Abstract
Tendinopathy is a process of chaotic extracellular matrix remodeling followed by increased secretion of enzymes and mediators of inflammation. The histopathological assessment of tendinous tissue is crucial to formulate the diagnosis and establish the severity of tendon degeneration. Nevertheless, the microscopic analysis of tendinous tissue features is often challenging. In this review, we aimed to compare the most popular scales used in tendon pathology assessment and reevaluate the role of the neovascularization process. The following scores were evaluated: the Bonar score, the Movin score, the Astrom and Rausing Score, and the Soslowsky score. Moreover, the role of neovascularization in tendon degeneration was reassessed. The Bonar system is the most commonly used in tendon pathology. According to the literature, hematoxylin and eosin with additional Alcian Blue staining seems to provide satisfactory results. Furthermore, two observers experienced in musculoskeletal pathology are sufficient for tendinopathy microscopic evaluation. The control, due to similar and typical alterations in tendinous tissue, is not necessary. Neovascularization plays an ambiguous role in tendon disorders. The neovascularization process is crucial in the tendon healing process. On the other hand, it is also an important component of the degeneration of tendinous tissue when the regeneration is incomplete and insufficient. The microscopic analysis of tendinous tissue features is often challenging. The assessment of tendinous tissue using the Bonar system is the most universal. The neovascularization variable in tendinopathy scoring systems should be reconsidered due to discrepancies in studies.
1. Tendon Histology and Pathology
Tendons are structures necessary to distribute the force generated by muscles [1,2,3]. They are subjected to extraordinary loads, which may lead to the development of pathology. Tendons are designed to contribute to human body movements, to stabilize joints, and to absorb the kinetic energy [3]. The structure of tendon is firm and fibro-elastic in texture, with a brilliant white color [4]. There are various types of tendons, such as flat, cylindrical, fan, and ribbon shaped [3]. Franchi et al. emphasized that short and thick tendons are responsible for transmitting high torques or resistive forces. On the contrary, long and thin tendons are responsible for soft and delicate movements [3]. The adaptive properties of tendinous tissue result from the highly organized structure, which can be described as a morphology resembling a synthetic climbing rope [5]. This special construction of tendons into subsequent subunits ensures a more uniform spread of loads and decreases the possibility of damage [5,6].
In the structure of the tendon, two elements can be distinguished: cells and extracellular matrix. The extracellular matrix acts as a scaffolding for cells, vessels, and nerves and is responsible for the strength of tendon [4,7]. It contains collagen molecules together with some non-collagenous substances, e.g., elastin, glycosaminoglycans, proteoglycans, glycoproteins, and water [8,9]. Type I collagen accounts for approximately 60–90% of the tendon dry mass. However, some other collagen types can also be found in the tendon structure, which are mainly III and V types [4,5]. Type I collagen is responsible for tendon tensile forces. Synergistically with type III, it forms heterotypic fibrils. Type III collagen is involved in tendon healing and forms cross-links. On the other hand, type V collagen forms a core for type I collagen fibrils and controls the lateral growth of the tendon [8].
Moreover, the collagen fibrils are surrounded by loose connective tissue, called endotenon. It complements the tendon structure and functions as energy storage. In turn, every collagen fascicle is surrounded by another connective tissue sheath: peritenon. These connective tissue elements provide access to blood and lymphatic vessels and innervation. The whole tendon is covered by epitenon, which is an additional connective tissue barrier [10].
The most frequent non-fibrous proteins in tendons are proteoglycans, which constitute 1–5% of a tendon dry mass [6,9,11,12]. Their main components are core proteins connected with polysaccharide chains, known as glycosaminoglycans side chains. Due to the possibility of binding large amounts of water, they guarantee strong hydration of the tendon. As a result, 55–70% of its mass is water. The most abundant proteoglycans are small leucine-rich types, of which decorin is the most widespread. Additionally, some other proteins, e.g., fibromodulin, biglycan, lumican, and keratocan, occur in smaller amounts. They lubricate and separate the elements of the tendon and participate in fibrillogenesis and matrix assembly. Other extracellular matrix proteins are glycoproteins. Among them, the most common is the cartilage oligomeric matrix protein (COMP) [6,13,14,15]. Glycoproteins control the cell-to-cell interactions as well as adhesion and transduce mechanical stimuli to the cytoplasm in the mechanotransduction process [6]. During the mechanotransduction process, tendons collect information about the load and signals from the local environment. As a result, the metabolism of tenocytes and the influence on the local mediators can be easily regulated [5]. Tendon disorders can be divided into acute and chronic [1,5]. Various factors may increase the risk of tendinous tissue pathology. Among them are sex, age, smoking, chronic diseases (e.g., type 2 diabetes, thyroid diseases, rheumatologic diseases, inherited diseases, mellitus, obesity), nutrition, occupation, hyperthermia, fluoroquinolone antibiotics, corticosteroids, joint instability, bone impingements, and tendon overloading during exercise, sport, and other physical activity [4,5,11,16,17,18]. Both acute and chronic injuries may completely or partially break tendon continuity. However, acute injuries have a higher probability of complete regeneration [19,20]. In turn, chronic injuries, which arise from overload and lead to tissue degeneration, are known as tendinopathies. They cause mechanical dysfunction of the tendon [21]. Tendinopathy is often associated with physical exercises and represents approximately 30–50% of all injuries connected with sport [1,22]. Injuries or repeated strain may lead to pain and swelling, which usually cause difficulties in movement [23,24]. Joseph et al. noted that tendinopathy is characterized by impaired healing of the extracellular matrix, with a limited amount of inflammatory cells, collagen degeneration, and increased proteoglycans and type III collagen levels [2,25,26]. Collagen fibers lose their hierarchical structure and become more irregular and spacious. Moreover, some pathological alterations of cells, non-collagenous ECM, nerves, and the vascular bed appear [17,21,27,28,29]. There is no clear link between clinics and histopathology in tendinopathy.
Similarly, the ultrastructure of tenocytes in tendinopathy has revealed a few characteristic changes. They become randomly scattered around cells, with disrupted localization and morphology of the nuclei [7,30]. The transformation of the shape of tenocytes into a more oval shape could be evidence of cartilage metaplasia and adaptation. It is thought that the cytoplasm and cellular shape variations reflect the gradual chondroid transformation [7,29]. Furthermore, some authors have noted the presence of apoptotic-like features in tenocytes, such as chromatin alterations and nuclei fragmentation [4,31].
Regarding the ECM, a replacement of collagen fibrils by non-collagenous ECM, with a subsequent decrease in the number of crimps, has been observed [6]. Tendinopathy is a process of a chaotic extracellular matrix remodeling and the increased secretion of enzymes, proteins, and mediators of inflammation, such as metalloproteinases, TNF-α, IL-1β, IL-6, IL-10, VEGF, TGF-β, and PGE2 [2,25]. There is a group of tendons especially prone to injuries due to their specific biology and localization [1,5,18,24,32,33,34,35]. Usually, these tendons are exposed to increased forces with various vectors of action, e.g., rotator cuff tendons, long head of the biceps tendon, Achilles tendon, posterior tibialis tendon, patellar tendon, gluteal tendons, and tibialis anterior tendon [26,35,36,37,38,39]. Moreover, these tendons contain hypovascular or avascular areas, so-called watershed regions, additionally predisposing them to pathology [2].
In this review, we aimed to compare the most popular scales used in tendon pathology assessment and reevaluate the role of the neovascularization process in tendinous tissue degeneration.
2. Classification of Histopathology and Current Trends
The histopathological assessment of tendinous tissue is crucial to formulate a diagnosis and establish the severity of tendon degeneration [29,40]. Nevertheless, microscopic analysis of the tendinous tissue features is often challenging and difficult. In this review, we present a few of the most popular scales used in tendon pathology assessment: the Bonar score, the Movin score, the Astrom and Rausing Score, and the Soslowsky score (Table 1).

Table 1.
The most popular scoring systems in tendon pathology assessment.
Assessment using the Movin and Bonar systems is the most commonly used in tendon pathology [28,29]. The variables in the Movin scale are the fiber structure, fiber arrangement, rounding of the nuclei, regional variations in cellularity, increased vascularity, decreased collagen stainability, hyalinization, and glycosaminoglycans content. Each parameter is scored with a four-point scoring system. The final score is between 0 (healthy tendon) and 24 (severely degenerated tendon). Tissue can be classified as slightly abnormal when the final score is between 1 and 8; moderately abnormal when the final score reaches values between 9 and 16; markedly abnormal when the final score is between 17 and 24. On the other hand, the variables in the Bonar scale are tenocyte morphology alterations, ground substance accumulation, extent of neovascularization, and collagen bundle architecture. Each variable is scored with a four-point scale, where 0 indicates healthy tissue and 3 indicates severely degenerated tissue. The total score ranges from 0 (normal tendon) to 12 (the most severe abnormality).
The less popular scales are the Astrom and Rausing Score and Soslowsky score [40,41,42]. The Astrom and Rausing Score is also a semiquantitative score to evaluate tendinopathy. It includes five parameters scored with a four-point scale. The final score varies between 0 (normal tendon) and 20 (the most severe degeneration). The Soslowsky score, used to investigate rotor cuff diseases, considers five histologic features of tendinosis, scored with a four-point scale. The final score reaches values between 0 (normal tendon) and 16 (the most severe degeneration).
These scoring systems introduce similar variables, but they do not determine some of the pivotal issues. These include specimen preparation, staining methods, additional immunohistochemical reactions, the number of investigators, the experience of investigators, the area of investigation, and a certain magnification. These features have not yet been well-established, and they are usually selected randomly by authors [27,28,37,43,44,45,46,47].
Regarding the staining methods, the H&E is the gold standard, but some authors support it with Alcian Blue and Masson Trichrome staining to visualize the ECM alterations [21,29,48,49,50]. Moreover, some of the authors have included in the evaluation of tendinous tissue IHC techniques to assess neovascularization, using CD31 and CD34 [44,50] type I collagen [51], or the apoptosis process [52].
The number of microscopic investigators in various papers differs from one to three observers. However, in the majority of articles, the number of investigators was not specified [27,28,37,43,51]. In our opinion, at least two observers experienced in MSK pathology are necessary to avoid bias. The inter-observer reliability in the Bonar system was evaluated by Fearon et al. The authors revealed that after a few modifications, the revised Bonar score was characterized by satisfactory inter-tester reliability; r2 = 0.71 [28].
Furthermore, authors usually randomly select the evaluated area of the slide. In some papers, the most severely degenerated area was chosen, while in other cases the entire slide was evaluated [29,44,47,49]. Regarding the modifications of the scoring systems, the four-variable Bonar scale was most commonly applied. Some of the authors introduced a fifth variable, counting the number of tenocytes [28,37,51,52,53]. Additionally, in some studies, a control group was included. Alterations in the course of tendinopathy are usually similar in all tendon structures. The characteristic changes include disrupted collagen architecture, tenocyte morphological alterations, neovascularization process, and expansion of the ground substance. Thus, the control group should not be obligatory, especially when experienced musculoskeletal observers assess the specimen.
3. The Issue of Neovascularization
The neovascularization process is typical for osteoarthritis, retinopathy, inflammation, tumors, and tendon disorders [18]. In healthy tendons, vascularization is modest, with a small number of capillaries localized between bundles of collagen in the ECM. These capillaries mainly arise from the musculotendinous junction, osteotendinous junction, and connective tissue sheath [54]. Tendons are metabolically active structures, and like other tissues, they require a blood supply. However, the vascular perfusion is relatively weak compared to other types of connective tissue [16,54]. Moreover, tendons usually contain specific hypovascular regions, which heal poorly and are especially prone to degeneration [55]. On the other hand, tendinopathy manifests microscopically as a degenerative process. It is also characterized by the expansion of newly formed capillaries, followed by chaotic production of ECM components [17,44,56].
Recent studies have revealed that neovascularization in tendon disorders has a mythological status and does not necessarily agree with clinics [55,57]. The Achilles tendon is the most often injured tendon in humans. Its tendinopathy usually occurs with an abundant neovascularization process [58]. Hypothetically, concomitant nerve ending ingrowth is responsible for pain in this disorder. Neurovascular ingrowth in clinically painful areas of tendons has been described in a few studies, which revealed a positive correlation of the pain level and the density of capillary vessels [16,59,60]. Numerous authors presented US-guided techniques to decrease the level of neovascularization and inject the sclerosants in the most vascularized areas of the tendon [58]. However, some studies clearly showed that there is no connection between neoangiogenesis and pain [44,61,62]. Neovascularization was also found in Achilles tendons among asymptomatic athletes [63].
Some authors observed in a tendinous tissue a surprising phenomenon: cigarette smoking inhibits the neovascularization process [17,64]. Although the level of neovascularization in smoking individuals showed greater resemblance to healthy tissue and underestimated the microscopic evaluation score, it was not consistent with the clinical outcome. Nevertheless, nicotine increases the neovascularization process in macular degeneration, which is associated with obstetric complications in pregnancy or spontaneous miscarriage [65,66,67]. The issue of smoking shows the importance of the neovascularization process in tendinopathy. However, the microscopic evaluation of the pathological role of the new vessel formation is not simple and may not be linked with clinics. Regarding the pathology and microscopic assessment of tendinous tissue, Fearon et al. revealed that the four-variable Bonar score may be biased by several factors [28]. The authors suggested that the complete lack of vascularity in the obtained pathological tendons, as well as the excessive expansion of new capillaries, should be graded as 3 points (extreme pathology). Moreover, the area of the neoangiogenesis investigation is also a subject of dispute. Some authors assess the most pathological area of tendinous tissue, while others evaluate the entire specimen [28,44,48,49]. Furthermore, some also supported their studies with immunohistochemical methods, with the use of CD31, CD34, factor VIII-related antigen, CD105, or smooth muscle actin [44,68,69]. Tendinous tissue after the injury undergoes a regeneration process, which consists of three main phases. In the formation phase, an intensive neoangiogenesis is usually observed [54] (Figure 1).
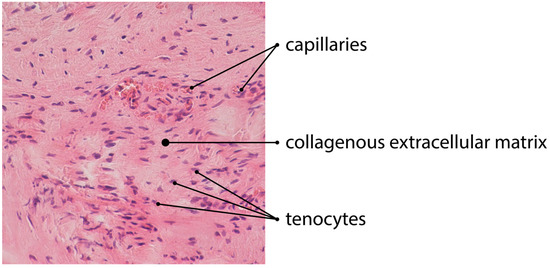
Figure 1.
H&E staining of tendinous tissue showing angiogenesis (magnification of the objective: 20×).
The process is specifically regulated by the local microenvironment components, such as mediators and ECM [62]. However, the exact levels of these mediators are still unknown. The ECM regulates the topology and elongation of vessels, using the proteases to pave the way for new capillaries [70]. Some authors observed that angiogenesis is significantly reduced in the areas of the tendinous tissue, with the increased production and aggregation of non-collagenous ECM [17,71] (Figure 2 and Figure 3).
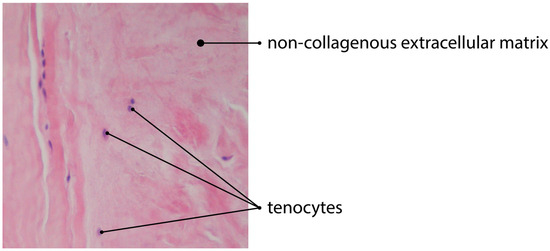
Figure 2.
H&E staining of tendinous tissue showing impaired angiogenesis due to deposition of non-collagenous ECM (magnification of the objective: 20×).
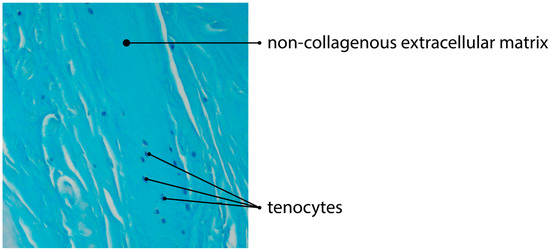
Figure 3.
Alcian blue staining of tendinous tissue showing impaired angiogenesis due to deposition of non-collagenous ECM (magnification of the objective: 20×).
Koehler et al., using a 3D angiogenesis model, demonstrated that glycosaminoglycan accumulation impaired the biological activity of VEGF [72]. Furthermore, Cheng et al. revealed that non-collagenous components of the ECM inhibit VEGF receptor signaling [73]. VEGF levels are elevated after the inflammatory phase, during the formation and remodeling phases [5]. Its main role is the stimulation of the angiogenesis process [74]. The neovascularization process is crucial in the tendon healing process. On the other hand, it is also an important component of the degeneration of tendinous tissue, when the regeneration is incomplete and insufficient.
4. Conclusions
The microscopic analysis of the tendinous tissue features is often challenging and difficult. The assessment using the Bonar system is the most commonly used in tendon pathology. According to the literature, H&E staining with additional Alcian Blue seems to provide sufficient material for the observer. Moreover, two investigators experienced in musculoskeletal pathology ensure a satisfying microscopic evaluation of tendinopathy. The control, due to similar and typical alterations in tendinous tissue, is not necessary. Neovascularization plays an ambiguous role in tendon disorders. Thus, this uncertain variable should be reconsidered and updated in tendinopathy scoring systems.
Author Contributions
Conceptualization, Ł.J., M.G. and D.G.; methodology, Ł.J. and M.G.; software, M.Z.; validation, D.G., A.K.-W. and W.Z.; formal analysis, D.G. and M.G.; investigation, M.G., M.Z. and Ł.J.; resources, M.G. and M.Z.; data curation, M.G. and Ł.J.; writing—original draft preparation, M.G., W.Z. and Ł.J.; writing—review and editing, D.G.; visualization, M.Z.; supervision, D.G., A.K.-W. and M.G.; project administration, M.Z. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon Basic Science: Development, Repair, Regeneration, and Healing: Tendon Development, Injury, and Repair. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabrzyński, J.; Zabrzyńska, A.; Grzanka, D. Tendinopathy–a Disease of Tendons. Orthop. Trauma Surg. Related Res. 2016, 3, 024–030. [Google Scholar]
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen Structure of Tendon Relates to Function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Murrell, G.A.C. The Basic Science of Tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Zabrzyński, J.; Łapaj, Ł.; Paczesny, Ł.; Zabrzyńska, A.; Grzanka, D. Tendon-Function-Related Structure, Simple Healing Process and Mysterious Ageing. Folia Morphol. 2018, 77, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Gagat, M.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D. Electron Microscope Study of the Advanced Tendinopathy Process of the Long Head of the Biceps Brachii Tendon Treated Arthroscopically. Folia Morphol. 2018, 77, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birk, D.E.; Mayne, R. Localization of Collagen Types I, III and V during Tendon Development. Changes in Collagen Types I and III Are Correlated with Changes in Fibril Diameter. Eur. J. Cell Biol. 1997, 72, 352–361. [Google Scholar]
- Vogel, K.G.; Ordog, A.; Pogany, G.; Oláh, J. Proteoglycans in the Compressed Region of Human Tibialis Posterior Tendon and in Ligaments. J. Orthop. Res. 1993, 11, 68–77. [Google Scholar] [CrossRef]
- Jozsa, L.; Kannus, P.; Balint, J.B.; Reffy, A. Three-Dimensional Ultrastructure of Human Tendons. Acta Anat. 1991, 142, 306–312. [Google Scholar] [CrossRef]
- Riley, G. The Pathogenesis of Tendinopathy. A Molecular Perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waggett, A.D.; Ralphs, J.R.; Kwan, A.P.L.; Woodnutt, D.; Benjamin, M. Characterization of Collagens and Proteoglycans at the Insertion of the Human Achilles Tendon. Matrix Biol. 1998, 16, 457–470. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. In Metabolic Influences on Risk for Tendon Disorders; Ackermann, P.W., Hart, D.A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 920, pp. 3–10. ISBN 978-3-319-33941-2. [Google Scholar]
- Zhang, G.; Chen, S.; Goldoni, S.; Calder, B.W.; Simpson, H.C.; Owens, R.T.; McQuillan, D.J.; Young, M.F.; Iozzo, R.V.; Birk, D.E. Genetic Evidence for the Coordinated Regulation of Collagen Fibrillogenesis in the Cornea by Decorin and Biglycan. J. Biol. Chem. 2009, 284, 8888–8897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. The Role of the Non-Collagenous Matrix in Tendon Function. Int. J. Exp. Path. 2013, 94, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of Tendinopathies: Inflammation or Degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabrzynski, J.; Gagat, M.; Paczesny, L.; Grzanka, D.; Huri, G. Correlation between Smoking and Neovascularization in Biceps Tendinopathy: A Functional Preoperative and Immunohistochemical Study. Ther. Adv. Chronic Dis. 2020, 11, 204062232095641. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Wilson, A.M.; Wolman, R.L. Current Concepts in the Management of Tendon Disorders. Rheumatology 2006, 45, 508–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for Tendon Repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-N.; Huang, Y.-C.; Ni, G.-X. Mechanotransduction of Stem Cells for Tendon Repair. WJSC 2020, 12, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Is the Inflammation Process Absolutely Absent in Tendinopathy of the Long Head of the Biceps Tendon? Histopathologic Study of the Long Head of the Biceps Tendon after Arthroscopic Treatment. Pol. J. Pathol. 2017, 68, 318–325. [Google Scholar] [CrossRef]
- Kannus, P.; Józsa, L.; Natri, A.; Järvinen, M. Effects of Training, Immobilization and Remobilization on Tendons. Scand. J. Med. Sci. Sports 1997, 7, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Backman, L.J.; Speed, C. Tendinopathy: Update on Pathophysiology. J. Orthop. Sports Phys. Ther. 2015, 45, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaux, J.-F.; Forthomme, B.; Goff, C.L.; Crielaard, J.-M.; Croisier, J.-L. Current Opinions on Tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar] [PubMed]
- Joseph, M.; Maresh, C.M.; McCarthy, M.B.; Kraemer, W.J.; Ledgard, F.; Arciero, C.L.; Anderson, J.M.; Nindl, B.C.; Mazzocca, A.D. Histological and Molecular Analysis of the Biceps Tendon Long Head Post-Tenotomy. J. Orthop. Res. 2009, 27, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Huri, G.; Gryckiewicz, S.; Çetik, R.M.; Szwedowski, D.; Łapaj, Ł.; Gagat, M.; Paczesny, Ł. Biceps Tenodesis versus Tenotomy with Fast Rehabilitation Protocol-A Functional Perspective in Chronic Tendinopathy. J. Clin. Med. 2020, 9, 3938. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Feller, J.A.; Bonar, S.F.; Khan, K.M. Abnormal Tenocyte Morphology Is More Prevalent than Collagen Disruption in Asymptomatic Athletes’ Patellar Tendons. J. Orthop. Res. 2004, 22, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.; Dahlstrom, J.E.; Twin, J.; Cook, J.; Scott, A. The Bonar Score Revisited: Region of Evaluation Significantly Influences the Standardized Assessment of Tendon Degeneration. J. Sci. Med. Sport 2014, 17, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Maffulli, N.; Longo, U.G.; Franceschi, F.; Rabitti, C.; Denaro, V. Movin and Bonar Scores Assess the Same Characteristics of Tendon Histology. Clin. Orthop. Relat. Res. 2008, 466, 1605–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingel, J.; Lu, Y.; Starborg, T.; Fredberg, U.; Langberg, H.; Nedergaard, A.; Weis, M.; Eyre, D.; Kjaer, M.; Kadler, K.E. 3-D Ultrastructure and Collagen Composition of Healthy and Overloaded Human Tendon: Evidence of Tenocyte and Matrix Buckling. J. Anat. 2014, 224, 548–555. [Google Scholar] [CrossRef]
- Galliani, I.; Burattini, S.; Mariani, A.; Riccio, M.; Cassiani, G.; Falcieri, E. Morpho-Functional Changes in Human Tendon Tissue. Eur. J. Histochem. 2009, 46, 3. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Maffulli, N. Biology of Tendon Injury: Healing, Modeling and Remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Gruchow, H.W.; Pelletier, D. An Epidemiologic Study of Tennis Elbow. Incidence, Recurrence, and Effectiveness of Prevention Strategies. Am. J. Sports Med. 1979, 7, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, P.; Polguj, M.; Podgórski, M.; Majos, A.; Krochmalski, M.; Domżalski, M. Patellar Ligament Hypertrophy Evaluated by Magnetic Resonance Imaging in a Group of Professional Weightlifters. Folia Morphol. 2012, 71, 240–244. [Google Scholar]
- Albano, D.; Martinelli, N.; Bianchi, A.; Romeo, G.; Bulfamante, G.; Galia, M.; Sconfienza, L.M. Posterior Tibial Tendon Dysfunction: Clinical and Magnetic Resonance Imaging Findings Having Histology as Reference Standard. Eur. J. Radiol. 2018, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, A.; Pozza, S.; Cenna, E.; Cavallo, F.; Gays, G.; Simbula, L.; De Petro, P.; Massè, A.; Massazza, G. In Achilles Tendinopathy, the Neovascularization, Detected by Contrast-Enhanced Ultrasound (CEUS), Is Abundant but Not Related to Symptoms. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Cook, J.; Chen, S.; Scarvell, J.; Cormick, W.; Smith, P.; Fearon, A. Identification and Differentiation of Gluteus Medius Tendon Pathology Using Ultrasound and Magnetic Resonance Imaging. Musculoskelet. Sci. Pract. 2019, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Lian, Ø.; Roberts, C.; Cook, J.; Handley, C.; Bahr, R.; Samiric, T.; Ilic, M.; Parkinson, J.; Hart, D.; et al. Increased Versican Content Is Associated with Tendinosis Pathology in the Patellar Tendon of Athletes with Jumper’s Knee. Scand. J. Med. Sci. Sports 2008, 18, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagat, M.; Zabrzyńska, A.; Zabrzyńska, M.; Zielińska, W. The Advances in Diagnostic Modalities of Disorders of the Long Head of the Biceps Tendon–Review. Med. Stud. 2021, 37, 83–90. [Google Scholar] [CrossRef]
- Loppini, M.; Longo, U.; Niccoli, G.; Khan, W.; Maffulli, N.; Denaro, V. Histopathological Scores for Tissue-Engineered, Repaired and Degenerated Tendon: A Systematic Review of the Literature. CSCR 2014, 10, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Aström, M.; Rausing, A. Chronic Achilles Tendinopathy. A Survey of Surgical and Histopathologic Findings. Clin. Orthop. Relat. Res. 1995, 316, 151–164. [Google Scholar] [CrossRef]
- Soslowsky, L.J.; Carpenter, J.E.; DeBano, C.M.; Banerji, I.; Moalli, M.R. Development and Use of an Animal Model for Investigations on Rotator Cuff Disease. J. Shoulder Elb. Surg. 1996, 5, 383–392. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Paczesny, Ł.; Zabrzyńska, A.; Grzanka, D.; Łapaj, Ł. Sonography in the Instability of the Long Head of the Biceps Tendon Confronted with Histopathologic and Arthroscopic Findings. Folia Morphol. 2018, 77, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Process of Neovascularisation Compared with Pain Intensity in Tendinopathy of the Long Head of the Biceps Brachii Tendon Associated with Concomitant Shoulder Disorders, after Arthroscopic Treatment. Microscopic Evaluation Supported by Immunohistochemical. Folia Morphol. 2018, 77, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgreen, K.; Lian, O.B.; Scott, A.; Nassab, P.; Fearon, A.; Engebretsen, L. Rotator Cuff Tear Degeneration and Cell Apoptosis in Smokers versus Nonsmokers. Arthroscopy 2014, 30, 936–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, L.; Vachon, P.; Beaudry, F.; Langelier, E. Histopathological, Biomechanical, and Behavioral Pain Findings of Achilles Tendinopathy Using an Animal Model of Overuse Injury. Physiol. Rep. 2015, 3, e12265. [Google Scholar] [CrossRef]
- Kurdziel, M.D.; Moravek, J.E.; Wiater, B.P.; Davidson, A.; Seta, J.; Maerz, T.; Baker, K.C.; Wiater, J.M. The Impact of Rotator Cuff Deficiency on Structure, Mechanical Properties, and Gene Expression Profiles of the Long Head of the Biceps Tendon (LHBT): Implications for Management of the LHBT during Primary Shoulder Arthroplasty: The Impact of Rotator Cuff Deficiency. J. Orthop. Res. 2015, 33, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Sethi, P.M.; Sheth, C.D.; Pauzenberger, L.; McCarthy, M.B.R.; Cote, M.P.; Soneson, E.; Miller, S.; Mazzocca, A.D. Macroscopic Rotator Cuff Tendinopathy and Histopathology Do Not Predict Repair Outcomes of Rotator Cuff Tears. Am. J. Sports Med. 2018, 46, 779–785. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ochiai, N.; Hashimoto, E.; Sasaki, Y.; Yamaguchi, T.; Kijima, T.; Akimoto, K.; Ohtori, S.; Takahashi, K. Relationship between Neuropathy Proximal to the Suprascapular Nerve and Rotator Cuff Tear in a Rodent Model. J. Orthop. Sci. 2018, 23, 414–419. [Google Scholar] [CrossRef]
- Nuelle, C.W.; Stokes, D.C.; Kuroki, K.; Crim, J.R.; Sherman, S.L. Radiologic and Histologic Evaluation of Proximal Bicep Pathology in Patients With Chronic Biceps Tendinopathy Undergoing Open Subpectoral Biceps Tenodesis. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1790–1796. [Google Scholar] [CrossRef]
- Okazaki, Y.; Furumatsu, T.; Maehara, A.; Miyazawa, S.; Kamatsuki, Y.; Hino, T.; Ozaki, T. Histological Alterations to the Hamstring Tendon Caused by Cleaning during Autograft Preparation. Muscle Ligaments Tendons J. 2019, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Lundgreen, K.; Lian, Ø.; Scott, A.; Engebretsen, L. Increased Levels of Apoptosis and P53 in Partial-Thickness Supraspinatus Tendon Tears. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.M.; Twin, J.; Dahlstrom, J.E.; Cook, J.L.; Cormick, W.; Smith, P.N.; Scott, A. Increased Substance P Expression in the Trochanteric Bursa of Patients with Greater Trochanteric Pain Syndrome. Rheumatol. Int. 2014, 34, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, S.A.; Hazleman, B.L.; Riley, G.P. The Vasculature and Its Role in the Damaged and Healing Tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditsios, K.; Agathangelidis, F.; Boutsiadis, A.; Karataglis, D.; Papadopoulos, P. Long Head of the Biceps Pathology Combined with Rotator Cuff Tears. Available online: https://www.hindawi.com/journals/aorth/2012/405472/ (accessed on 7 November 2018).
- Lewis, J.S.; Raza, S.A.; Pilcher, J.; Heron, C.; Poloniecki, J.D. The Prevalence of Neovascularity in Patients Clinically Diagnosed with Rotator Cuff Tendinopathy. BMC Musculoskelet. Disord. 2009, 10, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tol, J.L.; Spiezia, F.; Maffulli, N. Neovascularization in Achilles Tendinopathy: Have We Been Chasing a Red Herring? Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1891–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfredson, H.; Öhberg, L. Sclerosing Injections to Areas of Neo-Vascularisation Reduce Pain in Chronic Achilles Tendinopathy: A Double-Blind Randomised Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Danielson, P. An Emerging Role for Angiogenesis in Tendinopathy. Eur. Musculoskelet. Rev. 2009, 4, 75–76. [Google Scholar]
- Alfredson, H.; Ohberg, L.; Forsgren, S. Is Vasculo-Neural Ingrowth the Cause of Pain in Chronic Achilles Tendinosis? An Investigation Using Ultrasonography and Colour Doppler, Immunohistochemistry, and Diagnostic Injections. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Singaraju, V.M.; Kang, R.W.; Yanke, A.B.; McNickle, A.G.; Lewis, P.B.; Wang, V.M.; Williams, J.M.; Chubinskaya, S.; Romeo, A.A.; Cole, B.J. Biceps Tendinitis in Chronic Rotator Cuff Tears: A Histologic Perspective. J. Shoulder Elbow Surg. 2008, 17, 898–904. [Google Scholar] [CrossRef]
- de Vos, R.-J.; Weir, A.; Cobben, L.P.J.; Tol, J.L. The Value of Power Doppler Ultrasonography in Achilles Tendinopathy: A Prospective Study. Am. J. Sports Med. 2007, 35, 1696–1701. [Google Scholar] [CrossRef]
- Sengkerij, P.M.; de Vos, R.-J.; Weir, A.; van Weelde, B.J.G.; Tol, J.L. Interobserver Reliability of Neovascularization Score Using Power Doppler Ultrasonography in Midportion Achilles Tendinopathy. Am. J. Sports Med. 2009, 37, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.N.; Newton, J.B.; Boorman-Padgett, J.F.; Weiss, S.N.; Nuss, C.A.; Gittings, D.J.; Farber, D.C.; Soslowsky, L.J. Nicotine Impairs Intra-substance Tendon Healing after Full Thickness Injury in a Rat Model. J. Orthop. Res. 2019, 37, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, D.; Ambati, B.; Adamis, A.P.; Miller, J.W. Mechanisms of Age-Related Macular Degeneration. Ophthalmol. Clin. North Am. 2002, 15, 87–91. [Google Scholar] [CrossRef]
- Chheda, L.V.; Ferketich, A.K.; Carroll, C.P.; Moyer, P.D.; Kurz, D.E.; Kurz, P.A. Smoking as a Risk Factor for Choroidal Neovascularization Secondary to Presumed Ocular Histoplasmosis Syndrome. Ophthalmology 2012, 119, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Koide, K.; Ventura, W.; Hori, K.; Takenaka, S.; Maruyama, D.; Matsuoka, R.; Ichizuka, K.; Sekizawa, A. Effects of Maternal Smoking on the Placental Expression of Genes Related to Angiogenesis and Apoptosis during the First Trimester. PLoS ONE 2014, 9, e106140. [Google Scholar] [CrossRef] [PubMed]
- Bjur, D.; Alfredson, H.; Forsgren, S. The Innervation Pattern of the Human Achilles Tendon: Studies of the Normal and Tendinosis Tendon with Markers for General and Sensory Innervation. Cell Tissue Res. 2005, 320, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Florence, M.E.B.; Massuda, J.Y.; Bröcker, E.-B.; Metze, K.; Cintra, M.L.; de Souza, E.M. Angiogenesis in the Progression of Cutaneous Squamous Cell Carcinoma: An Immunohistochemical Study of Endothelial Markers. Clinics 2011, 66, 465–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivilis, I.; Milkiewicz, M.; Boyd, P.; Goldstein, J.; Brown, M.D.; Egginton, S.; Hansen, F.M.; Hudlicka, O.; Haas, T.L. Differential Involvement of MMP-2 and VEGF during Muscle Stretch- versus Shear Stress-Induced Angiogenesis. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H1430–H1438. [Google Scholar] [CrossRef] [Green Version]
- Edgar, L.T.; Underwood, C.J.; Guilkey, J.E.; Hoying, J.B.; Weiss, J.A. Extracellular Matrix Density Regulates the Rate of Neovessel Growth and Branching in Sprouting Angiogenesis. PLoS ONE 2014, 9, e85178. [Google Scholar] [CrossRef] [Green Version]
- Koehler, L.; Ruiz-Gómez, G.; Balamurugan, K.; Rother, S.; Freyse, J.; Möller, S.; Schnabelrauch, M.; Köhling, S.; Djordjevic, S.; Scharnweber, D.; et al. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci. Rep. 2019, 9, 18143. [Google Scholar] [CrossRef]
- Cheng, J.-J.; Huang, N.-K.; Chang, T.-T.; Ling Wang, D.; Lu, M.-K. Study for Anti-Angiogenic Activities of Polysaccharides Isolated from Antrodia Cinnamomea in Endothelial Cells. Life Sci. 2005, 76, 3029–3042. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T.; Wang, Y.; Murrell, G. The Roles of Growth Factors in Tendon and Ligament Healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).