Angiogenic Imbalance and Inflammatory Biomarkers in the Prediction of Hypertension as Well as Obstetric and Perinatal Complications in Women with Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
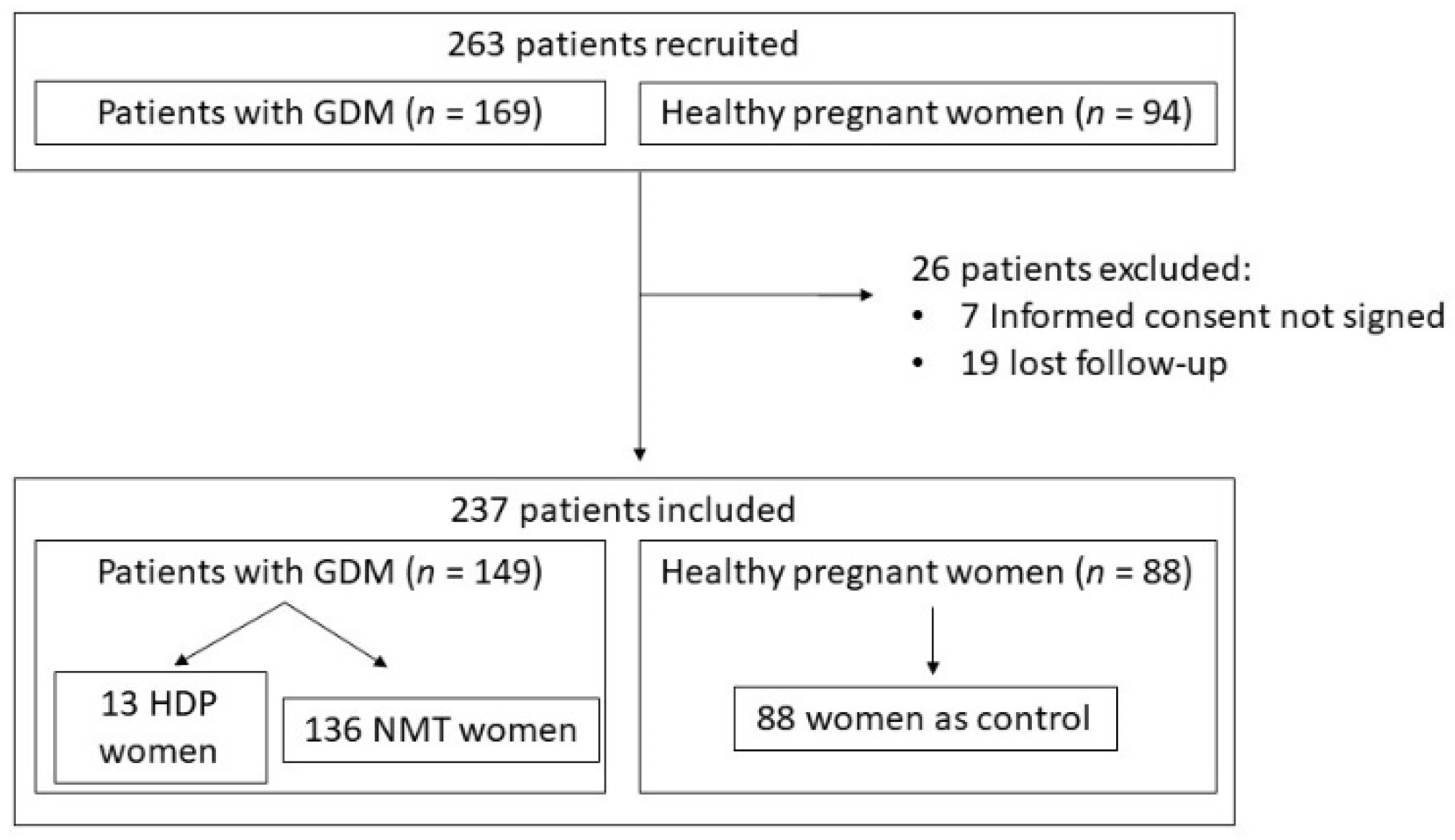
2.1. Study Design and Study Population
2.2. Evaluation of Variables Collected
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| BMI | body mass index |
| BP | blood pressure |
| FGF2 | fibroblast growth factor 2 |
| GDM | gestational diabetes mellitus |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HDP | hypertensive disorders of pregnancy |
| HGF | hepatocyte growth factor |
| IUGR | intrauterine growth restriction |
| LDL | low-density lipoprotein |
| LGA | large for gestational age |
| MCP-1 | monocyte chemoattractant protein-1 |
| NGF | nerve growth factor |
| NOS | nitric oxide synthase |
| NMT | normotensive |
| NPV | negative predictive value |
| ns | not statically significant |
| PAI-1 | plasminogen activator inhibitor-1 |
| PlGF | placental growth factor |
| PPV | positive predictive value |
| SD | standard deviation |
| sFlt-1 | soluble fms-like tyrosine kinase-1 |
| SGA | small for gestational age |
| TNFα | tumor necrosis factor alpha |
References
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the Management of Cardiovascular Diseases during Pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.B.; Hookham, M.B.; Yu, J.Y.; Lockhart, S.M.; Du, M.; Jenkins, A.; Nankervis, A.; Hanssen, K.F.; Henriksen, T.; Garg, S.K.; et al. Circulating Adipokines Are Associated with Pre-Eclampsia in Women with Type 1 Diabetes. Diabetologia 2017, 60, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Nerenberg, K.A.; Johnson, J.; Leung, B.; Savu, A.; Ryan, E.A.; Chik, C.L.; Kaul, P. Risks of Gestational Diabetes and Preeclampsia Over the Last Decade in a Cohort of Alberta Women. J. Obstet. Gynaecol. Can. 2013, 35, 986–994. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Umans, J.G.; Ratner, R. Hypertension Complicating Diabetic Pregnancies: Pathophysiology, Management, and Controversies. J. Clin. Hypertens. 2011, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Freerksen, N.; Röhrig, S.; Hoeft, B.; Maul, H. Gestational Diabetes and Preeclampsia—Similar Risk Factor Profiles? Early Hum. Dev. 2011, 88, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sircar, M.; Thadhani, R.; Karumanchi, S.A. Pathogenesis of Preeclampsia. Curr. Opin. Nephrol. Hypertens. 2015, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef] [PubMed]
- López-Tinoco, C.; Roca, M.; Fernández-Deudero, A.; García-Valero, A.; Bugatto, F.; Aguilar-Diosdado, M.; Bartha, J. Cytokine Profile, Metabolic Syndrome and Cardiovascular Disease Risk in Women with Late-Onset Gestational Diabetes Mellitus. Cytokine 2012, 58, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Basu, A.; Fu, D.; Wu, M.; Centola, M.; Jenkins, A.J.; Hanssen, K.F.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum Inflammatory Markers and Preeclampsia in Type 1 Diabetes. Diabetes Care 2013, 36, 2054–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An Automated Method for the Determination of the sFlt-1/PIGF Ratio in the Assessment of Preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, C.; Bacârea, A.; Huțanu, A.; Gabor, R.; Dobreanu, M. Placental Growth Factor, Soluble fms-Like Tyrosine Kinase 1, Soluble Endoglin, IL-6, and IL-16 as Biomarkers in Preeclampsia. Mediat. Inflamm. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekmekçi, B.; Ekmekci, H.; Gungor, Z.; Tüten, A.; Toprak, M.S.; Korkmaz, M.; Öncül, M.; Çalışkan, O.; Kucur, M.; Donma, O.; et al. Evaluation of Lp-PLA2 Mass, Vitronectin and PAI-1 Activity Levels in Patients with Preeclampsia. Arch. Gynecol. Obstet. 2014, 292, 53–58. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Khedagi, A.M.; Bello, N.A. Hypertensive Disorders of Pregnancy. Cardiol. Clin. 2020, 39, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Vambergue, A.; Nuttens, M.; Goeusse, P.; Biausque, S.; Lepeut, M.; Fontaine, P. Pregnancy Induced Hypertension in Women with Gestational Carbohydrate Intolerance: The Diagest Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 102, 31–35. [Google Scholar] [CrossRef]
- Kvetny, J.; Poulsen, H.F. Incidence of Gestational Hypertension in Gestational Diabetes Mellitus. Arch. Gynecol. Obstet. 2003, 267, 153–157. [Google Scholar] [CrossRef]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; De Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care 2007, 30, S251–S260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.L.; Ford, J.B.; Algert, C.S.; Antonsen, S.; Chalmers, J.; Cnattingius, S.; Gokhale, M.; Kotelchuck, M.; Melve, K.K.; Langridge, A.; et al. Population-Based Trends in Pregnancy Hypertension and Pre-Eclampsia: An International Comparative Study. BMJ Open 2011, 1, e000101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefevre, M.L. Low-Dose Aspirin Use for the Prevention of Morbidity and Mortality from Preeclampsia: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2014, 161, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M., Jr.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Xenakis, E.M.; Langer, O. The Association between Preeclampsia and the Severity of Gestational Diabetes: The Impact of Glycemic Control. Am. J. Obstet. Gynecol. 2004, 191, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Poolsup, N.; Suksomboon, N.; Amin, M. Effect of Treatment of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e92485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, N.; Green, M.S.; Yefet, E.; Nachum, Z. Modifiable Risk Factors for Gestational Diabetes Recurrence. Endocrine 2016, 54, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Lekva, T.; Michelsen, A.E.; Aukrust, P.; Henriksen, T.; Bollerslev, J.; Ueland, T. Leptin and Adiponectin as Predictors of Cardiovascular Risk after Gestational Diabetes Mellitus. Cardiovasc. Diabetol. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortelazzi, D.; Corbetta, S.; Ronzoni, S.; Pelle, F.; Marconi, A.; Cozzi, V.; Cetin, I.; Cortelazzi, R.; Beck-Peccoz, P.; Spada, A. Maternal and Foetal Resistin and Adiponectin Concentrations in Normal and Complicated Pregnancies. Clin. Endocrinol. 2007, 66, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.E.; Jamieson, N.; Greer, I.A.; Sattar, N. Paradoxical Elevation in Adiponectin Concentrations in Women with Preeclampsia. Hypertension 2003, 42, 891–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajantie, E.; Kaaja, R.; Ylikorkala, O.; Andersson, S.; Laivouri, H. Adiponectin Concentrations in Maternal Serum: Elevated in Preeclampsis but Unrelated to Insulin Sensitivity. J. Soc. Gynecol. Investig. 2005, 12, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Dʼanna, R.; Baviera, G.; Corrado, F.; Giordano, D.; Di Benedetto, A.; Jasonni, V.M. Plasma Adiponectin Concentration in Early Pregnancy and Subsequent Risk of Hypertensive Disorders. Obstet. Gynecol. 2005, 106, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Hendler, I.; Blackwell, S.C.; Mehta, S.H.; Whitty, J.E.; Russell, E.; Sorokin, Y.; Cotton, D.B. The Levels of Leptin, Adiponectin, and Resistin in Normal Weight, Overweight, and Obese Pregnant Women with and without Preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Hunt, H.; Melhorn, S.; Gammill, H.S.; Schur, E.A. Adipokine Profiles in Preeclampsia. J. Matern. Neonatal Med. 2019, 33, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, N.; Chrystodoulacos, G.; Kouskouni, E.; Salamalekis, E.; Creatsas, G. Alterations of Maternal and Fetal Leptin Concentrations in Hypertensive Disorders of Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 59–62. [Google Scholar] [CrossRef]
- Al-Musharaf, S.; Sabico, S.; Hussain, S.D.; Al-Tawashi, F.; Al Waily, H.B.; Al-Daghri, N.M.; McTernan, P. Inflammatory and Adipokine Status from Early to Midpregnancy in Arab Women and Its Associations with Gestational Diabetes Mellitus. Dis. Markers 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Satler, M.; Elhenicky, M.; Brix, J.; Krzyzanowska, K.; Schernthaner, G.; Husslein, P.W.; Schernthaner, G.-H. Circulating Levels of MCP-1 Are Increased in Women with Gestational Diabetes. Prenat. Diagn. 2008, 28, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, R.V.; Chepanov, S.V.; Babakov, V.N.; Rogovskaya, N.Y.; Kopteeva, E.V.; Alekseenkova, E.N.; Arzhanova, O.N. Maternal Serum Leptin, Adiponectin, Resistin and Monocyte Chemoattractant Protein-1 Levels in Different Types of Diabetes Mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mordwinkin, N.M.; Ouzounian, J.G.; Yedigarova, L.; Montoro, M.N.; Louie, S.G.; Rodgers, K.E. Alteration of Endothelial Function Markers in Women with Gestational Diabetes and Their Fetuses. J. Matern. Neonatal Med. 2012, 26, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myatt, L.; Clifton, R.G.; Roberts, J.M.; Spong, C.Y.; Hauth, J.C.; Varner, M.W.; Thorp, J.M.; Mercer, B.M.; Peaceman, A.M.; Ramin, S.M.; et al. First-Trimester Prediction of Preeclampsia in Nulliparous Women at Low Risk. Obstet. Gynecol. 2012, 119, 1234–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, A.M.; Giuffrida, D.; Moretti, L.; Re, P.; Grassi, G.; Menato, G.; Rolfo, A. Placental and Maternal sFlt1/PlGF Expression in Gestational Diabetes Mellitus. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Hoeller, A.; Ehrlich, L.; Golic, M.; Herse, F.; Perschel, F.H.; Siwetz, M.; Henrich, W.; Dechend, R.; Huppertz, B.; Verlohren, S. Placental Expression of sFlt-1 and PlGF in Early Preeclampsia vs. Early IUGR vs. Age-Matched Healthy Pregnancies. Hypertens. Pregnancy 2017, 36, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic Accuracy of sFlt1/PlGF Ratio as a Marker for Preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Molvarec, A.; Szarka, A.; Walentin, S.; Szűcs, E.; Nagy, B.; Rigó, J. Circulating Angiogenic Factors Determined by Electrochemiluminescence Immunoassay in Relation to the Clinical Features and Laboratory Parameters in Women with Pre-Eclampsia. Hypertens. Res. 2010, 33, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Staff, A.C.; Braekke, K.; Harsem, N.K.; Lyberg, T.; Holthe, M.R. Circulating Concentrations of sFlt1 (Soluble fms-Like Tyrosine Kinase 1) in Fetal and Maternal Serum during Pre-Eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF Ratio in Different Types of Hypertensive Pregnancy Disorders and Its Prognostic Potential in Preeclamptic Patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.N.; Lee, D.S.; Jeong, D.H.; Sung, M.S.; Kim, K.T. The Relationship of the Level of Circulating Antiangiogenic Factors to the Clinical Manifestations of Preeclampsia. Prenat. Diagn. 2009, 29, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Boutsikou, T.; Mastorakos, G.; Kyriakakou, M.; Margeli, A.; Hassiakos, D.; Papassotiriou, I.; Kanaka-Gantenbein, C.; Malamitsi-Puchner, A. Circulating Levels of Inflammatory Markers in Intrauterine Growth Restriction. Mediat. Inflamm. 2010, 2010, 790605. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.; Tong, S.; Palmer, K.R. Is Fetal Growth Restriction Associated with a More Severe Maternal Phenotype in the Setting of Early Onset Pre-Eclampsia? A Retrospective Study. PLoS ONE 2011, 6, e26937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helske, S.; Vuorela, P.; Carpén, O.; Hornig, C.; Weich, H.; Halmesmäki, E. Expression of Vascular Endothelial Growth Factor Receptors 1, 2 and 3 in Placentas from Normal and Complicated Pregnancies. Mol. Hum. Reprod. 2001, 7, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- March, M.I.; Geahchan, C.; Wenger, J.; Raghuraman, N.; Berg, A.; Haddow, H.; McKeon, B.A.; Narcisse, R.; David, J.L.; Scott, J.; et al. Circulating Angiogenic Factors and the Risk of Adverse Outcomes among Haitian Women with Preeclampsia. PLoS ONE 2015, 10, e0126815. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Whitten, A.E.; Korzeniewski, S.; Chaemsaithong, P.; Hernandez-Andrade, E.; Yeo, L.; Hassan, S.S. The Use of Angiogenic Biomarkers in Maternal Blood to Identify Which SGA Fetuses Will Require a Preterm Delivery and Mothers Who Will Develop Pre-Eclampsia. J. Matern. Neonatal Med. 2015, 29, 1214–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappas, M. Markers of Endothelial Cell Dysfunction Are Increased in Human Omental Adipose Tissue from Women with Pre-Existing Maternal Obesity and Gestational Diabetes. Metabolism 2014, 63, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Guller, S.; Ma, Y.Y.; Fu, H.-H.; Krikun, G.; Abrahams, V.M.; Mor, G. The Placental Syncytium and the Pathophysiology of Preeclampsia and Intrauterine Growth Restriction: A Novel Assay to Assess Syncytial Protein Expression. Ann. N. Y. Acad. Sci. 2008, 1127, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, B.; Bonnar, J. Uteroplacental Hemostasis in Intrauterine Fetal Growth Retardation. Semin. Thromb. Hemost. 1999, 25, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L.; Giannubilo, S.R. Blood Pressure Is Elevated in Normotensive Pregnant Women with Intrauterine Growth Restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Wenger, J.B.; James-Todd, T.; Lamparello, B.M.; Halprin, E.; Serdy, S.; Fan, S.; Horowitz, G.L.; Lim, K.-H.; Rana, S.; et al. The Association of Circulating Angiogenic Factors and HbA1c with the Risk of Preeclampsia in Women with Preexisting Diabetes. Hypertens. Pregnancy 2013, 33, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zen, M.; Padmanabhan, S.; Zhang, K.; Kirby, A.; Cheung, N.W.; Lee, V.W.; Alahakoon, T.I. Urinary and Serum Angiogenic Markers in Women with Preexisting Diabetes During Pregnancy and Their Role in Preeclampsia Prediction. Diabetes Care 2019, 43, 67–73. [Google Scholar] [CrossRef] [PubMed]


| Variable * | GDM-HDP (n = 13) | GDM-NMT (n = 136) | Control (n = 88) | p-Value |
|---|---|---|---|---|
| Age (y) | 33.9 ± 3.7 | 34.6 ± 4.3 | 33.1 ± 5.1 | ns |
| Pregestational BMI (kg/m2) | 30.1 ± 6.8 | 26.8 ± 4.9 | 24.5 ± 3.8 | <0.001 a,c |
| Systolic BP (mmHg) | 117.9 ± 16.1 | 109.4 ± 14.1 | 109.4 ± 11.9 | ns |
| Diastolic BP (mmHg) | 73.2 ± 10.9 | 64.8 ± 8.6 | 63.4 ± 8.7 | 0.001 a,b |
| Basal glucose (mmol/L) | 4.98 ± 0.49 | 4.97 ± 0.62 | 4.65 ± 0.32 | 0.042 c |
| HbA1c (%) | 5.24 ± 0.5 | 5.0 ± 0.4 | 4.8 ± 0.3 | <0.001 a,c |
| HOMA-IR | 2.4 ± 0.9 | 1.6 ± 1.3 | 1.9 ± 1.1 | ns |
| Albumin/creatinine (mg/g) | 30.2 ± 62.1 | 66.7 ± 7.5 | 4.9 ± 3.6 | <0.001 |
| Uric acid (mmol/L) | 0.25 ± 0.07 | 0.23 ± 0.13 | 0.23 ± 0.23 | ns |
| Total cholesterol (mmol/L) | 6.41 ± 0.65 | 6.40 ± 1.29 | 6.48 ± 1.0 | ns |
| LDL cholesterol (mmol/L) | 3.66 ± 0.49 | 3.82 ± 1.39 | 3.51 ± 0.98 | ns |
| HDL cholesterol (mmol/L) | 1.99 ± 0.44 | 1.85 ± 0.45 | 1.99 ± 0.5 | ns |
| Triglycerides (mmol/L) | 2.36 ± 0.62 | 2.27 ± 0.9 | 2.05 ± 0.72 | ns |
| Variable | GDM-HDP (n = 13) | GDM-NMT (n = 136) | Control (n = 88) | p-Value |
|---|---|---|---|---|
| Systolic BP (mmHg) * | 151.7 ± 29.9 | 120 ± 12.3 | 119.8 ± 11.4 | <0.001 a,b |
| Diastolic BP (mmHg) * | 90.5 ± 16.6 | 71.5 ± 10.2 | 70.2 ± 7.5 | <0.001 a,b |
| Gestational age at delivery (wk) * | 37.9 ± 1.7 | 38.9 ± 1.4 | 39.6 ± 1.3 | <0.001 a,b,c |
| Cesarean section † | 5 (38.5%) | 43 (31.6%) | 21 (2.9%) | ns |
| Birthweight (g) * | 2701 ± 591 | 3256 ± 477 | 3323 ± 429 | <0.001 a,b |
| Customized percentile * | 24.3 ± 25 | 48.7 ± 28.7 | 48.4 ± 28.5 | 0.017 a,b |
| Apgar < 6 at 5 min † | 0 | 2 (1.5%) | 0 | ns |
| Macrosomia † | 0 | 17 (12.5%) | 10 (11.4%) | ns |
| LGA † | 0 | 16 (11.8%) | 8 (9.1%) | ns |
| SGA † | 6 (46.2%) | 11 (8.1%) | 11 (12.5%) | <0.001 |
| IUGR † | 4 (30.8%) | 5 (3.7%) | 7 (8%) | 0.001 |
| Variable * | GDM-HDP (n = 13) | GDM-NMT (n = 136) | Control (n = 88) | p-Value |
|---|---|---|---|---|
| Adiponectin (pg/mL) | 10.52 ± 1.33 | 12.94 ± 2.78 | 13.18 ± 2.97 | 0.031 a,b |
| Resistin (pg/mL) | 6.69 + 3.69 | 7.21 + 3 | 8.31 + 3.31 | 0.028 c |
| PAI-1 (pg/mL) | 7.31 ± 3.96 | 7.65 ± 3.15 | 8.79 ± 3.45 | 0.034 c |
| NGF (pg/mL) | 0.34 ± 0.75 | 0.63 ± 0.83 | 0.92 ± 0.92 | ns |
| Leptin (pg/mL) | 10.97 ± 0.85 | 10.08 ± 1.14 | 10.16 ± 0.99 | 0.038 b |
| HGF (pg/mL) | 6.51 ± 1.08 | 7.1 ± 1.01 | 6.87 ± 1.91 | ns |
| MCP-1 (pg/mL) | 5.23 ± 0.61 | 5.03 ± 0.53 | 4.81 ± 0.58 | 0.023 |
| TNFα (pg/mL) | 0.18 ± 2.05 | 0.51 ± 1.11 | 0.79 ± 0.75 | ns |
| sFlt-1 (pg/mL) | 7.58 ± 1.08 | 7.7 ± 0.95 | 7.41 ± 1.0 | ns |
| PIGF (pg/mL) | 2.66 ± 1.95 | 5.05 ± 1.05 | 5.32 ± 1.06 | <0.001 a,b |
| FGF2 (pg/mL) | 3.79 ± 0.75 | 4.08 ± 0.63 | 4.06 ± 0.65 | ns |
| sFlt-1/PlGF ratio | 4.92 ± 2.63 | 2.34 ± 1.33 | 2.21 ± 1.56 | <0.001 a,b |
| Variable | OR | Z-Score | p-Value | CI 95% |
|---|---|---|---|---|
| Age (y) | 0.94 | −0.05 | 0.76 | (0.67–1.32) |
| Pregestational BMI (kg/m2) | 1.14 | 0.13 | 0.32 | (0.87–1.50) |
| Adiponectin (pg/mL) | 0.45 | −0.80 | 0.012 | (0.23–0.83) |
| Leptin (pg/mL) | 1.04 | 0.04 | 0.96 | (0.18–5.86) |
| sFlt-1/PIGF ratio | 2.70 | 0.99 | 0.012 | (1.24–5.86) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Barea, A.; Sánchez-Lechuga, B.; Campos-Caro, A.; Córdoba-Doña, J.A.; de la Varga-Martínez, R.; Arroba, A.I.; Bugatto, F.; Aguilar-Diosdado, M.; López-Tinoco, C. Angiogenic Imbalance and Inflammatory Biomarkers in the Prediction of Hypertension as Well as Obstetric and Perinatal Complications in Women with Gestational Diabetes Mellitus. J. Clin. Med. 2022, 11, 1514. https://doi.org/10.3390/jcm11061514
Lara-Barea A, Sánchez-Lechuga B, Campos-Caro A, Córdoba-Doña JA, de la Varga-Martínez R, Arroba AI, Bugatto F, Aguilar-Diosdado M, López-Tinoco C. Angiogenic Imbalance and Inflammatory Biomarkers in the Prediction of Hypertension as Well as Obstetric and Perinatal Complications in Women with Gestational Diabetes Mellitus. Journal of Clinical Medicine. 2022; 11(6):1514. https://doi.org/10.3390/jcm11061514
Chicago/Turabian StyleLara-Barea, Almudena, Begoña Sánchez-Lechuga, Antonio Campos-Caro, Juan Antonio Córdoba-Doña, Raquel de la Varga-Martínez, Ana I. Arroba, Fernando Bugatto, Manuel Aguilar-Diosdado, and Cristina López-Tinoco. 2022. "Angiogenic Imbalance and Inflammatory Biomarkers in the Prediction of Hypertension as Well as Obstetric and Perinatal Complications in Women with Gestational Diabetes Mellitus" Journal of Clinical Medicine 11, no. 6: 1514. https://doi.org/10.3390/jcm11061514
APA StyleLara-Barea, A., Sánchez-Lechuga, B., Campos-Caro, A., Córdoba-Doña, J. A., de la Varga-Martínez, R., Arroba, A. I., Bugatto, F., Aguilar-Diosdado, M., & López-Tinoco, C. (2022). Angiogenic Imbalance and Inflammatory Biomarkers in the Prediction of Hypertension as Well as Obstetric and Perinatal Complications in Women with Gestational Diabetes Mellitus. Journal of Clinical Medicine, 11(6), 1514. https://doi.org/10.3390/jcm11061514






