (n = 232) | 55.9 ± 87.5 | 42.5 ± 22.0 | 73.1 ± 127.9 | 0.004 |
| Lipase at 12 h (n = 331) | 69.2 ± 194.7 | 31.1 ± 24.2 | 112.9 ± 278.3 | <0.001 |
| Lipase at 24 h (n = 328) | 80.0 ± 209.8 | 29.2 ± 24.8 | 135.3 ± 292.7 | <0.001 |
| Maximum lipase | 108.0 ± 242.9 | 46.1 ± 29.7 | 178.5 ± 340.9 | <0.001 |
| Outcome | ||||
| Unfavorable neurologic outcome | 219 (61.7) | 84 (44.4) | 135 (81.3) | <0.001 |
| 28-day mortality | 141 (39.7) | 48 (25.4) | 93 (56) | <0.001 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Shockable rhythm (VF/VT) | 0.62 | 0.39–0.98 | 0.04 |
| Time from collapse to ROSC (per minute) | 1.02 | 1.01–1.04 | <0.001 |
| Coronary artery disease | 1.7 | 1.01–2.87 | 0.046 |
| Neurologic Outcome | 28-Day Mortality | |||||
|---|---|---|---|---|---|---|
| Favorable | Unfavorable | p-Value | Survival | Death | p-Value | |
| (n = 136, 38.3%) | (n = 219, 61.7%) | (n = 214, 60.3%) | (n = 141, 39.7%) | |||
| Patients’ demographic data | ||||||
| Age (years) | 61.0 ± 14.9 | 64.5 ± 16.0 | 0.04 | 61.1 ± 15.8 | 66.2 ± 14.6 | 0.003 |
| Sex, male | 113 (83.1) | 153 (69.9) | 0.005 | 166 (77.6) | 100 (70.9) | 0.16 |
| Weight (kg) | 65.3 ± 13.4 | 64.7 ± 11.8 | 0.61 | 64.2 ± 13.1 | 65.9 ± 11.3 | 0.21 |
| Height (cm) | 167.9 ± 8.7 | 165.5 ± 9.2 | 0.01 | 167 ± 10.0 | 166 ± 7.4 | 0.43 |
| CA information | ||||||
| Witnessed | 112 (82.4) | 143 (65.3) | <0.001 | 170 (79.4) | 85 (60.3) | <0.001 |
| Bystander | 114 (83.8) | 134 (61.2) | <0.001 | 166 (77.6) | 82 (58.2) | <0.001 |
| Shockable rhythm (VF/VT) | 82 (60.3) | 56 (25.6) | <0.001 | 108 (50.5) | 30 (21.3) | <0.001 |
| Cardiac cause | 108 (79.4) | 117 (53.4) | <0.001 | 149 (69.6) | 76 (53.9) | 0.003 |
| Time from collapse to ROSC (minutes) | 18.8 ± 15.6 | 35.7 ± 20.7 | <0.001 | 23 ± 18.4 | 38.5 ± 20.2 | <0.001 |
| Total dose of epinephrine (mg) | 1.4 ± 2.7 | 3.2 ± 3.4 | <0.001 | 1.8 ± 2.7 | 3.6 ± 3.7 | <0.001 |
| Comorbidities | ||||||
| Hypertension | 50 (36.8) | 85 (38.8) | 0.7 | 76 (35.5) | 59 (41.8) | 0.23 |
| Diabetes mellitus | 26 (19.1) | 64 (29.2) | 0.03 | 43 (20.1) | 47 (33.3) | 0.005 |
| Coronary artery disease | 38 (27.9) | 48 (21.9) | 0.2 | 53 (24.8) | 33 (23.4) | 0.77 |
| Cerebrovascular accident | 8 (5.9) | 20 (9.1) | 0.27 | 11 (5.1) | 17 (12.1) | 0.02 |
| Pulmonary disease | 9 (6.6) | 24 (11.0) | 0.17 | 20 (9.3) | 13 (9.2) | 0.97 |
| Renal disease | 6 (4.4) | 40 (18.3) | <0.001 | 19 (8.9) | 27 (19.1) | 0.005 |
| Liver cirrhosis | 0 | 2 (0.9) | 0.53 | 0 | 2 (1.4) | 0.16 |
| Other cancer | 8 (5.9) | 19 (8.7) | 0.33 | 15 (7.0) | 12 (8.5) | 0.60 |
| Laboratory test levels (U/L) | ||||||
| Amylase at 0 h (n = 232) | 106.8 ± 66.9 | 236.9 ± 473.0 | 0.001 | 120.6 ± 92.3 | 274.3 ± 560.8 | <0.001 |
| Amylase at 12 h (n = 333) | 225.7 ± 295.8 | 544.6 ± 667.0 | <0.001 | 328.3 ± 493.7 | 572.2 ± 660.9 | 0.001 |
| Amylase at 24 h (n = 326) | 224.9 ± 269.7 | 540.1 ± 585.0 | <0.001 | 332.0 ± 482.6 | 554.4 ± 525.5 | 0.03 |
| Maximum amylase | 258.7 ± 297.5 | 629.5 ± 730.5 | <0.001 | 375.8 ± 538.9 | 656.8 ± 713.4 | 0.001 |
| Lipase at 0 h (n = 232) | 45.0 ± 36.8 | 64.4 ± 111.4 | 0.12 | 47.6 ± 37.9 | 69.2 ± 131.6 | 0.048 |
| Lipase at 12 h (n = 331) | 68.8 ± 224.0 | 69.4 ± 174.2 | 0.59 | 67.5 ± 224.2 | 71.9 ± 135.1 | 0.84 |
| Lipase at 24 h (n = 328) | 45.3 ± 88.2 | 101.9 ± 256.6 | <0.001 | 58.8 ± 161.9 | 113.9 ± 266.7 | 0.001 |
| Maximum Lipase | 86.9 ± 219.7 | 121.1 ± 255.9 | 0.098 | 90.8 ± 224.4 | 134.1 ± 267.3 | 0.045 |
| Elevated pancreatic enzyme levels | ||||||
| None | 105 (77.2) | 84 (38.4) | 141 (65.9) | 48 (34.0) | ||
| Amylase alone | 22 (16.2) | 105 (47.9) | <0.001 a | 55 (25.7) | 72 (51.1) | <0.001 a |
| Lipase alone | 5 (3.7) | 8 (3.7) | 0.29 a | 7 (3.3) | 6 (4.3) | 0.11 a |
| Both | 4 (2.9) | 22 (10.0) | <0.001 a | 11 (5.1) | 15 (10.6) | 0.001 a |
| Post-CA shock | 97 (71.3) | 188 (85.8) | 0.001 | 157 (73.4) | 128 (90.8) | <0.001 |
| CRRT | 8 (5.9) | 63 (28.8) | <0.001 | 22 (10.3) | 49 (34.8) | <0.001 |
| ECMO | 13 (9.6) | 26 (11.9) | 0.5 | 18 (8.4) | 21 (14.9) | 0.06 |
| CPC | Mortality | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex, male | 2.96 | 1.20–7.28 | 0.02 | |||
| Witnessed | 0.51 | 0.28–0.91 | 0.02 | |||
| Bystander | 0.39 | 0.19–0.81 | 0.01 | |||
| Shockable rhythm (VF/VT) | 0.44 | 0.22–0.85 | 0.01 | 0.48 | 0.25–0.94 | 0.03 |
| Time from collapse to ROSC (per minute) | 1.05 | 1.04–1.07 | <0.001 | 1.04 | 1.02–1.05 | <0.001 |
| Cardiac cause | 0.26 | 0.12–0.53 | <0.001 | |||
| Elevated pancreatic enzyme levels | ||||||
| Amylase alone | 6.03 | 3.03–12.00 | <0.001 a | 2.8 | 1.58–4.93 | <0.001 a |
| Lipase alone | 0.56 | 0.09–3.37 | 0.52 a | 1.05 | 0.24–4.56 | 0.94 a |
| Both | 7.08 | 1.73–29.02 | 0.007 a | 2.05 | 0.73–5.77 | 0.17 a |
| Post-CA shock | 2.78 | 1.12–6.92 | 0.03 | |||
| CRRT | 3.77 | 1.22–11.65 | 0.02 | 4.05 | 1.69–9.72 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Kim, M.J.; Park, I.; Kim, H.Y.; Lee, M.; Park, Y.S.; Chung, S.P. Predisposing Factors and Neurologic Outcomes of Patients with Elevated Serum Amylase and/or Lipase after Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 1426. https://doi.org/10.3390/jcm11051426
Park SY, Kim MJ, Park I, Kim HY, Lee M, Park YS, Chung SP. Predisposing Factors and Neurologic Outcomes of Patients with Elevated Serum Amylase and/or Lipase after Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(5):1426. https://doi.org/10.3390/jcm11051426
Chicago/Turabian StylePark, Shin Young, Min Joung Kim, Incheol Park, Ha Yan Kim, Myeongjee Lee, Yoo Seok Park, and Sung Phil Chung. 2022. "Predisposing Factors and Neurologic Outcomes of Patients with Elevated Serum Amylase and/or Lipase after Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 5: 1426. https://doi.org/10.3390/jcm11051426
APA StylePark, S. Y., Kim, M. J., Park, I., Kim, H. Y., Lee, M., Park, Y. S., & Chung, S. P. (2022). Predisposing Factors and Neurologic Outcomes of Patients with Elevated Serum Amylase and/or Lipase after Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(5), 1426. https://doi.org/10.3390/jcm11051426






