Diffusion-Weighted Magnetic Resonance Imaging in Ovarian Cancer: Exploiting Strengths and Understanding Limitations
Abstract
:1. Introduction
2. Optimizing the DW-MRI Technique
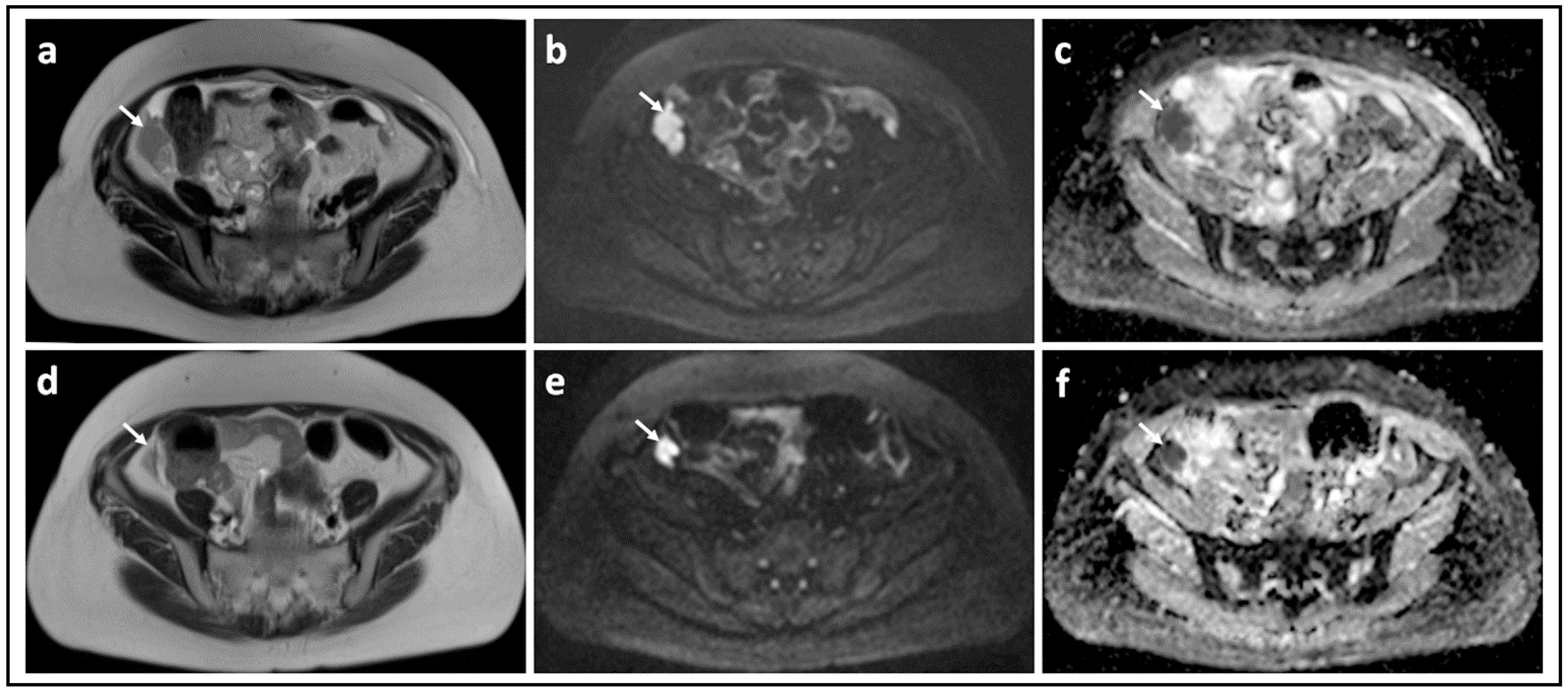
3. DW-MRI for Differentiating Benign from Malignant Adnexal Masses
4. The Role of DW-MRI in Disease Staging and Predicting Resectability
5. DW-MRI for Longitudinal Follow-Up and to Assess Response
6. Introducing DW-MRI into Clinical Trials?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UK CR. CancerStats. 2020. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ (accessed on 17 August 2020).
- United States Cancer Statistics: Data Visualizations. Available online: www.cdc.gov/cancer/dataviz (accessed on 10 January 2022).
- Thigpen, T. The If and When of Surgical Debulking for Ovarian Carcinoma. N. Engl. J. Med. 2004, 351, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; et al. European Society of Gynaecological Oncology (ESGO) Guidelines for Ovarian Cancer Surgery. Int. J. Gynecol. Cancer 2017, 27, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J. Prediction of optimal debulking surgery in ovarian cancer. Gland Surg. 2021, 10, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.; Borràs, E.; Pérez-Benavente, A.; Gil-Moreno, A.; Santamaria, A.; Sabidó, E. Proteomic Studies on the Management of High-Grade Serous Ovarian Cancer Patients: A Mini-Review. Cancers 2021, 13, 2067. [Google Scholar] [CrossRef] [PubMed]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, CD007565. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.T. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl. Cancer Inst. Monogr. 1975, 42, 101–104. [Google Scholar] [PubMed]
- Hoskins, W.J. Epithelial Ovarian Carcinoma: Principles of Primary Surgery. Gynecol. Oncol. 1994, 55, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Tian, W.-J.; Chi, D.S.; Sehouli, J.; Trope, C.G.; Jiang, R.; Ayhan, A.; Cormio, G.; Xing, Y.; Breitbach, G.-P.; Braicu, E.I.; et al. A Risk Model for Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer: An Evidence-Based Proposal for Patient Selection. Ann. Surg. Oncol. 2011, 19, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Barrett, T.; Sala, E. MRI of ovarian masses. J. Magn. Reson. Imaging 2013, 37, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Kyriazi, S.; Collins, D.J.; Morgan, V.A.; Giles, S.L.; DeSouza, N.M. Diffusion-weighted Imaging of Peritoneal Disease for Noninvasive Staging of Advanced Ovarian Cancer. Radiographics 2010, 30, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- LeBihan, D. IVIM method measures diffusion and perfusion. Diagn. Imaging 1990, 12, 133–136. [Google Scholar]
- Le Bihan, D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology 2013, 268, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.C.; Downey, K.; Kyriazi, S.; Desouza, N.M. New MR Techniques in Gynecologic Cancer. Am. J. Roentgenol. 2013, 200, 249–260. [Google Scholar] [CrossRef]
- Cooper, R.L.; Chang, D.B.; Young, A.C.; Martin, C.J.; Ancker-Johnson, B. Restricted Diffusion in Biophysical Systems: Experiment. Biophys. J. 1974, 14, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Kuai, Z.-X.; Liu, W.-Y.; Zhu, Y.-M. Effect of multiple perfusion components on pseudo-diffusion coefficient in intravoxel incoherent motion imaging. Phys. Med. Biol. 2017, 62, 8197–8209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wang, Y.; Zhou, Y.; Liu, C.; Xie, L.; Zhou, Z.; Liang, N.; Shen, Y.; Yao, Z.; Liu, J. Comparison between types I and II epithelial ovarian cancer using histogram analysis of monoexponential, biexponential, and stretched-exponential diffusion models. J. Magn. Reson. Imaging 2017, 46, 1797–1809. [Google Scholar] [CrossRef]
- Winfield, J.M.; Desouza, N.M.; Priest, A.N.; Wakefield, J.C.; Hodgkin, C.; Freeman, S.; Orton, M.R.; Collins, D.J. Modelling DW-MRI data from primary and metastatic ovarian tumours. Eur. Radiol. 2015, 25, 2033–2040. [Google Scholar] [CrossRef] [Green Version]
- Charles-Edwards, E.M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging 2006, 6, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollineni, V.; Kramer, G.; Liu, Y.; Melidis, C.; Desouza, N. A literature review of the association between diffusion-weighted MRI derived apparent diffusion coefficient and tumour aggressiveness in pelvic cancer. Cancer Treat. Rev. 2015, 41, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.M.; Douglas, N.H.M.; DeSouza, N.M.; Collins, D.J. Phantom for assessment of fat suppression in large field-of-view diffusion-weighted magnetic resonance imaging. Phys. Med. Biol. 2014, 59, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.; Anttila, M.; Rautiainen, S.; Arponen, O.; Kivelä, A.; Mäkinen, P.; Härmä, K.; Hämäläinen, K.; Kosma, V.-M.; Ylä-Herttuala, S.; et al. Primary and metastatic ovarian cancer: Characterization by 3.0T diffusion-weighted MRI. Eur. Radiol. 2017, 27, 4002–4012. [Google Scholar] [CrossRef] [Green Version]
- Pi, S.; Cao, R.; Qiang, J.W.; Guo, Y.H. Utility of DWI with quantitative ADC values in ovarian tumors: A meta-analysis of diagnostic test performance. Acta Radiol. 2018, 59, 1386–1394. [Google Scholar] [CrossRef]
- Davarpanah, A.H.; Kambadakone, A.; Holalkere, N.S.; Guimaraes, A.R.; Hahn, P.F.; Lee, S.I. Diffusion MRI of uterine and ovarian masses: Identifying the benign lesions. Abdom. Radiol. 2016, 41, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wang, T.; Zhang, G. Diffusion-Weighted Magnetic Resonance Imaging (DWI) Parameters in Benign and Malignant Ovarian Tumors with Solid and Cystic Components. J. Coll. Physicians Surg. Pak. 2019, 29, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, S.; Kayan, M. Differentiation between benign and malignant ovarian masses using multiparametric MRI. Diagn. Interv. Imaging 2020, 101, 147–155. [Google Scholar] [CrossRef]
- Kierans, A.S.; Bennett, G.L.; Mussi, T.; Babb, J.; Rusinek, H.; Melamed, J.; Rosenkrantz, A.B. Characterization of malignancy of adnexal lesions using ADC entropy: Comparison with mean ADC and qualitative DWI assessment. J. Magn. Reson. Imaging 2013, 37, 164–171. [Google Scholar] [CrossRef]
- Zhao, S.-H.; Li, H.-M.; Qiang, J.-W.; Wang, D.-B.; Fan, H. The value of MRI for differentiating benign from malignant sex cord-stromal tumors of the ovary: Emphasis on diffusion-weighted MR imaging. J. Ovarian Res. 2018, 11, 73. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Daraï, E.; Cuenod, C.A.; Fournier, L.; Toussaint, I.; Marsault, C.; Bazot, M. Contribution of diffusion-weighted MR imaging for predicting benignity of complex adnexal masses. Eur. Radiol. 2009, 19, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kanematsu, M.; Ono, H.; Yano, R.; Furui, T.; Morishige, K.-I.; Hatano, Y. Ovarian fibromas: MR imaging findings with emphasis on intratumoral cyst formation. Eur. J. Radiol. 2013, 82, e417–e421. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, A.F.; Nabil, M.; Khah, H.H.; Alviri, M.; Heidari-Sooreshjaani, M.; Gity, M.; Malek, M.; Rad, H.S. ADC-derived spatial features can accurately classify adnexal lesions. J. Magn. Reson. Imaging 2017, 47, 1061–1071. [Google Scholar] [CrossRef]
- Mokry, T.; Mlynarska-Bujny, A.; Kuder, T.A.; Hasse, F.C.; Hog, R.; Wallwiener, M.; Dinkic, C.; Brucker, J.; Sinn, P.; Gnirs, R.; et al. Ultra-High-b-Value Kurtosis Imaging for Noninvasive Tissue Characterization of Ovarian Lesions. Radiology 2020, 296, 358–369. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kato, H.; Noda, Y.; Suzui, N.; Miyazaki, T.; Furui, T.; Morishige, K.-I.; Matsuo, M. CT and MRI characteristics of ovarian mature teratoma in patients with anti-N-methyl-D-aspartate receptor encephalitis. Diagn. Interv. Imaging 2021, 102, 447–453. [Google Scholar] [CrossRef]
- Acs, G. Serous and Mucinous Borderline (Low Malignant Potential) Tumors of the Ovary. Pathol. Patterns Rev. 2005, 123, S13–S57. [Google Scholar] [CrossRef]
- Ye, Z. Editorial for “Histogram Analysis Comparison of Monoexponential, Advanced Diffusion—Weighted Imaging, and Dynamic Contrast—Enhanced MRI for Differentiating Borderline from Malignant Epithelial Ovarian Tumors”. J. Magn. Reson. Imaging 2020, 52, 269–270. [Google Scholar] [CrossRef]
- Lu, J.J.; Pi, S.; Ma, F.H.; Zhang, G.F.; Qiang, J.W. Apparent diffusion coefficients measured using different regions of interest in differentiating borderline from malignant ovarian tumors. Acta Radiol. 2018, 60, 1020–1027. [Google Scholar] [CrossRef]
- Li, H.; Zhang, R.; Gu, W.; Zhao, S.; Lu, N.; Zhang, G.; Peng, W.; Qiang, J. Whole solid tumour volume histogram analysis of the apparent diffusion coefficient for differentiating high-grade from low-grade serous ovarian carcinoma: Correlation with Ki-67 proliferation status. Clin. Radiol. 2019, 74, 918–925. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Song, Y.; Li, H.; Lu, J.; Li, Y.; Duan, S.; Qiang, J. Histogram Analysis Comparison of Monoexponential, Advanced Diffusion—Weighted Imaging, and Dynamic Contrast—Enhanced MRI for Differentiating Borderline from Malignant Epithelial Ovarian Tumors. J. Magn. Reson. Imaging 2020, 52, 257–268. [Google Scholar] [CrossRef]
- Cacioppa, L.M.; Crusco, F.; Marchetti, F.; Duranti, M.; Renzulli, M.; Golfieri, R. Magnetic resonance imaging of pure ovarian dysgerminoma: A series of eight cases. Cancer Imaging 2021, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sun, F.; Bao, L.; Chu, C.; Li, H.; Yin, Q.; Guan, W.; Wang, D. Pure dysgerminoma of the ovary: CT and MRI features with pathological correlation in 13 tumors. J. Ovarian Res. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Matsuzaki, K.; Uehara, H. Primary carcinoid tumor of the ovary: MR imaging characteristics with pathologic correlation. Magn. Reson. Med. Sci. 2011, 10, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, T.; Kishimoto, K.; Tajima, S.; Maeda, I.; Takagi, M.; Suzuki, N.; Mimura, H. Apparent diffusion coefficient (ADC) values of serous, endometrioid, and clear cell carcinoma of the ovary: Pathological correlation. Acta Radiol. 2019, 61, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; FIGO Committee on Gynecologic Oncology. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Forstner, R.; Sala, E.; Kinkel, K.; Spencer, J.A. ESUR guidelines: Ovarian cancer staging and follow-up. Eur. Radiol. 2010, 20, 2773–2780. [Google Scholar] [CrossRef]
- Lee, E.Y.P.; An, H.; Tse, K.Y.; Khong, P.-L. Molecular Imaging of Peritoneal Carcinomatosis in Ovarian Carcinoma. Am. J. Roentgenol. 2020, 215, 305–312. [Google Scholar] [CrossRef]
- Coakley, F.V.; Choi, P.H.; Gougoutas, C.A.; Pothuri, B.; Venkatraman, E.; Chi, D.; Bergman, A.; Hricak, H. Peritoneal Metastases: Detection with Spiral CT in Patients with Ovarian Cancer. Radiology 2002, 223, 495–499. [Google Scholar] [CrossRef]
- Forstner, R.; Hricak, H.; Occhipinti, K.A.; Powell, C.B.; Frankel, S.D.; Stern, J.L. Ovarian cancer: Staging with CT and MR imaging. Radiology 1995, 197, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tempany, C.M.C.; Zou, K.H.; Silverman, S.G.; Brown, D.L.; Kurtz, A.B.; McNeil, B.J. Staging of Advanced Ovarian Cancer: Comparison of Imaging Modalities—Report from the Radiological Diagnostic Oncology Group. Radiology 2000, 215, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Sebrechts, C.P.; Barone, R.M.; Muller, W. Diffusion-Weighted MRI of Peritoneal Tumors: Comparison With Conventional MRI and Surgical and Histopathologic Findings—A Feasibility Study. Am. J. Roentgenol. 2009, 193, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.G.; Hernando, C.G.; Delgado, D.V.; Martínez, R.S.; Bhosale, P.; Sanchez, J.B.; Chiva, L. Diffusion-weighted magnetic resonance imaging in peritoneal carcinomatosis from suspected ovarian cancer: Diagnostic performance in correlation with surgical findings. Eur. J. Radiol. 2019, 121, 108696. [Google Scholar] [CrossRef]
- Rizzo, S.; De Piano, F.; Buscarino, V.; Pagan, E.; Bagnardi, V.; Zanagnolo, V.; Colombo, N.; Maggioni, A.; Del Grande, M.; Del Grande, F.; et al. Pre-operative evaluation of epithelial ovarian cancer patients: Role of whole body diffusion weighted imaging MR and CT scans in the selection of patients suitable for primary debulking surgery. A single-centre study. Eur. J. Radiol. 2020, 123, 108786. [Google Scholar] [CrossRef]
- Michielsen, K.; Vergote, I.; De Beeck, K.O.; Amant, F.; Leunen, K.; Moerman, P.; Deroose, C.; Souverijns, G.; Dymarkowski, S.; De Keyzer, F.; et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: A clinical feasibility study in comparison to CT and FDG-PET/CT. Eur. Radiol. 2013, 24, 889–901. [Google Scholar] [CrossRef]
- Michielsen, K.; Dresen, R.; Vanslembrouck, R.; De Keyzer, F.; Amant, F.; Mussen, E.; Leunen, K.; Berteloot, P.; Moerman, P.; Vergote, I.; et al. Diagnostic value of whole body diffusion-weighted MRI compared to computed tomography for pre-operative assessment of patients suspected for ovarian cancer. Eur. J. Cancer 2017, 83, 88–98. [Google Scholar] [CrossRef]
- Engbersen, M.; Sant, I.V.T.; Lok, C.; Lambregts, D.; Sonke, G.; Beets-Tan, R.; van Driel, W.; Lahaye, M. MRI with diffusion-weighted imaging to predict feasibility of complete cytoreduction with the peritoneal cancer index (PCI) in advanced stage ovarian cancer patients. Eur. J. Radiol. 2019, 114, 146–151. [Google Scholar] [CrossRef]
- Jacquet, P.; Jelinek, J.S.; Steves, M.A.; Sugarbaker, P.H. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 1993, 72, 1631–1636. [Google Scholar] [CrossRef]
- Michielsen, K.L.M.; Vergote, I.; Dresen, R.; De Beeck, K.O.; Vanslembrouck, R.; Amant, F.; Leunen, K.; Moerman, P.; Fieuws, S.; De Keyzer, F.; et al. Whole-body diffusion-weighted magnetic resonance imaging in the diagnosis of rdecurrent ovarian cancer: A clinical feasibility study. Br. J. Radiol. 2016, 89, 20160468. [Google Scholar] [CrossRef] [Green Version]
- Results. GfEaEtUoNM. NIST Technical Note. 1994. Available online: http://www.nist.gov/pml/pubs/tn1297/index.cfm (accessed on 28 September 2021).
- Winfield, J.M.; Wakefield, J.C.; Dolling, D.; Hall, M.; Freeman, S.; Brenton, J.D.; Lutchman-Singh, K.; Pace, E.; Priest, A.N.; Quest, R.A.; et al. Diffusion-weighted MRI in Advanced Epithelial Ovarian Cancer: Apparent Diffusion Coefficient as a Response Marker. Radiology 2019, 293, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Gauquelin, L.; Nougaret, S.; Chicart, M.; Pulido, M.; Floquet, A.; Guyon, F.; Croce, S.; Kind, M.; Cazeau, A.-L. Diffusion-weighted MRI and PET/CT reproducibility in epithelial ovarian cancers during neoadjuvant chemotherapy. Diagn. Interv. Imaging 2021, 102, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, X.-P.; Sun, Y.-S.; Tang, L.; Shen, L. Apparent Diffusion Coefficient: Potential Imaging Biomarker for Prediction and Early Detection of Response to Chemotherapy in Hepatic Metastases. Radiology 2008, 248, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Harry, V.N.; Semple, S.I.; Gilbert, F.; Parkin, D.E. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol. Oncol. 2008, 111, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tourell, M.C.; Shokoohmand, A.; Landgraf, M.; Holzapfel, N.P.; Poh, P.S.P.; Loessner, D.; Momot, K.I. The distribution of the apparent diffusion coefficient as an indicator of the response to chemotherapeutics in ovarian tumour xenografts. Sci. Rep. 2017, 7, 42905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, E.; Kataoka, M.Y.; Priest, A.N.; Gill, A.B.; McLean, M.A.; Joubert, I.; Graves, M.J.; Crawford, R.A.F.; Jimenez-Linan, M.; Earl, H.M.; et al. Advanced Ovarian Cancer: Multiparametric MR Imaging Demonstrates Response- and Metastasis-specific Effects. Radiology 2012, 263, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winfield, J.M.; Wakefield, J.C.; Brenton, J.D.; AbdulJabbar, K.; Savio, A.; Freeman, S.; Pace, E.; Lutchman-Singh, K.; Vroobel, K.M.; Yuan, Y.; et al. Biomarkers for site-specific response to neoadjuvant chemotherapy in epithelial ovarian cancer: Relating MRI changes to tumour cell load and necrosis. Br. J. Cancer 2021, 124, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- De Piano, F.; Buscarino, V.; Maresca, D.; Maisonneuve, P.; Aletti, G.; Lazzari, R.; Vavassori, A.; Bellomi, M.; Rizzo, S. Do DWI and quantitative DCE perfusion MR have a prognostic value in high-grade serous ovarian cancer? La Radiol. Med. 2019, 124, 1315–1323. [Google Scholar]
- Kyriazi, S.; Collins, D.; Messiou, C.; Pennert, K.; Davidson, R.; Giles, S.; Kaye, S.B.; DeSouza, N.M. Metastatic Ovarian and Primary Peritoneal Cancer: Assessing Chemotherapy Response with Diffusion-weighted MR Imaging—Value of Histogram Analysis of Apparent Diffusion Coefficients. Radiology 2011, 261, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barajas, R.; Rubenstein, J.; Chang, J.; Hwang, J.; Cha, S. Diffusion-Weighted MR Imaging Derived Apparent Diffusion Coefficient Is Predictive of Clinical Outcome in Primary Central Nervous System Lymphoma. Am. J. Neuroradiol. 2009, 31, 60–66. [Google Scholar] [CrossRef]
- Pope, W.B.; Kim, H.J.; Huo, J.; Alger, J.; Brown, M.S.; Gjertson, D.; Sai, V.; Young, J.; Tekchandani, L.; Cloughesy, T.; et al. Recurrent Glioblastoma Multiforme: ADC Histogram Analysis Predicts Response to Bevacizumab Treatment. Radiology 2009, 252, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSouza, N.M.; Winfield, J.M.; Waterton, J.C.; Weller, A.; Papoutsaki, M.-V.; Doran, S.J.; Collins, D.J.; Fournier, L.; Sullivan, D.; Chenevert, T.; et al. Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: Current considerations and future perspectives. Eur. Radiol. 2018, 28, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
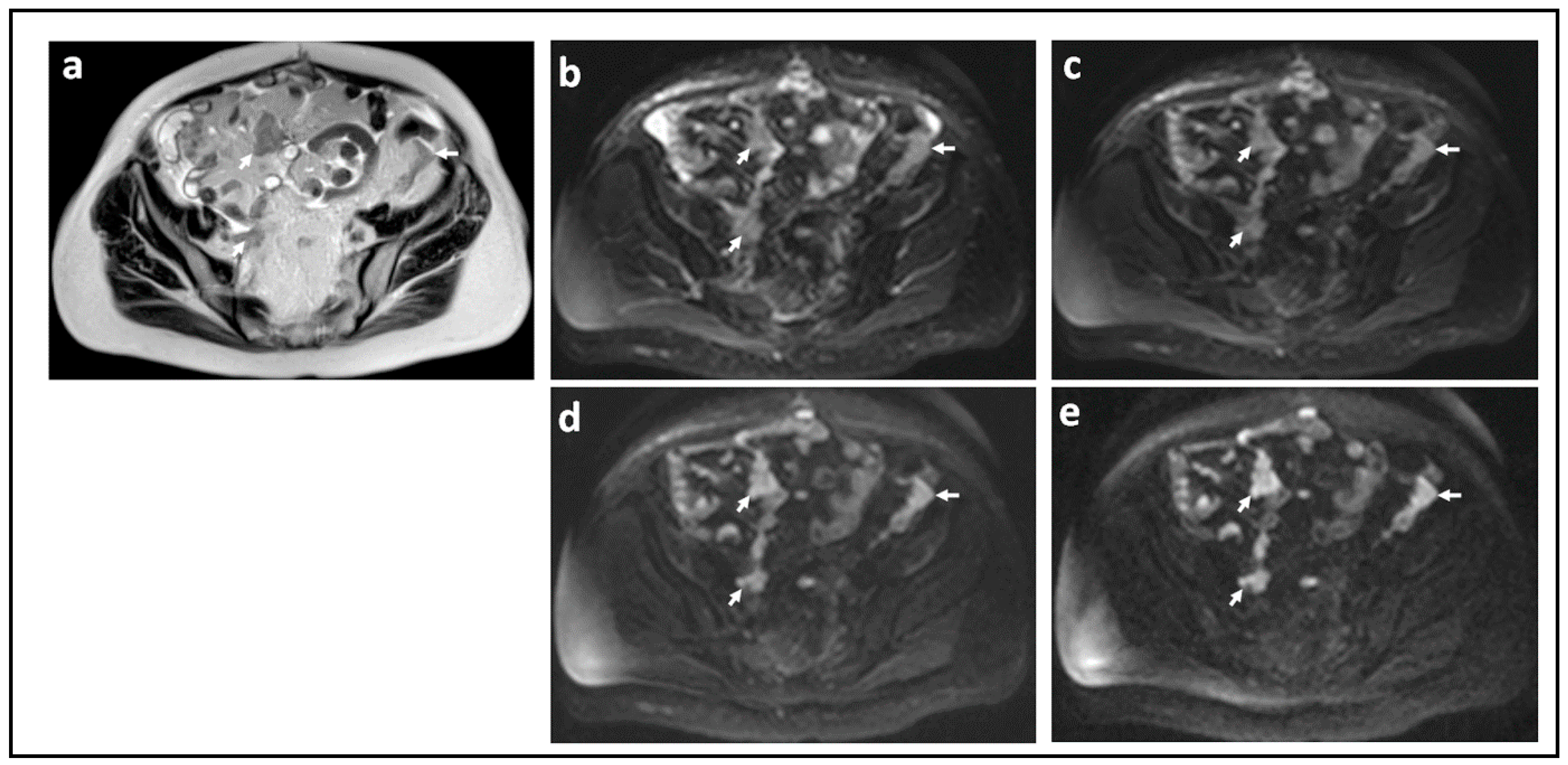
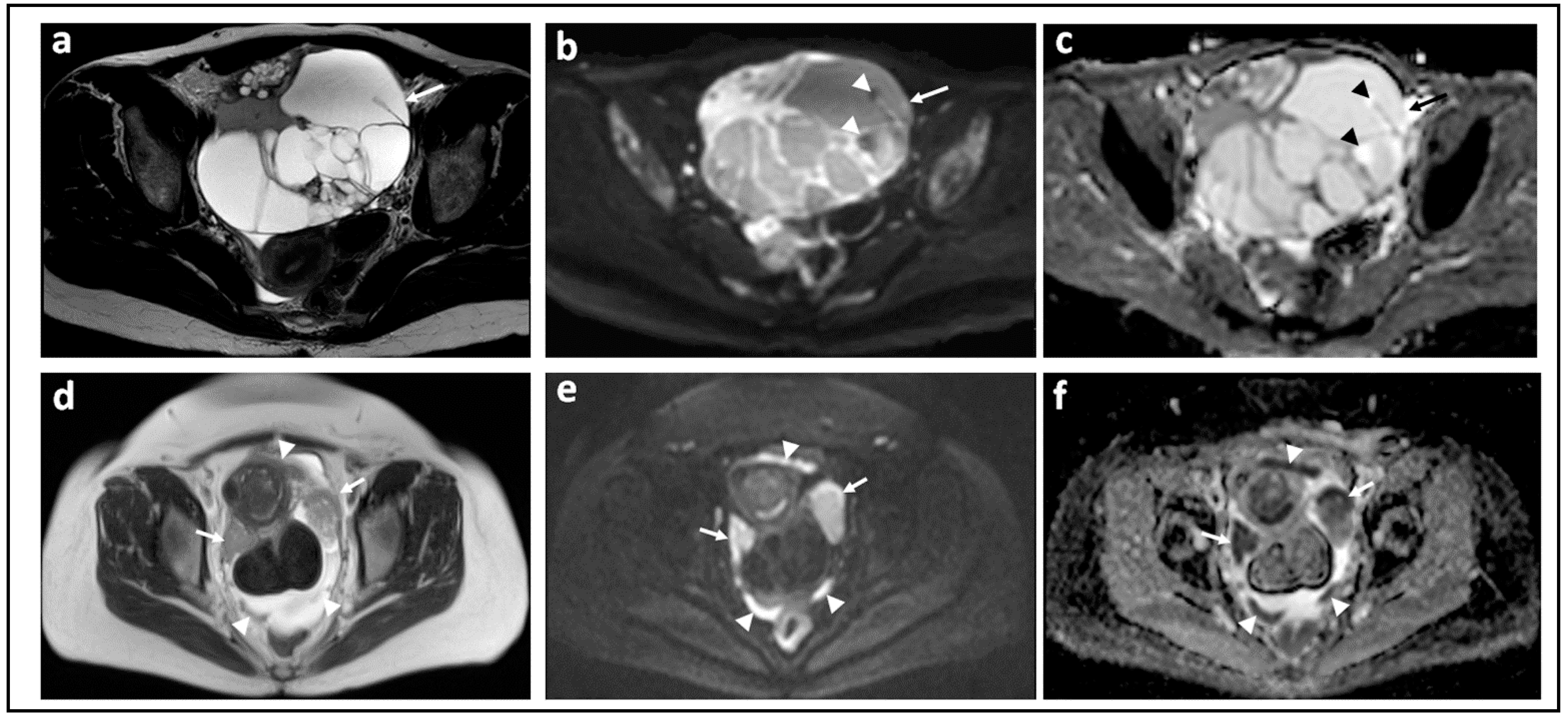
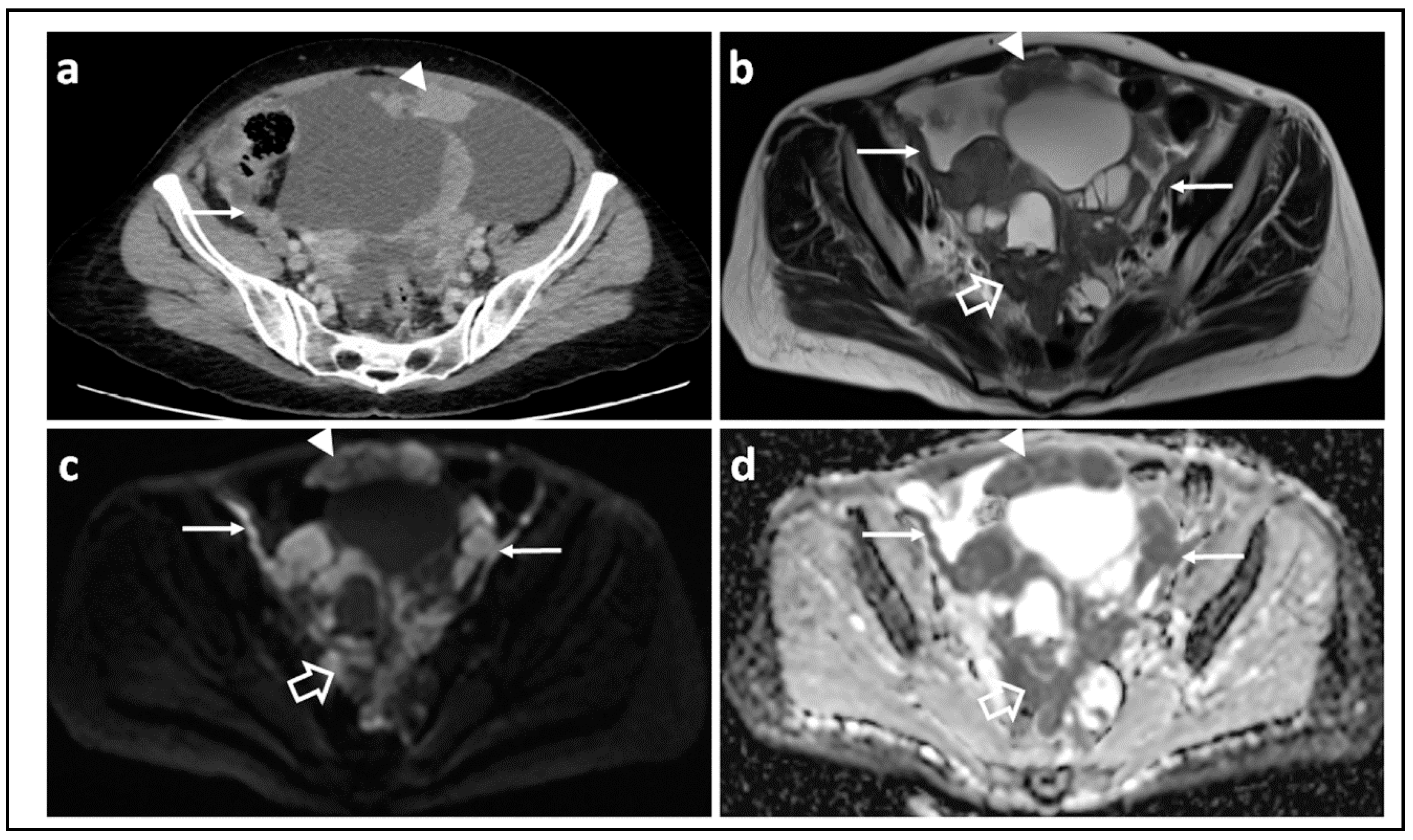
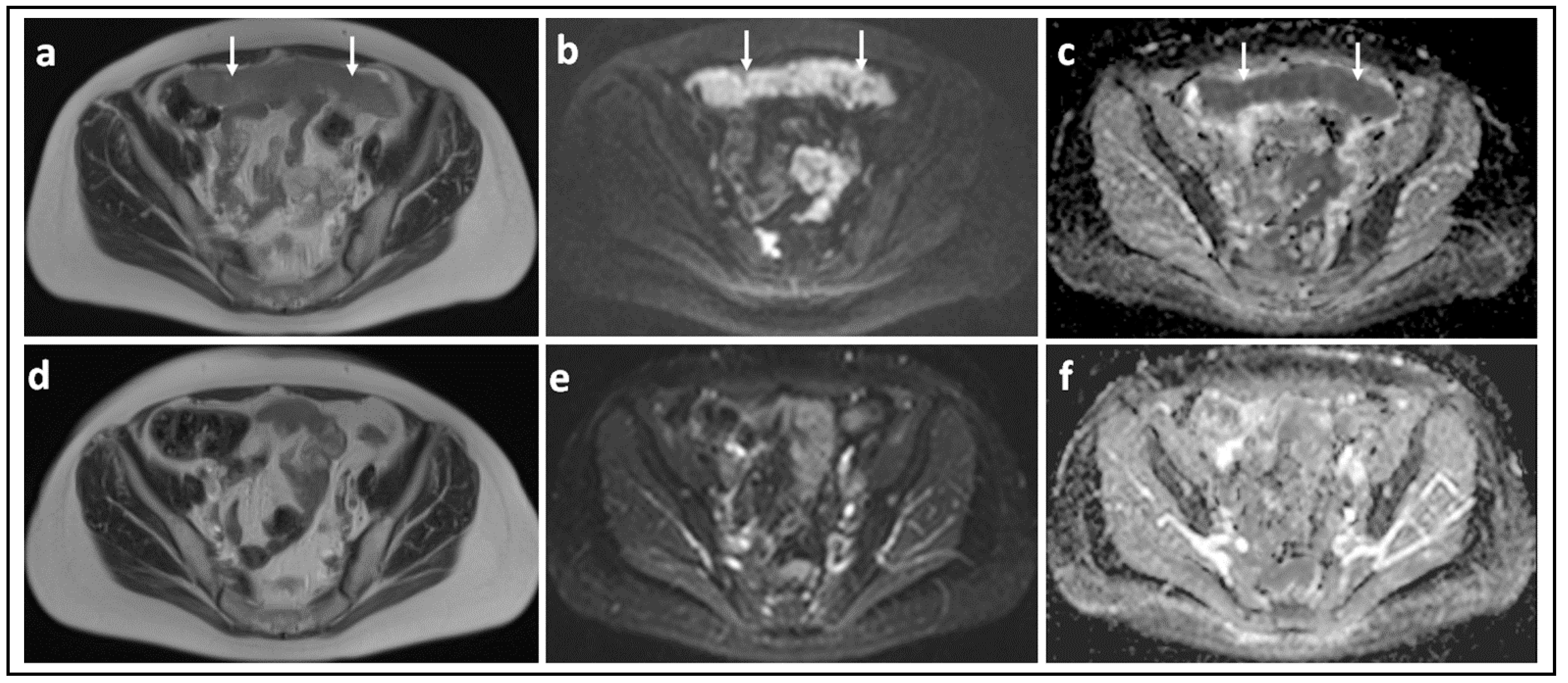
| Parameter | 1.5 T a | 3.0 T |
|---|---|---|
| Receive coil | anterior body matrix and posterior spine matrix; 32 channel body array | body coil [Sense-XL-Torso] b; 8 channel cardiac array c |
| Slice orientation | axial | axial b,c |
| Breathing | Free breathing | Breath-hold [upper abdomen] or free-breathing [pelvis] b; free-breathing c |
| Sequences | Single shot EPI | Single shot EPI b,c |
| Averages | 4 | |
| FOV/mm [read] × mm [phase] | 380 × 332 | 340 × 340 |
| Acquired matrix | 128 | 160 c |
| Reconstructed matrix | 256 | 160 c |
| Acquired pixel size/mm × mm | 3 × 3 | 1.8 × 1.8 b |
| Slice thickness/mm | 6 | 5 [0.5 gap] b; 5 [1 mm gap] c |
| No. of slices | 26 | 48–56 b |
| Parallel imaging | GRAPPA [reduction factor 2; 36 ACS lines]; ASSET reduction factor 2 | SENSE factor 2 b,c |
| Phase encode direction | AP | not available |
| Receive bandwidth | 1776 Hz/pixel; ±125 kHz [1953 Hz/pixel] | 250 kHz c |
| TR/ms | 8000 | 2600 c |
| TE/ms | 75; 81 | 71.5 c |
| Fat suppression | SPAIR; water selective excitation | STIR b |
| Diffusion gradient scheme | Bipolar; DSE | not available |
| Diffusion encoding scheme | Three-scan trace; ALL | not available |
| Diffusion weightings [b-values] for full volume coverage/s mm−2 | 0, 100, 500, 900 | 0, 300, 600 b |
| Diffusion weightings [b-values] for additional station/s mm−2 | 0, 50, 100, 150, 200, 250, 300, 500, 700, 900 | 0, 30, 50, 100, 150, 200, 400, 600, 800, 1000, 1500 c |
| Stage 1 Tumor Confined to Ovaries or Fallopian Tube[s] | T1-N0-M0 |
|---|---|
| IA: tumor limited to one ovary [capsule intact] or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings | T1a-N0-M0 |
| IB: tumor limited to both ovaries [capsules intact] or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings | T1b-N0-M0 |
IC: tumor limited to one or both ovaries or fallopian tubes, with any of the following:
| T1c1-N0-M0 T1c2-N0-M0 T1c3-N0-M0 |
| Stage II. Tumor involves one or both ovaries or fallopian tubes with pelvic extension [below pelvic brim] or primary peritoneal cancer | T2-N0-M0 |
| IIA: extension and/or implants on uterus and/or fallopian tubes and/or ovaries | T2a-N0-M0 |
| IIB: extension to other pelvic intraperitoneal tissues | T2b-N0-M0 |
| Stage III. Tumor involves one or both ovaries or fallopian tubes, or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes | |
IIIA1: positive retroperitoneal lymph nodes only [cytologically or histologically proven]:
| T1/T2-N1-M0 |
| IIIA2: microscopic extrapelvic [above the pelvic brim] peritoneal involvement with or without positive retroperitoneal lymph node | T3a2-N0/N1-M0 |
| IIIB: macroscopic peritoneal metastasis beyond the pelvis up to 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes | T3b-N0/N1-M0 |
| IIIC: macroscopic peritoneal metastasis beyond the pelvis more than 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes [includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ] | T3c-N0/N1-M0 |
| Stage IV. Distant metastasis excluding peritoneal metastases | |
| Stage IVA: pleural effusion with positive cytology | Any T, any N, M1a |
| Stage IVB: parenchymal metastases and metastases to extra-abdominal organs [including inguinal lymph nodes and lymph nodes outside of the abdominal cavity] | Any T, any N, M1b |
| Title | Conditions | Interventions | No. Participants Planned | Primary Outcome Measure | DWI Assessments | Single/ Multicentre | Location |
|---|---|---|---|---|---|---|---|
| Imaging Study in Advanced Ovarian Cancer | Ovarian cancer | Diagnostic Test: Ultrasound, CT and WBDWI/MR | 400 | Preoperative identification of patients with ovarian/tubal cancer in whom optimal debulking (R0/R1) can not be achieved by US and CT scan | Qualitative | Single | Gynecologic Oncology Center in Prague, Prague, Czechia |
| Clinical Impact of Dedicated MR Staging of Ovarian Cancer | Ovarian cancer | Other: MRI | 270 | Diagnostic performance of DW-MRI to predict a complete cytoreductive surgery | Qualitative | Single | NKI-AVL, Amsterdam, Netherlands |
| Value of MRI in the Characterization of Ovarian Masses Unable to Classify With Ultrasound Using the International Ovarian Tumor Analysis (IOTA) Simple Rules | Patients With a Sonographically Unclassifiable Adnexal Mass Using the IOTA Simple Rules | Other: Diffusion/Perfusion-weighted Magnetic Resonance Imaging | 250 | The sensitivity and specificity of the ADNEXMR SCORING system in classifying adnexal masses as malignant or benign using MRI with diffusion- and perfusion-weighted sequences in masses unclassified by the IOTA Simple Rules. Gold standard is histopathology diagnosis within 120 days after ultrasound examination. | Radiologist scoring | Single | University Hospitals Leuven, Leuven, Belgium |
| Benchmarking Intra-tumor Heterogeneity In Ovarian Cancer: Linking In-vivo Imaging Phenotypes With Histology And Genomics | Ovarian cancer | Procedure: 18FDG-PET/CT Scan Procedure: MRI | 26 | Genomic markers of spatial heterogeneity by evaluating spatially explicit phenotypic clusters based on a combination of perfusion, diffusion and metabolic tumor profiles (maps) in both ovarian tumors and metastatic peritoneal/omental implants of patients with HGSOC undergoing primary debulking surgery. | Quantitative | Single | Memorial Sloan Kettering West Harrison, Harrison, New York, United States
|
| Whole-body Diffusion MRI for Staging, Response Prediction and Detecting Tumor Recurrence in Patients With Ovarian Cancer | Ovarian carcinoma | Other: Whole body DW-MRI | 350 | WB-DW-MRI for tumor characterization and staging at primary diagnosis and response prediction to neoadjuvant chemotherapy | Qualitative | Single | University Hospitals UZ Leuven, Gasthuisberg, Leuven, Belgium |
| Diffusion-weighted Imaging Study in Cancer of the Ovary | Ovarian Cancer Peritoneal Metastases | DW-MRI | 134 | To assess the reproducibility of quantitative diffusion-weighted magnetic resonane imaging (DW-MRI) for visualising peritoneal metastases in a multi-centre setting and biologically validate the measurements by correlating scan data (ADC change) following chemotherapy with histology of the tumor (amount of cell death) at surgery | Quantitative | Multicentre |
|
| Whole Body Diffusion MRI for Non-invasive Lesion Detection and Therapy Follow-up: Study With Patients With Ovarian Cancer and Peritoneal Metastasis | Ovarian Cancer Peritoneal Metastases | Procedure: intravenous contrast administration | 50 | To evaluate which of the two treatments (primary debulking surgery followed chemotherapy versus platinum-based neoadjuvant chemotherapy followed by interval debulking surgery, followed in turn by chemotherapy) is the best option for a particular type of patient. | Qualitative | Single | University Hospital Gasthuisberg, Leuven, Belgium |
| Evaluation of Response to the Neoadjuvant Chemotherapy for Advanced Ovarian Cancer by Multimodal Functional Imaging | Ovarian carcinoma | Procedure: 18FDG-PET/CT and DW-MRI before and after 4 cycles of neoadjuvant chemotherapy | 11 | Inter-rater Reliability of Magnetic Resonance Imaging (MRI) Apparent Diffusion Coefficient (ADC) | Quantitative | Single |
|
| Clinical Need | Pearls | Pitfalls |
|---|---|---|
| Technical performance |
|
|
| Detecting and Characterising malignant lesions |
|
|
| Staging and Resectability |
|
|
| Response assessment |
|
|
| Incorporation into clinical trials |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, T.; Adejolu, M.; deSouza, N.M. Diffusion-Weighted Magnetic Resonance Imaging in Ovarian Cancer: Exploiting Strengths and Understanding Limitations. J. Clin. Med. 2022, 11, 1524. https://doi.org/10.3390/jcm11061524
Gagliardi T, Adejolu M, deSouza NM. Diffusion-Weighted Magnetic Resonance Imaging in Ovarian Cancer: Exploiting Strengths and Understanding Limitations. Journal of Clinical Medicine. 2022; 11(6):1524. https://doi.org/10.3390/jcm11061524
Chicago/Turabian StyleGagliardi, Tanja, Margaret Adejolu, and Nandita M. deSouza. 2022. "Diffusion-Weighted Magnetic Resonance Imaging in Ovarian Cancer: Exploiting Strengths and Understanding Limitations" Journal of Clinical Medicine 11, no. 6: 1524. https://doi.org/10.3390/jcm11061524
APA StyleGagliardi, T., Adejolu, M., & deSouza, N. M. (2022). Diffusion-Weighted Magnetic Resonance Imaging in Ovarian Cancer: Exploiting Strengths and Understanding Limitations. Journal of Clinical Medicine, 11(6), 1524. https://doi.org/10.3390/jcm11061524






