Decreased Need for Correction Boluses with Universal Utilisation of Dual-Wave Boluses in Children with Type 1 Diabetes
Abstract
:1. Introduction
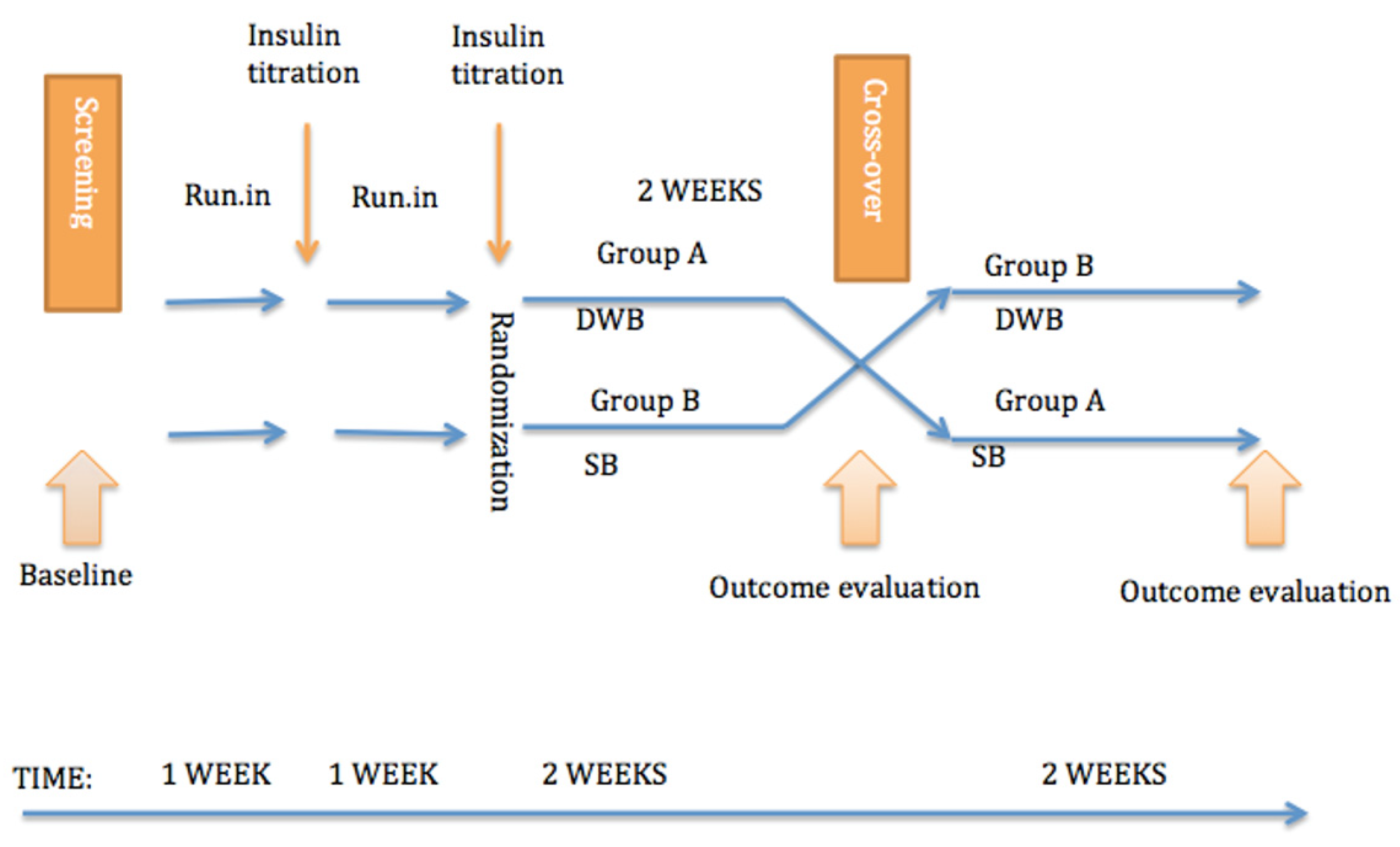
2. Materials and Methods
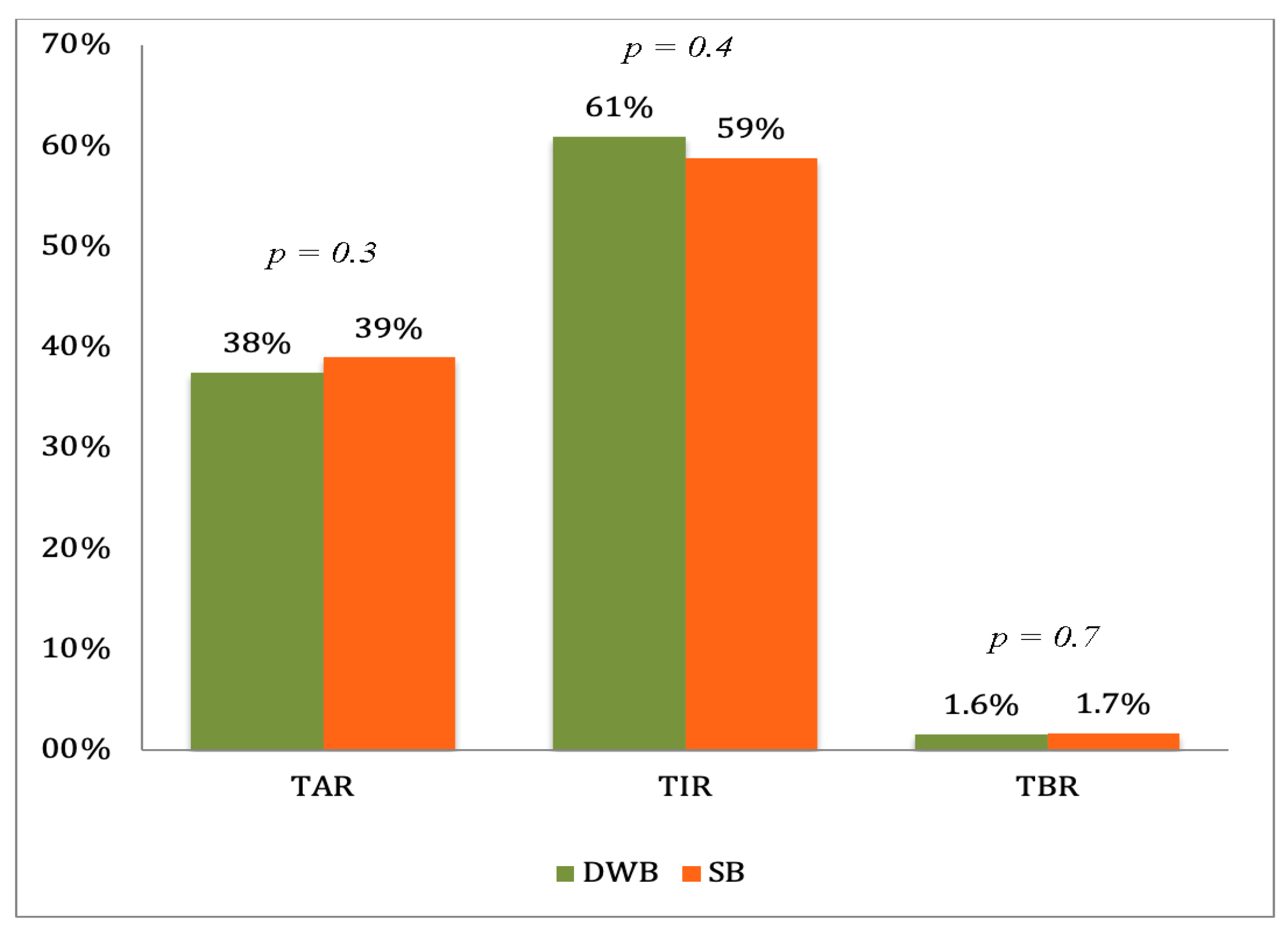
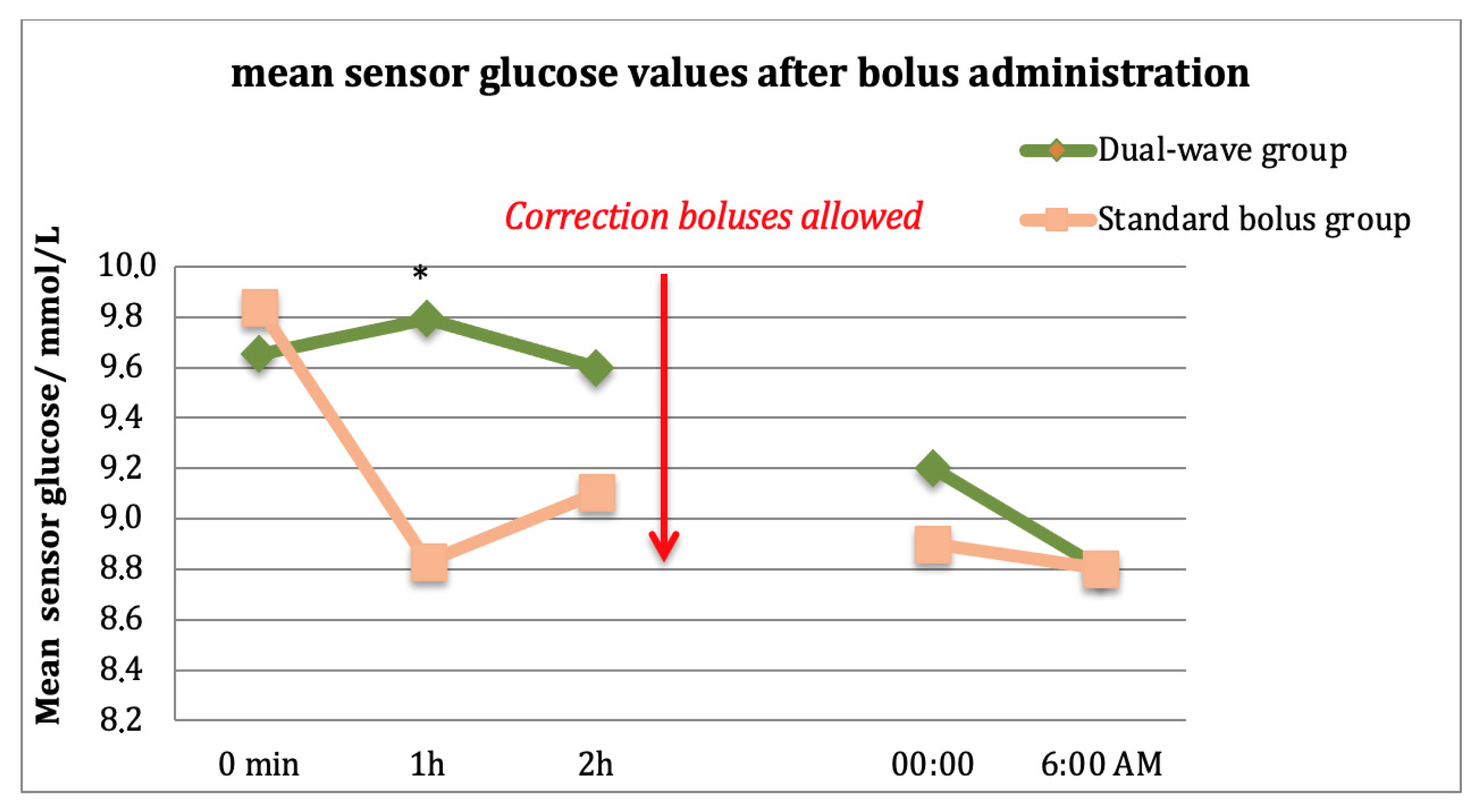
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prahalad, P.; Tanenbaum, M.; Hood, K.; Maahs, D.M. Diabetes technology: Improving care, improving patient-reported outcomes and preventing complications in young people with Type 1 diabetes. Diabet. Med. 2018, 35, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pańkowska, E.; Szypowska, A.; Lipka, M.; Szpotańska, M.; Błazik, M.; Groele, L. Application of novel dual wave meal bolus and its impact on glycated hemoglobin A1c level in children with type 1 diabetes. Pediatr. Diabetes 2009, 10, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of Fat, Protein, and Glycemic Index on Postprandial Glucose Control in Type 1 Diabetes: Implications for Intensive Diabetes Management in the Continuous Glucose Monitoring Era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, K.; Dżygało, K.; Szypowska, A. The additional dose of insulin for high-protein mixed meal provides better glycemic control in children with type 1 diabetes on insulin pumps: Randomized cross-over study. Pediatr. Diabetes 2017, 18, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Pańkowska, E.; Błazik, M.; Groele, L. Does the Fat-Protein Meal Increase Postprandial Glucose Level in Type 1 Diabetes Patients on Insulin Pump: The Conclusion of a Randomized Study. Diabetes Technol. Ther. 2012, 14, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Cao, M.; Sajid, S.; Hayes, M.; Choi, L.; Rother, C.; De León, R. The dual-wave bolus feature in continuous subcutaneous insulin infusion pumps controls prolonged post-prandial hyperglycaemia better than standard bolus in Type 1 diabetes. Diabetes Nutr. Metab. 2004, 17, 211–216. [Google Scholar] [PubMed]
- Jones, S.M.; Quarry, J.L.; Caldwell-McMillan, M.; Mauger, D.T.; Gabbay, R.A. Optimal Insulin Pump Dosing and Postprandial Glycemia Following a Pizza Meal Using the Continuous Glucose Monitoring System. Diabetes Technol. Ther. 2005, 7, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.P.; Saib, S.Z.; MacKenzie, T.; Hansen, M.M.; Garg, S.K. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet. Med. 2002, 19, 317–321. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.A.; Gilbertson, H.R.; Donath, S.M.; Cameron, F.J. Optimizing Postprandial Glycemia in Pediatric Patients With Type 1 Diabetes Using Insulin Pump Therapy. Diabetes Care 2008, 31, 1491–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curry, D.L.; Morris, J.G.; Rogers, Q.R.; Stern, J.S. Dynamics of insulin and glucagon secretion by the isolated perfused cat pancreas. Comp. Biochem. Physiol. Part A Physiol. 1982, 72, 333–338. [Google Scholar] [CrossRef]
- Lopez, P.E.; Smart, C.E.; McElduff, P.; Foskett, D.C.; Price, D.A.; Paterson, M.A.; King, B.R. Optimising the combination insulin bolus split for a high fat, high protein meal in children and adolescents using insulin pump therapy. Diabet. Med. 2017, 34, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Toschi, E.; Steil, G.M.; Wolpert, H.A. Optimized Mealtime Insulin Dosing for Fat and Protein in Type 1 Diabetes: Application of a Model-Based Approach to Derive Insulin Doses for Open-Loop Diabetes Management. Diabetes Care 2016, 39, 1631–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolpert, H.A.; Atakov-Castillo, A.; Smith, S.A.; Steil, G.M. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: Implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care 2013, 36, 810–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neu, A.; Behret, F.; Braun, R.; Herrlich, S.; Liebrich, F.; Loesch-Binder, M.; Schneider, A.; Schweizer, R. Higher glucose concentrations following protein- and fat-rich meals-the Tuebingen Grill Study: A pilot study in adolescents with type 1 diabetes. Pediatr. Diabetes 2014, 16, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Rigby, A.S.; Vail, A. Statistical methods in epidemiology. II: A commonsense approach to sample size estimation. Disabil. Rehabil. 1998, 20, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Purves, R.D. Optimum numerical integration methods for estimation of area-un-der-the-curve (AUC) and area-under-the-moment-curve (AUMC). J. Pharmacokinet. Biopharm. 1992, 20, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; Walker, M.; King, D.; Gonzalez, J.T.; Allerton, D.; Stevenson, E.J.; Shaw, J.A.; West, D.J. Carbohydrate Counting at Meal Time Followed by a Small Secondary Postprandial Bolus Injection at 3 Hours Prevents Late Hyperglycemia, Without Hypoglycemia, After a High-Carbohydrate, High-Fat Meal in Type 1 Diabetes. Diabetes Care 2016, 39, e141–e142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taber’s Medical Dictionary. Available online: https://medical-dictionary.thefreedictionary.com/Western+diet#:~:text=A%20diet%20with%20inadequate%20fruits,snacks%2C%20eggs%2C%20and%20butter (accessed on 10 February 2022).
| Number of Study Subjects = 24 | |
|---|---|
| Gender (boys/girls) | 10/14 |
| Age (years) | 10.8 ± 2.0 |
| Weight (kg) | 44.2 ± 12.6 |
| BMI (kg/m2) | 18.7 ± 3.4 |
| Predicted glycated hemoglobin A1c (%) | 7.6 ± 0.7 |
| Mean sensor glucose (mmol/L) | 9.5 ± 1.1 |
| Daily insulin dose per kg (IU/kg/d) | 0.8 ± 0.1 |
| Basal insulin (%) | 37.8 ± 10.7 |
| Bolus insulin (%) | 62.2 ± 10.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukka, M.; Tillmann, V.; Peet, A. Decreased Need for Correction Boluses with Universal Utilisation of Dual-Wave Boluses in Children with Type 1 Diabetes. J. Clin. Med. 2022, 11, 1689. https://doi.org/10.3390/jcm11061689
Lukka M, Tillmann V, Peet A. Decreased Need for Correction Boluses with Universal Utilisation of Dual-Wave Boluses in Children with Type 1 Diabetes. Journal of Clinical Medicine. 2022; 11(6):1689. https://doi.org/10.3390/jcm11061689
Chicago/Turabian StyleLukka, Mari, Vallo Tillmann, and Aleksandr Peet. 2022. "Decreased Need for Correction Boluses with Universal Utilisation of Dual-Wave Boluses in Children with Type 1 Diabetes" Journal of Clinical Medicine 11, no. 6: 1689. https://doi.org/10.3390/jcm11061689






