Does the Serum Concentration of Angiotensin II Type 1 Receptor Have an Effect on the Severity of COVID-19? A Prospective Preliminary Observational Study among Healthcare Professionals
Abstract
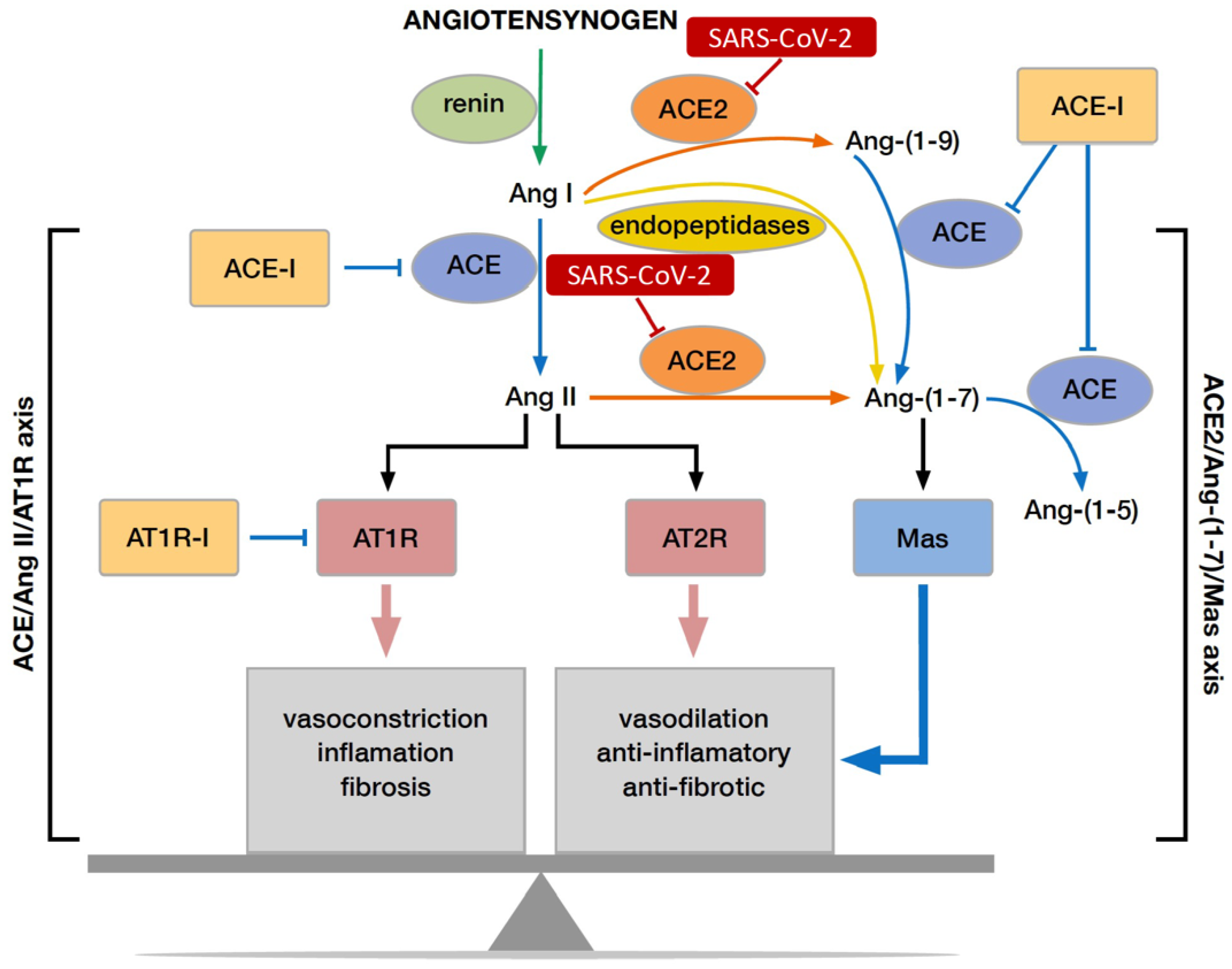
:1. Introduction
2. Materials and Methods
2.1. Design and Settings
2.2. Ethics
2.3. Participants
2.4. Outcomes
2.4.1. AT1R Serum Concentration
2.4.2. COVID Severity
- Asymptomatic or Presymptomatic Infection: Individuals who tested positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test or an antigen test) but had no symptoms that were consistent with COVID-19;
- Mild Illness: Individuals who had any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhoea, loss of taste and smell) but who did not have shortness of breath, dyspnoea, or abnormal chest imaging;
- Moderate Illness: Individuals who showed lower respiratory disease evidence during clinical assessment or imaging and had a saturation of oxygen (SpO2) ≥ 94% on room air at sea level;
- Severe Illness: Individuals who had SpO2 < 94% on room air at sea level, a ratio of the arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mmHg, a respiratory rate >30 breaths per minute, or lung infiltrates >50%;
- Critical Illness: Individuals who had respiratory failure, septic shock, and/or multiple organ dysfunction.
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Analysis of AT1R Serum Concentration and Selected Values in Both Groups
3.2. Analysis of AT1R Serum Concentration and Selected Variables in the COVID-19 Recovered
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio-Med. Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease (COVID-19) Treatment Guidelines: Clinical Spectrum of SARS-CoV-2 Infection; National Institutes of Health: Bethesda, MA, USA, 2021.
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin System in Kidney Physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [CrossRef] [Green Version]
- Bernard, A.; Broeckaert, F.; De Poorter, G.; De Cock, A.; Hermans, C.; Saegerman, C.; Houins, G. The Belgian PCB/Dioxin Incident: Analysis of the Food Chain Contamination and Health Risk Evaluation. Environ. Res. 2002, 88, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guang, C.; Phillips, R.D.; Jiang, B.; Milani, F. Three Key Proteases--Angiotensin-I-Converting Enzyme (ACE), ACE2 and Renin--within and beyond the Renin-Angiotensin System. Arch. Cardiovasc. Dis. 2012, 105, 373–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ardes, D.; Boccatonda, A.; Rossi, I.; Guagnano, M.T.; Santilli, F.; Cipollone, F.; Bucci, M. COVID-19 and RAS: Unravelling an Unclear Relationship. Int. J. Mol. Sci. 2020, 21, 3003. [Google Scholar] [CrossRef]
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.D.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; et al. A Pilot Clinical Trial of Recombinant Human Angiotensin-Converting Enzyme 2 in Acute Respiratory Distress Syndrome. Crit. Care Lond. Engl. 2017, 21, 234. [Google Scholar] [CrossRef] [Green Version]
- Lundström, A.; Ziegler, L.; Havervall, S.; Rudberg, A.-S.; von Meijenfeldt, F.; Lisman, T.; Mackman, N.; Sandén, P.; Thålin, C. Soluble Angiotensin-Converting Enzyme 2 Is Transiently Elevated in COVID-19 and Correlates with Specific Inflammatory and Endothelial Markers. J. Med. Virol. 2021, 93, 5908–5916. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic Value of Interleukin-6, C-Reactive Protein, and Procalcitonin in Patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Dandona, P.; Dhindsa, S.; Ghanim, H.; Chaudhuri, A. Angiotensin II and Inflammation: The Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockade. J. Hum. Hypertens. 2007, 21, 20–27. [Google Scholar] [CrossRef]
- Gromotowicz-Poplawska, A.; Stankiewicz, A.; Kramkowski, K.; Gradzka, A.; Wojewodzka-Zelezniakowicz, M.; Dzieciol, J.; Szemraj, J.; Chabielska, E. The Acute Prothrombotic Effect of Aldosterone in Rats Is Partially Mediated via Angiotensin II Receptor Type 1. Thromb. Res. 2016, 138, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sawathiparnich, P.; Murphey, L.J.; Kumar, S.; Vaughan, D.E.; Brown, N.J. Effect of Combined AT1 Receptor and Aldosterone Receptor Antagonism on Plasminogen Activator Inhibitor-1. J. Clin. Endocrinol. Metab. 2003, 88, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Ducros, E.; Berthaut, A.; Mirshahi, S.S.; Faussat, A.M.; Soria, J.; Agarwal, M.K.; Mirshahi, M. Aldosterone Modifies Hemostasis via Upregulation of the Protein-C Receptor in Human Vascular Endothelium. Biochem. Biophys. Res. Commun. 2008, 373, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Remková, A.; Remko, M. The Role of Renin-Angiotensin System in Prothrombotic State in Essential Hypertension. Physiol. Res. 2010, 59, 13–23. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Tiwari, S.; Riazi, S.; Hu, X.; Wang, X.; Ecelbarger, C.M. Regulation of Angiotensin II Type I Receptor (AT1R) Protein Levels in the Obese Zucker Rat Kidney and Urine. Clin. Exp. Hypertens. 2009, 31, 49–63. [Google Scholar] [CrossRef]
- Bansal, S.; Tokman, S.; Fleming, T.; Maine, G.N.; Sanborn, K.; Hachem, R.; Bharat, A.; Smith, M.A.; Bremner, R.M.; Mohanakumar, T. SARS-CoV-2 Infection in Lung Transplant Recipients Induces Circulating Exosomes with SARS-CoV-2 Spike Protein S2. Clin. Transl. Med. 2021, 11, e576. [Google Scholar] [CrossRef]
- Guzzi, P.H.; Mercatelli, D.; Ceraolo, C.; Giorgi, F.M. Master Regulator Analysis of the SARS-CoV-2/Human Interactome. J. Clin. Med. 2020, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Speth, R.C. Angiotensin II Administration to COVID-19 Patients Is Not Advisable. Crit. Care Lond. Engl. 2020, 24, 296. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Dworakowska, D.; Grossman, A.B. Renin-Angiotensin System Inhibitors in Management of Hypertension during the COVID-19 Pandemic. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet Lond. Engl. 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Dublin, S.; Walker, R.; Floyd, J.S.; Shortreed, S.M.; Fuller, S.; Albertson-Junkans, L.; Harrington, L.B.; Greenwood-Hickman, M.A.; Green, B.B.; Psaty, B.M. Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19 Infection or Hospitalization: A Cohort Study. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Yasuda, N.; Komuro, I. Mechanisms and Functions of Agonist-Independent Activation in the Angiotensin II Type 1 Receptor. Mol. Cell. Endocrinol. 2009, 302, 140–147. [Google Scholar] [CrossRef]
- Gurwitz, D. Angiotensin Receptor Blockers as Tentative SARS-CoV-2 Therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Rothlin, R.P.; Duarte, M.; Pelorosso, F.G.; Nicolosi, L.; Salgado, M.V.; Vetulli, H.M.; Spitzer, E. Angiotensin Receptor Blockers for COVID-19: Pathophysiological and Pharmacological Considerations About Ongoing and Future Prospective Clinical Trials. Front. Pharmacol. 2021, 12, 603736. [Google Scholar] [CrossRef]
- Guillon, P.; Clément, M.; Sébille, V.; Rivain, J.-G.; Chou, C.-F.; Ruvoën-Clouet, N.; Le Pendu, J. Inhibition of the Interaction between the SARS-CoV Spike Protein and Its Cellular Receptor by Anti-Histo-Blood Group Antibodies. Glycobiology 2008, 18, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, G.; Harvey, D.J.; Feldmann, F.; Stroeher, U.; Feldmann, H.; Royle, L.; Dwek, R.A.; Rudd, P.M. Identification of N-Linked Carbohydrates from Severe Acute Respiratory Syndrome (SARS) Spike Glycoprotein. Virology 2010, 399, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Hoiland, R.L.; Fergusson, N.A.; Mitra, A.R.; Griesdale, D.E.G.; Devine, D.V.; Stukas, S.; Cooper, J.; Thiara, S.; Foster, D.; Chen, L.Y.C.; et al. The Association of ABO Blood Group with Indices of Disease Severity and Multiorgan Dysfunction in COVID-19. Blood Adv. 2020, 4, 4981–4989. [Google Scholar] [CrossRef] [PubMed]
- Latz, C.A.; DeCarlo, C.; Boitano, L.; Png, C.Y.M.; Patell, R.; Conrad, M.F.; Eagleton, M.; Dua, A. Blood Type and Outcomes in Patients with COVID-19. Ann. Hematol. 2020, 99, 2113–2118. [Google Scholar] [CrossRef]
- Leaf, R.K.; Al-Samkari, H.; Brenner, S.K.; Gupta, S.; Leaf, D.E. ABO Phenotype and Death in Critically Ill Patients with COVID-19. Br. J. Haematol. 2020, 190, e204–e208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Chen, J.; Cai, Y.; Deng, A.; Yang, M. Association between ABO Blood Groups and Risk of SARS-CoV-2 Pneumonia. Br. J. Haematol. 2020, 190, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Schull, M.J.; Vermeulen, M.J.; Park, A.L. Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness: A Population-Based Cohort Study. Ann. Intern. Med. 2021, 174, 308–315. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. Clin. Infect. Dis. 2021, 73, 328–331. [Google Scholar] [CrossRef]
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Testing the Association between Blood Type and COVID-19 Infection, Intubation, and Death. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Boudin, L.; Janvier, F.; Bylicki, O.; Dutasta, F. ABO Blood Groups Are Not Associated with Risk of Acquiring the SARS-CoV-2 Infection in Young Adults. Haematologica 2020, 105, 2841–2843. [Google Scholar] [CrossRef]
- Barnkob, M.B.; Pottegård, A.; Støvring, H.; Haunstrup, T.M.; Homburg, K.; Larsen, R.; Hansen, M.B.; Titlestad, K.; Aagaard, B.; Møller, B.K.; et al. Reduced Prevalence of SARS-CoV-2 Infection in ABO Blood Group O. Blood Adv. 2020, 4, 4990–4993. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, M.; Pal, R. ABO Blood Groups and Severe Outcomes in COVID-19: A Meta-Analysis. Postgrad. Med. J. 2020. [Google Scholar] [CrossRef]

| All n = 82 | COVID-19 Recovered n = 40 | Non-COVID-19 n = 42 | p | |
|---|---|---|---|---|
| Age (years) | 0.58 * | |||
| M ± SD | 39.9 ± 9.8 | 39.3 ± 10.7 | 39.9 ± 9.8 | |
| Me (Q1–Q3) | 38.0 (31.0–47.0) | 36.0 (29.5–48.0) | 38.0 (31.0–47.0) | |
| Min–Max | 25.0–64.0 | 25.0–64.0 | 25.0–64.0 | |
| Weight (kg) | 0.62 * | |||
| M ± SD | 77.9 ± 16.8 | 78.9 ± 18.6 | 77.0 ± 15.1 | |
| Me (Q1–Q3) | 77.5 (67.0–87.0) | 79.0 (66.0–89.5) | 76.5 (67.0–82.0) | |
| Min–Max | 45.0–135.0 | 45.0–135.0 | 50.0–116.0 | |
| Height (cm) | 0.43 * | |||
| M ± SD | 171.5 ± 8.9 | 172.4 ± 10.0 | 170.8 ± 7.8 | |
| Me (Q1–Q3) | 173.0 (164.0–180.0) | 173.0 (163.5–180.5) | 172.5 (164.0–176.0) | |
| Min–Max | 155.0–189.0 | 157.0–189.0 | 155.0–184.0 | |
| BMI (kg/m2) | 0.92 * | |||
| M ± SD | 26.4 ± 4.7 | 26.4 ± 5.2 | 26.3 ± 4.3 | |
| Me (Q1–Q3) | 26.3 (22.8–29.4) | 26.2 (22.8–29.7) | 26.3 (23.3–29.0) | |
| Min–Max | 17.4–37.8 | 17.4–37.8 | 18.9–36.3 | |
| Sex (female) n (%) | 49 (60%) | 23 (58%) | 26 (62%) | 0.68 ** |
| Blood group n (%) | 0.36 ** | |||
| O | 17 (23%) | 5 (14%) | 12 (31%) | |
| AB | 8 (11%) | 5 (14%) | 3 (8%) | |
| A | 33 (44%) | 17 (49%) | 16 (41%) | |
| B | 6 (22%) | 8 (23%) | 11 (20%) | |
| Rh factor n (%) | 0.12 ** | |||
| Rh − | 14 (19%) | 4 (11%) | 10 (26%) | |
| Rh + | 60 (81%) | 31 (89%) | 29 (74%) | |
| Chronic disease n (%) | ||||
| Hypertension (Yes) | 8 (10%) | 5 (13%) | 3 (7%) | 0.41 ** |
| Diabetes (Yes) | 2 (2%) | 1 (3%) | 1 (2%) | 0.97 ** |
| Thyroid disease (Yes) | 7 (9%) | 4 (10%) | 4 (7%) | 0.64 ** |
| AT1R Level—Linear Regression | ||||||
|---|---|---|---|---|---|---|
| Variables | B | SE | t | p-Value | ß | |
| Age | −0.01 | 0.02 | −0.63 | 0.53 | −0.07 | |
| Body height | 0.01 | 0.03 | 0.26 | 0.79 | 0.03 | |
| Body weight | −0.02 | 0.01 | −1.11 | 0.27 | −0.12 | |
| BMI | −0.07 | 0.05 | −1.43 | 0.16 | −0.16 | |
| Sex | F | Ref. | ||||
| M | −0.33 | 0.23 | −1.41 | 0.16 | −0.16 | |
| Blood group | O | Ref. | ||||
| A | 0.22 | 0.38 | 0.57 | 0.57 | 0.08 | |
| B | −0.18 | 0.46 | −0.39 | 0.70 | −0.06 | |
| AB | 0.03 | 0.60 | 0.05 | 0.96 | 0.01 | |
| Rh factor | – | Ref. | ||||
| + | 0.18 | 0.31 | 0.58 | 0.57 | 0.07 | |
| COVID-19 | No | Ref | ||||
| Yes | −0.23 | 0.23 | −1.01 | 0.32 | −0.11 | |
| Hypertension | No | Ref. | ||||
| Yes | −0.16 | 0.39 | −0.42 | 0.67 | −0.05 | |
| Diabetes | No | Ref. | ||||
| Yes | 0.84 | 0.75 | 1.12 | 0.27 | 0.12 | |
| Thyroid disease | No | Ref. | ||||
| Yes | 0.28 | 0.48 | 0.58 | 0.57 | 0.09 | |
| Variable | AT1R Concentration | p * | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 Recovered (n = 40) | Non-COVID-19 (n = 42) | |||||||||||||||
| M | Me | Min | Max | Q1 | Q3 | SD | M | Me | Min | Max | Q1 | Q3 | SD | |||
| Sex | M | 3.03 | 2.99 | 0.28 | 7.19 | 1.48 | 4.32 | 2.03 | 3.25 | 2.74 | 0.20 | 6.71 | 1.52 | 5.36 | 2.26 | 0.63 |
| F | 3.49 | 3.05 | 1.35 | 8.43 | 2.48 | 3.95 | 1.64 | 4.07 | 3.44 | 0.20 | 8.53 | 2.86 | 5.44 | 2.35 | 0.30 | |
| p-value * | 0.48 | 0.37 | ||||||||||||||
| Blood group | O | 4.02 | 3.98 | 1.82 | 5.55 | 3.43 | 5.31 | 1.52 | 3.19 | 3.13 | 0.49 | 6.55 | 1.17 | 5.45 | 2.21 | 1.00 |
| AB | 4.41 | 3.95 | 1.33 | 8.43 | 2.48 | 5.88 | 2.82 | 2.06 | 1.96 | 0.20 | 4.02 | 0.20 | 4.02 | 1.91 | 1.00 | |
| A | 2.59 | 2.87 | 0.88 | 5.04 | 1.78 | 3.05 | 1.02 | 4.91 | 4.90 | 1.15 | 8.53 | 2.68 | 7.14 | 2.52 | 0.08 | |
| B | 3.91 | 3.76 | 1.35 | 7.19 | 2.92 | 4.69 | 1.74 | 2.73 | 3.35 | 0.20 | 3.89 | 1.95 | 3.57 | 1.40 | 0.96 | |
| p-value ** | 0.72 | 0.42 | ||||||||||||||
| Rh | – | 3.57 | 2.88 | 1.35 | 7.19 | 1.56 | 5.59 | 2.67 | 3.13 | 3.35 | 0.49 | 8.13 | 1.13 | 3.89 | 2.30 | 0.62 |
| + | 3.33 | 3.05 | 0.88 | 8.43 | 2.34 | 3.95 | 1.61 | 3.91 | 3.32 | 0.20 | 8.53 | 2.06 | 5.79 | 2.39 | 0.34 | |
| p-value * | 0.98 | 0.41 | ||||||||||||||
| Hypertension | No | 3.23 | 2.99 | 0.28 | 8.43 | 1.80 | 3.98 | 1.77 | 3.86 | 3.42 | 0.20 | 8.53 | 1.98 | 5.44 | 2.28 | 0.23 |
| Yes | 3.71 | 3.43 | 1.78 | 7.19 | 1.82 | 4.33 | 2.23 | 2.44 | 1.13 | 0.20 | 5.99 | 0.20 | 5.99 | 3.11 | 0.37 | |
| p-value * | 0.59 | 0.31 | ||||||||||||||
| Diabetes | No | 3.27 | 2.99 | 0.28 | 8.43 | 1.80 | 3.98 | 1.82 | 3.70 | 3.32 | 0.20 | 8.53 | 1.96 | 5.23 | 2.33 | 0.41 |
| Yes | 4.33 | 4.33 | 4.33 | 4.33 | 4.33 | 4.33 | - | 5.99 | 5.99 | 5.99 | 5.99 | 5.99 | 5.99 | - | - | |
| p-value * | - | - | ||||||||||||||
| Thyroid disease | No | 3.24 | 2.98 | 0.28 | 8.43 | 1.79 | 4.15 | 1.86 | 3.67 | 3.42 | 0.20 | 8.53 | 1.91 | 5.44 | 2.30 | 0.42 |
| Yes | 3.79 | 3.19 | 2.91 | 5.88 | 2.95 | 4.63 | 1.41 | 4.84 | 3.28 | 3.11 | 8.13 | 3.11 | 8.13 | 2.85 | 0.60 | |
| p-value * | 0.43 | 0.56 | ||||||||||||||
| AT1R Level—Linear Regression | ||||||
|---|---|---|---|---|---|---|
| Variables | B | SE | t | p-Value | ß | |
| Age | 0.00 | 0.03 | 0.17 | 0.86 | 0.03 | |
| Body height | 0.01 | 0.03 | 0.41 | 0.69 | 0.07 | |
| Body weight | 0.00 | 0.02 | −0.01 | 0.99 | 0.00 | |
| BMI | −0.01 | 0.06 | −0.22 | 0.83 | −0.04 | |
| Sex | F | Ref. | ||||
| M | −0.23 | 0.29 | −0.78 | 0.44 | −0.13 | |
| Blood group | 0 | Ref. | ||||
| A | −1.14 | 0.41 | −2.78 | 0.009 | −0.48 | |
| B | 0.18 | 0.50 | 0.35 | 0.73 | 0.06 | |
| AB | 0.68 | 0.59 | 1.15 | 0.26 | 0.22 | |
| Rh factor | − | Ref. | ||||
| + | −0.12 | 0.46 | −0.26 | 0.79 | −0.05 | |
| Symptoms | 1–2 | Ref | ||||
| 2–3 | −0.15 | 0.30 | −0.49 | 0.63 | −0.08 | |
| Hypertension | No | Ref. | ||||
| Yes | 0.24 | 0.44 | 0.55 | 0.59 | 0.09 | |
| Diabetes | No | Ref. | ||||
| Yes | 0.53 | 0.92 | 0.58 | 0.57 | 0.09 | |
| Thyroid disease | No | Ref. | ||||
| Yes | 0.28 | 0.48 | 0.58 | 0.57 | 0.09 | |
| NIH Illness Category | n | AT1R Serum Concentration in COVID-19 Recovered Group (n = 40) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | Me | Min | Max | Q1 | Q3 | SD | |||
| 1 | 1 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | - | 0.21 * |
| 2 | 25 | 3.24 | 2.99 | 1.35 | 6.34 | 2.46 | 3.95 | 1.30 | |
| 3 | 10 | 3.24 | 3.28 | 0.28 | 5.55 | 1.71 | 5.04 | 1.72 | |
| 4 | 4 | 2.75 | 1.11 | 0.35 | 8.43 | 0.62 | 4.88 | 3.81 | |
| 1–2 | 26 | 3.40 | 3.02 | 1.35 | 7.19 | 2.46 | 3.98 | 1.49 | 0.45 ** |
| 3–4 | 14 | 3.10 | 3.01 | 0.28 | 8.43 | 1.33 | 5.04 | 2.33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janc, J.; Suchański, M.; Mierzchała-Pasierb, M.; Woźnica-Niesobska, E.; Łysenko, L.; Leśnik, P. Does the Serum Concentration of Angiotensin II Type 1 Receptor Have an Effect on the Severity of COVID-19? A Prospective Preliminary Observational Study among Healthcare Professionals. J. Clin. Med. 2022, 11, 1769. https://doi.org/10.3390/jcm11071769
Janc J, Suchański M, Mierzchała-Pasierb M, Woźnica-Niesobska E, Łysenko L, Leśnik P. Does the Serum Concentration of Angiotensin II Type 1 Receptor Have an Effect on the Severity of COVID-19? A Prospective Preliminary Observational Study among Healthcare Professionals. Journal of Clinical Medicine. 2022; 11(7):1769. https://doi.org/10.3390/jcm11071769
Chicago/Turabian StyleJanc, Jarosław, Michał Suchański, Magdalena Mierzchała-Pasierb, Ewa Woźnica-Niesobska, Lidia Łysenko, and Patrycja Leśnik. 2022. "Does the Serum Concentration of Angiotensin II Type 1 Receptor Have an Effect on the Severity of COVID-19? A Prospective Preliminary Observational Study among Healthcare Professionals" Journal of Clinical Medicine 11, no. 7: 1769. https://doi.org/10.3390/jcm11071769
APA StyleJanc, J., Suchański, M., Mierzchała-Pasierb, M., Woźnica-Niesobska, E., Łysenko, L., & Leśnik, P. (2022). Does the Serum Concentration of Angiotensin II Type 1 Receptor Have an Effect on the Severity of COVID-19? A Prospective Preliminary Observational Study among Healthcare Professionals. Journal of Clinical Medicine, 11(7), 1769. https://doi.org/10.3390/jcm11071769






