Advances in Microscopic Studies of Tendinopathy: Literature Review and Current Trends, with Special Reference to Neovascularization Process
Abstract
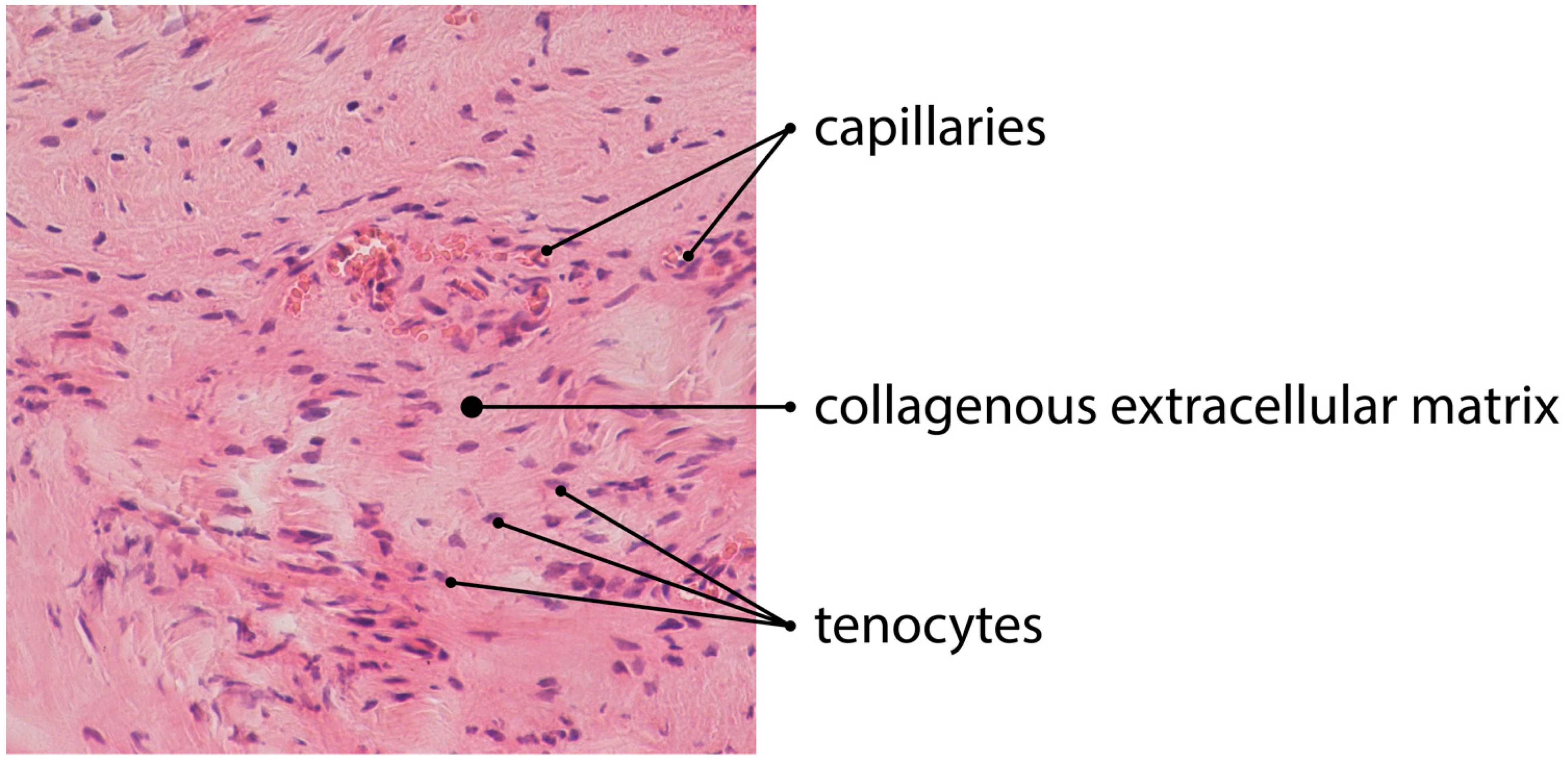
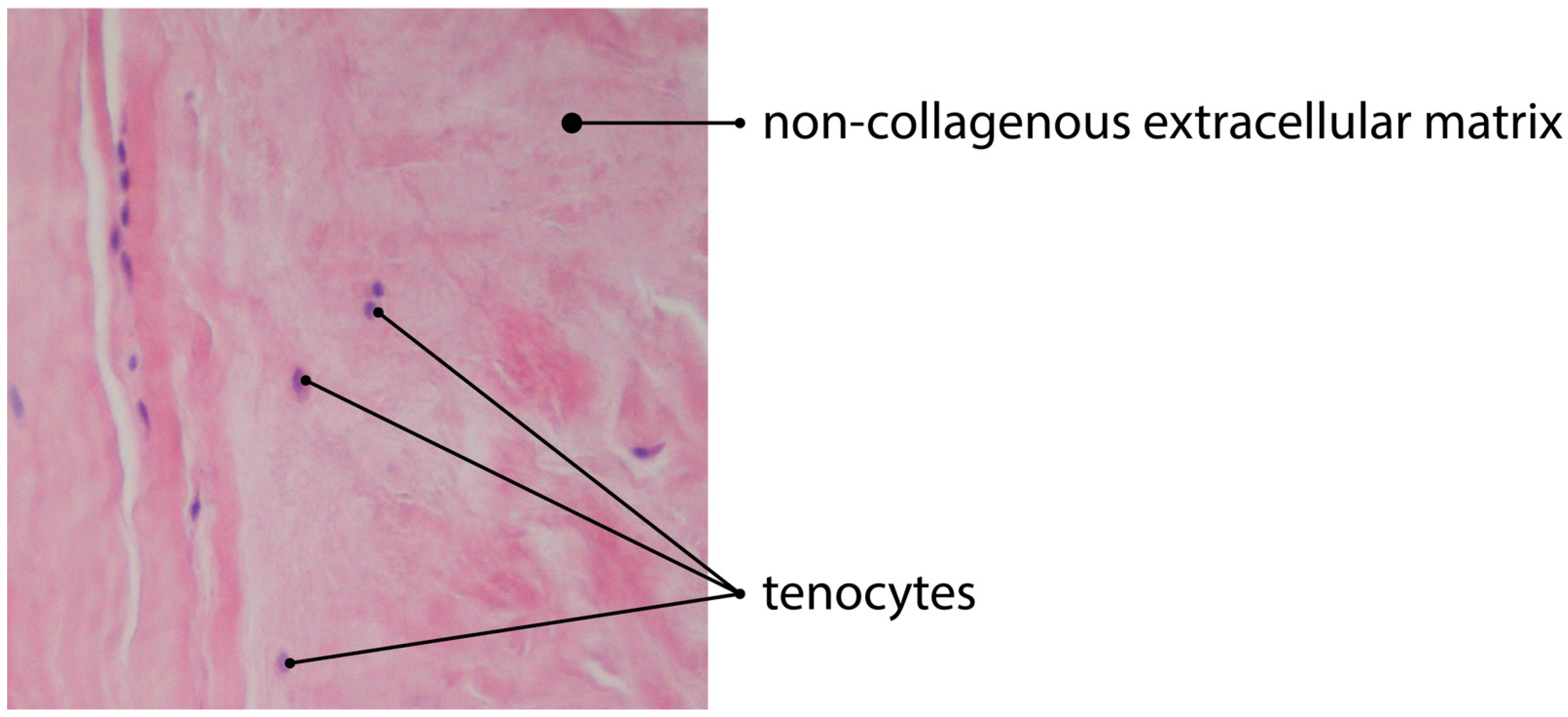
:1. Tendon Histology and Pathology
2. Classification of Histopathology and Current Trends
3. The Issue of Neovascularization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon Basic Science: Development, Repair, Regeneration, and Healing: Tendon Development, Injury, and Repair. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabrzyński, J.; Zabrzyńska, A.; Grzanka, D. Tendinopathy–a Disease of Tendons. Orthop. Trauma Surg. Related Res. 2016, 3, 024–030. [Google Scholar]
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen Structure of Tendon Relates to Function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Murrell, G.A.C. The Basic Science of Tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Zabrzyński, J.; Łapaj, Ł.; Paczesny, Ł.; Zabrzyńska, A.; Grzanka, D. Tendon-Function-Related Structure, Simple Healing Process and Mysterious Ageing. Folia Morphol. 2018, 77, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Gagat, M.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D. Electron Microscope Study of the Advanced Tendinopathy Process of the Long Head of the Biceps Brachii Tendon Treated Arthroscopically. Folia Morphol. 2018, 77, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birk, D.E.; Mayne, R. Localization of Collagen Types I, III and V during Tendon Development. Changes in Collagen Types I and III Are Correlated with Changes in Fibril Diameter. Eur. J. Cell Biol. 1997, 72, 352–361. [Google Scholar]
- Vogel, K.G.; Ordog, A.; Pogany, G.; Oláh, J. Proteoglycans in the Compressed Region of Human Tibialis Posterior Tendon and in Ligaments. J. Orthop. Res. 1993, 11, 68–77. [Google Scholar] [CrossRef]
- Jozsa, L.; Kannus, P.; Balint, J.B.; Reffy, A. Three-Dimensional Ultrastructure of Human Tendons. Acta Anat. 1991, 142, 306–312. [Google Scholar] [CrossRef]
- Riley, G. The Pathogenesis of Tendinopathy. A Molecular Perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waggett, A.D.; Ralphs, J.R.; Kwan, A.P.L.; Woodnutt, D.; Benjamin, M. Characterization of Collagens and Proteoglycans at the Insertion of the Human Achilles Tendon. Matrix Biol. 1998, 16, 457–470. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. In Metabolic Influences on Risk for Tendon Disorders; Ackermann, P.W., Hart, D.A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 920, pp. 3–10. ISBN 978-3-319-33941-2. [Google Scholar]
- Zhang, G.; Chen, S.; Goldoni, S.; Calder, B.W.; Simpson, H.C.; Owens, R.T.; McQuillan, D.J.; Young, M.F.; Iozzo, R.V.; Birk, D.E. Genetic Evidence for the Coordinated Regulation of Collagen Fibrillogenesis in the Cornea by Decorin and Biglycan. J. Biol. Chem. 2009, 284, 8888–8897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. The Role of the Non-Collagenous Matrix in Tendon Function. Int. J. Exp. Path. 2013, 94, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of Tendinopathies: Inflammation or Degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabrzynski, J.; Gagat, M.; Paczesny, L.; Grzanka, D.; Huri, G. Correlation between Smoking and Neovascularization in Biceps Tendinopathy: A Functional Preoperative and Immunohistochemical Study. Ther. Adv. Chronic Dis. 2020, 11, 204062232095641. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Wilson, A.M.; Wolman, R.L. Current Concepts in the Management of Tendon Disorders. Rheumatology 2006, 45, 508–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for Tendon Repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-N.; Huang, Y.-C.; Ni, G.-X. Mechanotransduction of Stem Cells for Tendon Repair. WJSC 2020, 12, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Is the Inflammation Process Absolutely Absent in Tendinopathy of the Long Head of the Biceps Tendon? Histopathologic Study of the Long Head of the Biceps Tendon after Arthroscopic Treatment. Pol. J. Pathol. 2017, 68, 318–325. [Google Scholar] [CrossRef]
- Kannus, P.; Józsa, L.; Natri, A.; Järvinen, M. Effects of Training, Immobilization and Remobilization on Tendons. Scand. J. Med. Sci. Sports 1997, 7, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Backman, L.J.; Speed, C. Tendinopathy: Update on Pathophysiology. J. Orthop. Sports Phys. Ther. 2015, 45, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaux, J.-F.; Forthomme, B.; Goff, C.L.; Crielaard, J.-M.; Croisier, J.-L. Current Opinions on Tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar] [PubMed]
- Joseph, M.; Maresh, C.M.; McCarthy, M.B.; Kraemer, W.J.; Ledgard, F.; Arciero, C.L.; Anderson, J.M.; Nindl, B.C.; Mazzocca, A.D. Histological and Molecular Analysis of the Biceps Tendon Long Head Post-Tenotomy. J. Orthop. Res. 2009, 27, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Huri, G.; Gryckiewicz, S.; Çetik, R.M.; Szwedowski, D.; Łapaj, Ł.; Gagat, M.; Paczesny, Ł. Biceps Tenodesis versus Tenotomy with Fast Rehabilitation Protocol-A Functional Perspective in Chronic Tendinopathy. J. Clin. Med. 2020, 9, 3938. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Feller, J.A.; Bonar, S.F.; Khan, K.M. Abnormal Tenocyte Morphology Is More Prevalent than Collagen Disruption in Asymptomatic Athletes’ Patellar Tendons. J. Orthop. Res. 2004, 22, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.; Dahlstrom, J.E.; Twin, J.; Cook, J.; Scott, A. The Bonar Score Revisited: Region of Evaluation Significantly Influences the Standardized Assessment of Tendon Degeneration. J. Sci. Med. Sport 2014, 17, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Maffulli, N.; Longo, U.G.; Franceschi, F.; Rabitti, C.; Denaro, V. Movin and Bonar Scores Assess the Same Characteristics of Tendon Histology. Clin. Orthop. Relat. Res. 2008, 466, 1605–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingel, J.; Lu, Y.; Starborg, T.; Fredberg, U.; Langberg, H.; Nedergaard, A.; Weis, M.; Eyre, D.; Kjaer, M.; Kadler, K.E. 3-D Ultrastructure and Collagen Composition of Healthy and Overloaded Human Tendon: Evidence of Tenocyte and Matrix Buckling. J. Anat. 2014, 224, 548–555. [Google Scholar] [CrossRef]
- Galliani, I.; Burattini, S.; Mariani, A.; Riccio, M.; Cassiani, G.; Falcieri, E. Morpho-Functional Changes in Human Tendon Tissue. Eur. J. Histochem. 2009, 46, 3. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Maffulli, N. Biology of Tendon Injury: Healing, Modeling and Remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Gruchow, H.W.; Pelletier, D. An Epidemiologic Study of Tennis Elbow. Incidence, Recurrence, and Effectiveness of Prevention Strategies. Am. J. Sports Med. 1979, 7, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, P.; Polguj, M.; Podgórski, M.; Majos, A.; Krochmalski, M.; Domżalski, M. Patellar Ligament Hypertrophy Evaluated by Magnetic Resonance Imaging in a Group of Professional Weightlifters. Folia Morphol. 2012, 71, 240–244. [Google Scholar]
- Albano, D.; Martinelli, N.; Bianchi, A.; Romeo, G.; Bulfamante, G.; Galia, M.; Sconfienza, L.M. Posterior Tibial Tendon Dysfunction: Clinical and Magnetic Resonance Imaging Findings Having Histology as Reference Standard. Eur. J. Radiol. 2018, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, A.; Pozza, S.; Cenna, E.; Cavallo, F.; Gays, G.; Simbula, L.; De Petro, P.; Massè, A.; Massazza, G. In Achilles Tendinopathy, the Neovascularization, Detected by Contrast-Enhanced Ultrasound (CEUS), Is Abundant but Not Related to Symptoms. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Cook, J.; Chen, S.; Scarvell, J.; Cormick, W.; Smith, P.; Fearon, A. Identification and Differentiation of Gluteus Medius Tendon Pathology Using Ultrasound and Magnetic Resonance Imaging. Musculoskelet. Sci. Pract. 2019, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Lian, Ø.; Roberts, C.; Cook, J.; Handley, C.; Bahr, R.; Samiric, T.; Ilic, M.; Parkinson, J.; Hart, D.; et al. Increased Versican Content Is Associated with Tendinosis Pathology in the Patellar Tendon of Athletes with Jumper’s Knee. Scand. J. Med. Sci. Sports 2008, 18, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagat, M.; Zabrzyńska, A.; Zabrzyńska, M.; Zielińska, W. The Advances in Diagnostic Modalities of Disorders of the Long Head of the Biceps Tendon–Review. Med. Stud. 2021, 37, 83–90. [Google Scholar] [CrossRef]
- Loppini, M.; Longo, U.; Niccoli, G.; Khan, W.; Maffulli, N.; Denaro, V. Histopathological Scores for Tissue-Engineered, Repaired and Degenerated Tendon: A Systematic Review of the Literature. CSCR 2014, 10, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Aström, M.; Rausing, A. Chronic Achilles Tendinopathy. A Survey of Surgical and Histopathologic Findings. Clin. Orthop. Relat. Res. 1995, 316, 151–164. [Google Scholar] [CrossRef]
- Soslowsky, L.J.; Carpenter, J.E.; DeBano, C.M.; Banerji, I.; Moalli, M.R. Development and Use of an Animal Model for Investigations on Rotator Cuff Disease. J. Shoulder Elb. Surg. 1996, 5, 383–392. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Paczesny, Ł.; Zabrzyńska, A.; Grzanka, D.; Łapaj, Ł. Sonography in the Instability of the Long Head of the Biceps Tendon Confronted with Histopathologic and Arthroscopic Findings. Folia Morphol. 2018, 77, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Process of Neovascularisation Compared with Pain Intensity in Tendinopathy of the Long Head of the Biceps Brachii Tendon Associated with Concomitant Shoulder Disorders, after Arthroscopic Treatment. Microscopic Evaluation Supported by Immunohistochemical. Folia Morphol. 2018, 77, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgreen, K.; Lian, O.B.; Scott, A.; Nassab, P.; Fearon, A.; Engebretsen, L. Rotator Cuff Tear Degeneration and Cell Apoptosis in Smokers versus Nonsmokers. Arthroscopy 2014, 30, 936–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, L.; Vachon, P.; Beaudry, F.; Langelier, E. Histopathological, Biomechanical, and Behavioral Pain Findings of Achilles Tendinopathy Using an Animal Model of Overuse Injury. Physiol. Rep. 2015, 3, e12265. [Google Scholar] [CrossRef]
- Kurdziel, M.D.; Moravek, J.E.; Wiater, B.P.; Davidson, A.; Seta, J.; Maerz, T.; Baker, K.C.; Wiater, J.M. The Impact of Rotator Cuff Deficiency on Structure, Mechanical Properties, and Gene Expression Profiles of the Long Head of the Biceps Tendon (LHBT): Implications for Management of the LHBT during Primary Shoulder Arthroplasty: The Impact of Rotator Cuff Deficiency. J. Orthop. Res. 2015, 33, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Sethi, P.M.; Sheth, C.D.; Pauzenberger, L.; McCarthy, M.B.R.; Cote, M.P.; Soneson, E.; Miller, S.; Mazzocca, A.D. Macroscopic Rotator Cuff Tendinopathy and Histopathology Do Not Predict Repair Outcomes of Rotator Cuff Tears. Am. J. Sports Med. 2018, 46, 779–785. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ochiai, N.; Hashimoto, E.; Sasaki, Y.; Yamaguchi, T.; Kijima, T.; Akimoto, K.; Ohtori, S.; Takahashi, K. Relationship between Neuropathy Proximal to the Suprascapular Nerve and Rotator Cuff Tear in a Rodent Model. J. Orthop. Sci. 2018, 23, 414–419. [Google Scholar] [CrossRef]
- Nuelle, C.W.; Stokes, D.C.; Kuroki, K.; Crim, J.R.; Sherman, S.L. Radiologic and Histologic Evaluation of Proximal Bicep Pathology in Patients With Chronic Biceps Tendinopathy Undergoing Open Subpectoral Biceps Tenodesis. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1790–1796. [Google Scholar] [CrossRef]
- Okazaki, Y.; Furumatsu, T.; Maehara, A.; Miyazawa, S.; Kamatsuki, Y.; Hino, T.; Ozaki, T. Histological Alterations to the Hamstring Tendon Caused by Cleaning during Autograft Preparation. Muscle Ligaments Tendons J. 2019, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Lundgreen, K.; Lian, Ø.; Scott, A.; Engebretsen, L. Increased Levels of Apoptosis and P53 in Partial-Thickness Supraspinatus Tendon Tears. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.M.; Twin, J.; Dahlstrom, J.E.; Cook, J.L.; Cormick, W.; Smith, P.N.; Scott, A. Increased Substance P Expression in the Trochanteric Bursa of Patients with Greater Trochanteric Pain Syndrome. Rheumatol. Int. 2014, 34, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, S.A.; Hazleman, B.L.; Riley, G.P. The Vasculature and Its Role in the Damaged and Healing Tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditsios, K.; Agathangelidis, F.; Boutsiadis, A.; Karataglis, D.; Papadopoulos, P. Long Head of the Biceps Pathology Combined with Rotator Cuff Tears. Available online: https://www.hindawi.com/journals/aorth/2012/405472/ (accessed on 7 November 2018).
- Lewis, J.S.; Raza, S.A.; Pilcher, J.; Heron, C.; Poloniecki, J.D. The Prevalence of Neovascularity in Patients Clinically Diagnosed with Rotator Cuff Tendinopathy. BMC Musculoskelet. Disord. 2009, 10, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tol, J.L.; Spiezia, F.; Maffulli, N. Neovascularization in Achilles Tendinopathy: Have We Been Chasing a Red Herring? Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1891–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfredson, H.; Öhberg, L. Sclerosing Injections to Areas of Neo-Vascularisation Reduce Pain in Chronic Achilles Tendinopathy: A Double-Blind Randomised Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Danielson, P. An Emerging Role for Angiogenesis in Tendinopathy. Eur. Musculoskelet. Rev. 2009, 4, 75–76. [Google Scholar]
- Alfredson, H.; Ohberg, L.; Forsgren, S. Is Vasculo-Neural Ingrowth the Cause of Pain in Chronic Achilles Tendinosis? An Investigation Using Ultrasonography and Colour Doppler, Immunohistochemistry, and Diagnostic Injections. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Singaraju, V.M.; Kang, R.W.; Yanke, A.B.; McNickle, A.G.; Lewis, P.B.; Wang, V.M.; Williams, J.M.; Chubinskaya, S.; Romeo, A.A.; Cole, B.J. Biceps Tendinitis in Chronic Rotator Cuff Tears: A Histologic Perspective. J. Shoulder Elbow Surg. 2008, 17, 898–904. [Google Scholar] [CrossRef]
- de Vos, R.-J.; Weir, A.; Cobben, L.P.J.; Tol, J.L. The Value of Power Doppler Ultrasonography in Achilles Tendinopathy: A Prospective Study. Am. J. Sports Med. 2007, 35, 1696–1701. [Google Scholar] [CrossRef]
- Sengkerij, P.M.; de Vos, R.-J.; Weir, A.; van Weelde, B.J.G.; Tol, J.L. Interobserver Reliability of Neovascularization Score Using Power Doppler Ultrasonography in Midportion Achilles Tendinopathy. Am. J. Sports Med. 2009, 37, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.N.; Newton, J.B.; Boorman-Padgett, J.F.; Weiss, S.N.; Nuss, C.A.; Gittings, D.J.; Farber, D.C.; Soslowsky, L.J. Nicotine Impairs Intra-substance Tendon Healing after Full Thickness Injury in a Rat Model. J. Orthop. Res. 2019, 37, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, D.; Ambati, B.; Adamis, A.P.; Miller, J.W. Mechanisms of Age-Related Macular Degeneration. Ophthalmol. Clin. North Am. 2002, 15, 87–91. [Google Scholar] [CrossRef]
- Chheda, L.V.; Ferketich, A.K.; Carroll, C.P.; Moyer, P.D.; Kurz, D.E.; Kurz, P.A. Smoking as a Risk Factor for Choroidal Neovascularization Secondary to Presumed Ocular Histoplasmosis Syndrome. Ophthalmology 2012, 119, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Koide, K.; Ventura, W.; Hori, K.; Takenaka, S.; Maruyama, D.; Matsuoka, R.; Ichizuka, K.; Sekizawa, A. Effects of Maternal Smoking on the Placental Expression of Genes Related to Angiogenesis and Apoptosis during the First Trimester. PLoS ONE 2014, 9, e106140. [Google Scholar] [CrossRef] [PubMed]
- Bjur, D.; Alfredson, H.; Forsgren, S. The Innervation Pattern of the Human Achilles Tendon: Studies of the Normal and Tendinosis Tendon with Markers for General and Sensory Innervation. Cell Tissue Res. 2005, 320, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Florence, M.E.B.; Massuda, J.Y.; Bröcker, E.-B.; Metze, K.; Cintra, M.L.; de Souza, E.M. Angiogenesis in the Progression of Cutaneous Squamous Cell Carcinoma: An Immunohistochemical Study of Endothelial Markers. Clinics 2011, 66, 465–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivilis, I.; Milkiewicz, M.; Boyd, P.; Goldstein, J.; Brown, M.D.; Egginton, S.; Hansen, F.M.; Hudlicka, O.; Haas, T.L. Differential Involvement of MMP-2 and VEGF during Muscle Stretch- versus Shear Stress-Induced Angiogenesis. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H1430–H1438. [Google Scholar] [CrossRef] [Green Version]
- Edgar, L.T.; Underwood, C.J.; Guilkey, J.E.; Hoying, J.B.; Weiss, J.A. Extracellular Matrix Density Regulates the Rate of Neovessel Growth and Branching in Sprouting Angiogenesis. PLoS ONE 2014, 9, e85178. [Google Scholar] [CrossRef] [Green Version]
- Koehler, L.; Ruiz-Gómez, G.; Balamurugan, K.; Rother, S.; Freyse, J.; Möller, S.; Schnabelrauch, M.; Köhling, S.; Djordjevic, S.; Scharnweber, D.; et al. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci. Rep. 2019, 9, 18143. [Google Scholar] [CrossRef]
- Cheng, J.-J.; Huang, N.-K.; Chang, T.-T.; Ling Wang, D.; Lu, M.-K. Study for Anti-Angiogenic Activities of Polysaccharides Isolated from Antrodia Cinnamomea in Endothelial Cells. Life Sci. 2005, 76, 3029–3042. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T.; Wang, Y.; Murrell, G. The Roles of Growth Factors in Tendon and Ligament Healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]

| Scale | Items | Points |
|---|---|---|
| Movin | Fiber structure | 0–3 |
| Fiber arrangement | 0–3 | |
| Rounding of the nuclei | 0–3 | |
| Regional variations in cellularity | 0–3 | |
| Increased vascularity | 0–3 | |
| Decreased collagen stainability | 0–3 | |
| Hyalinization | 0–3 | |
| Glycosaminoglycans | 0–3 | |
| Bonar | Tenocyte morphology | 0–3 |
| Ground substance | 0–3 | |
| Vascularity | 0–3 | |
| Collagen | 0–3 | |
| Astrom and Rausing | Fiber alignment | 0–3 |
| Fiber structure | 0–3 | |
| Morphology of tenocyte nuclei | 0–3 | |
| Variations in cell density | 0–3 | |
| Variations in cell density | 0–3 | |
| Soslowsky | Cellularity | 0–3 |
| Fibroblastic changes | 0–3 | |
| Collagen fiber orientation | 0–3 | |
| Disruption | 0–3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworski, Ł.; Zabrzyńska, M.; Klimaszewska-Wiśniewska, A.; Zielińska, W.; Grzanka, D.; Gagat, M. Advances in Microscopic Studies of Tendinopathy: Literature Review and Current Trends, with Special Reference to Neovascularization Process. J. Clin. Med. 2022, 11, 1572. https://doi.org/10.3390/jcm11061572
Jaworski Ł, Zabrzyńska M, Klimaszewska-Wiśniewska A, Zielińska W, Grzanka D, Gagat M. Advances in Microscopic Studies of Tendinopathy: Literature Review and Current Trends, with Special Reference to Neovascularization Process. Journal of Clinical Medicine. 2022; 11(6):1572. https://doi.org/10.3390/jcm11061572
Chicago/Turabian StyleJaworski, Łukasz, Maria Zabrzyńska, Anna Klimaszewska-Wiśniewska, Wioletta Zielińska, Dariusz Grzanka, and Maciej Gagat. 2022. "Advances in Microscopic Studies of Tendinopathy: Literature Review and Current Trends, with Special Reference to Neovascularization Process" Journal of Clinical Medicine 11, no. 6: 1572. https://doi.org/10.3390/jcm11061572
APA StyleJaworski, Ł., Zabrzyńska, M., Klimaszewska-Wiśniewska, A., Zielińska, W., Grzanka, D., & Gagat, M. (2022). Advances in Microscopic Studies of Tendinopathy: Literature Review and Current Trends, with Special Reference to Neovascularization Process. Journal of Clinical Medicine, 11(6), 1572. https://doi.org/10.3390/jcm11061572







