Treatment of Giant Cell Arteritis (GCA)
Abstract
:1. Introduction
2. Glucocorticoids
3. Immunosuppressants
4. Biologic Agents
5. Future Perspectives
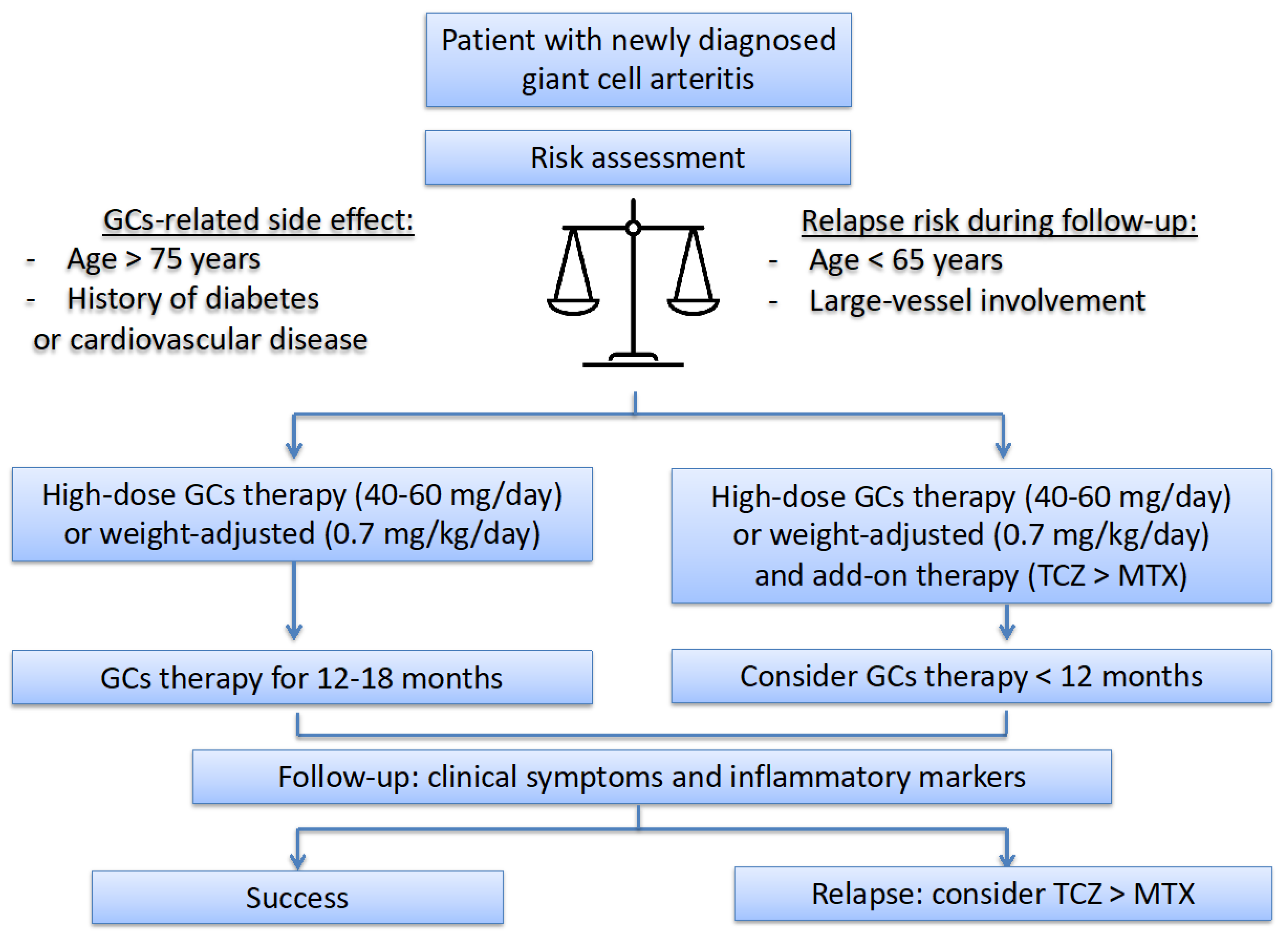
6. Therapeutic Strategy and International Recommendations
7. Non-Immunosuppressive Therapy
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AZA | azathioprine |
| GCs | glucocorticoids |
| GCA | giant cell arteritis |
| IL | interleukin |
| IS | immunosuppressant |
| IV | intravenous |
| LEF | leflunomide |
| MTX | methotrexate |
| RCT | randomised controlled trial |
| TCZ | tocilizumab |
| TNF | tumor necrosis factor |
References
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Lopez-Diaz, M.J.; Miranda-Filloy, J.A.; Gonzalez-Juanatey, C.; Martin, J.; Llorca, J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009, 61, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Blockmans, D.; Bley, T.; Schmidt, W. Imaging for large-vessel vasculitis. Curr. Opin. Rheumatol. 2009, 21, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Unizony, S.H.; Bao, M.; Luder, Y.; Han, J.; Pavlov, A.; Stone, J.H. Tocilizumab vs placebo for the treatment of giant cell arteritis with polymyalgia rheumatica symptoms, cranial symptoms or both in a randomized trial. Semin. Arthritis Rheum. 2021, 51, 469–476. [Google Scholar] [CrossRef] [PubMed]
- De Mornac, D.; Espitia, O.; Néel, A.; Connault, J.; Masseau, A.; Espitia-Thibault, A.; Artifoni, M.; Achille, A.; Wahbi, A.; Lacou, M.; et al. Large-vessel involvement is predictive of multiple relapses in giant cell arteritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211009029. [Google Scholar] [CrossRef] [PubMed]
- Birkhead, N.C.; Wagener, H.P.; Shick, R.M. Treatment of temporal arteritis with adrenal corticosteroids; results in fifty-five cases in which lesion was proved at biopsy. J. Am. Med. Assoc. 1957, 163, 821–827. [Google Scholar] [CrossRef]
- Miloslavsky, E.M.; Naden, R.P.; Bijlsma, J.W.J.; Brogan, P.A.; Brown, E.S.; Brunetta, P.; Buttgereit, F.; Choi, H.K.; DiCaire, J.-F.; Gelfand, J.M.; et al. Development of a glucocorticoid toxicity index (GTI) using multicriteria decision analysis. Ann. Rheum. Dis. 2017, 76, 543–546. [Google Scholar] [CrossRef]
- Proven, A.; Gabriel, S.E.; Orces, C.; O’Fallon, W.M.; Hunder, G.G. Glucocorticoid therapy in giant cell arteritis: Duration and adverse outcomes. Arthritis Rheum. 2003, 49, 703–708. [Google Scholar] [CrossRef]
- Perrineau, S.; Ghesquière, T.; Charles, P.; Paule, R.; Samson, M.; Gayraud, M.; Chauvin, A.; Terrier, B.; Guillevin, L.; Bonnotte, B.; et al. A French cohort of patients with giant cell arteritis: Glucocorticoid treatment and its associated side effects. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 129), 155–160. [Google Scholar]
- Mahr, A.D.; Jover, J.A.; Spiera, R.F.; Hernández-García, C.; Fernández-Gutiérrez, B.; Lavalley, M.P.; Merkel, P.A. Adjunctive methotrexate for treatment of giant cell arteritis: An individual patient data meta-analysis. Arthritis Rheum. 2007, 56, 2789–2797. [Google Scholar] [CrossRef]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of tocilizumab in giant-cell arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienvenu, B.; Ly, K.H.; Lambert, M.; Agard, C.; André, M.; Benhamou, Y.; Bonnotte, B.; de Boysson, H.; Espitia, O.; Fau, G.; et al. Management of giant cell arteritis: Recommendations of the French study group for large vessel vasculitis (GEFA). Rev. Med. Interne 2016, 37, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackie, S.L.; Dejaco, C.; Appenzeller, S.; Camellino, D.; Duftner, C.; Gonzalez-Chiappe, S.; Mahr, A.; Mukhtyar, C.; Reynolds, G.; de Souza, A.W.S.; et al. British society for rheumatology guideline on diagnosis and treatment of giant cell arteritis: Executive summary. Rheumatology 2020, 59, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American college of rheumatology/vasculitis foundation guideline for the management of giant cell arteritis and takayasu arteritis. Arthritis Rheumatol. 2021, 73, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.S. Giant cell arteritis. Ann. Intern. Med. 2016, 165, ITC65–ITC80. [Google Scholar] [CrossRef]
- Diamantopoulos, A.P.; Haugeberg, G.; Lindland, A.; Myklebust, G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: Towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016, 55, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Monti, S.; Bartoletti, A.; Bellis, E.; Delvino, P.; Montecucco, C. Fast-track ultrasound clinic for the diagnosis of giant cell arteritis changes the prognosis of the disease but not the risk of future relapse. Front. Med. 2020, 7, 589794. [Google Scholar] [CrossRef]
- Chevalet, P.; Barrier, J.H.; Pottier, P.; Magadur-Joly, G.; Pottier, M.A.; Hamidou, M.; Planchon, B.; El Kouri, D.; Connan, L.; Dupond, J.L.; et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: A one year followup study of 164 patients. J. Rheumatol. 2000, 27, 1484–1491. [Google Scholar]
- Mazlumzadeh, M.; Hunder, G.G.; Easley, K.A.; Calamia, K.T.; Matteson, E.L.; Griffing, W.L.; Younge, B.R.; Weyand, C.M.; Goronzy, J.J. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: A double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006, 54, 3310–3318. [Google Scholar] [CrossRef]
- Dasgupta, B.; Borg, F.A.; Hassan, N.; Alexander, L.; Barraclough, K.; Bourke, B.; Fulcher, J.; Hollywood, J.; Hutchings, A.; James, P.; et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology 2010, 49, 1594–1597. [Google Scholar] [CrossRef] [Green Version]
- Hunder, G.G.; Sheps, S.G.; Allen, G.L.; Joyce, J.W. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: Comparison in a prospective study. Ann. Intern. Med. 1975, 82, 613–618. [Google Scholar] [PubMed]
- Hayreh, S.S.; Zimmerman, B.; Kardon, R.H. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol. Scand. 2002, 80, 355–367. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; Blanco, R.; Rodríguez-Valverde, V.; Martínez-Taboada, V.M.; Delgado-Rodriguez, M.; Figueroa, M.; Uriarte, E. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: Predictors and response to treatment. Arthritis Rheum. 1998, 41, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Kyle, V.; Hazleman, B.L. Treatment of polymyalgia rheumatica and giant cell arteritis. I. Steroid regimens in the first two months. Ann. Rheum. Dis. 1989, 48, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lado, L.; Calviño-Díaz, C.; Piñeiro, A.; Dierssen, T.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Lopez-Diaz, M.J.; Blanco, R.; Llorca, J.; Gonzalez-Gay, M.A. Relapses and recurrences in giant cell arteritis: A population-based study of patients with biopsy-proven disease from Northwestern Spain. Medicine 2011, 90, 186–193. [Google Scholar] [CrossRef]
- Mendel, A.; Ennis, D.; Carette, S.; Pagnoux, C. Assessment of glucocorticoid tapering in large vessel and anti-neutrophil cytoplasmic antibody-associated vasculitides. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 129), 119–124. [Google Scholar]
- Spiera, R.F.; Mitnick, H.J.; Kupersmith, M.; Richmond, M.; Spiera, H.; Peterson, M.G.; Paget, S.A. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin. Exp. Rheumatol. 2001, 19, 495–501. [Google Scholar]
- Hoffman, G.S.; Cid, M.C.; Hellmann, D.B.; Guillevin, L.; Stone, J.H.; Schousboe, J.; Cohen, P.; Calabrese, L.H.; Dickler, H.; Merkel, P.A.; et al. A Multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002, 46, 1309–1318. [Google Scholar] [CrossRef]
- Jover, J.A.; Hernández-García, C.; Morado, I.C.; Vargas, E.; Bañares, A.; Fernández-Gutiérrez, B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2001, 134, 106–114. [Google Scholar]
- Gérard, A.-L.; Simon-Tillaux, N.; Yordanov, Y.; Cacoub, P.; Tubach, F.; Saadoun, D.; Dechartres, A. Efficacy and safety of steroid-sparing treatments in giant cell arteritis according to the glucocorticoids tapering regimen: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 88, 96–103. [Google Scholar] [CrossRef]
- Diamantopoulos, A.P.; Hetland, H.; Myklebust, G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: A case series. BioMed Res. Int. 2013, 2013, 120638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tengesdal, S.; Diamantopoulos, A.P.; Myklebust, G. Leflunomide versus methotrexate in treatment of giant cell arteritis: Comparison of efficacy, safety, and drug survival. Scand. J. Rheumatol. 2019, 48, 333–335. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.; Hazleman, B.L. Azathioprine in giant cell arteritis/polymyalgia rheumatica: A double-blind study. Ann. Rheum. Dis. 1986, 45, 136–138. [Google Scholar]
- Ly, K.H.; Dalmay, F.; Gondran, G.; Palat, S.; Bezanahary, H.; Cypierre, A.; Fauchais, A.-L.; Liozon, E. Steroid-sparing effect and toxicity of dapsone treatment in giant cell arteritis: A single-center, retrospective study of 70 patients. Medicine 2016, 95, e4974. [Google Scholar] [CrossRef] [PubMed]
- Liozon, F.; Vidal, E.; Barrier, J. Does dapsone have a role in the treatment of temporal arteritis with regard to efficacy and toxicity? Clin. Exp. Rheumatol. 1993, 11, 694–695. [Google Scholar] [PubMed]
- Sailler, L.; Pugnet, G.; Bienvenu, B. Treatment of giant cell arteritis. Rev. Med. Interne 2013, 34, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Piras, D.; Baldovino, S.; Russo, A.; Naretto, C.; Rossi, D.; Alpa, M.; Roccatello, D. Mycophenolate mofetil as steroid-sparing treatment for elderly patients with giant cell arteritis: Report of three cases. Aging Clin. Exp. Res 2012, 24, 273–277. [Google Scholar] [PubMed]
- Schaufelberger, C.; Möllby, H.; Uddhammar, A.; Bratt, J.; Nordborg, E. No additional steroid-sparing effect of cyclosporine A in giant cell arteritis. Scand. J. Rheumatol. 2006, 35, 327–329. [Google Scholar] [CrossRef]
- Seror, R.; Baron, G.; Hachulla, E.; Debandt, M.; Larroche, C.; Puéchal, X.; Maurier, F.; de Wazieres, B.; Quéméneur, T.; Ravaud, P.; et al. Adalimumab for steroid sparing in patients with giant-cell arteritis: Results of a multicentre randomised controlled trial. Ann. Rheum. Dis. 2014, 73, 2074–2081. [Google Scholar] [CrossRef]
- Martínez-Taboada, V.M.; Rodríguez-Valverde, V.; Carreño, L.; López-Longo, J.; Figueroa, M.; Belzunegui, J.; Mola, E.M.; Bonilla, G. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann. Rheum. Dis. 2008, 67, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, G.S.; Cid, M.C.; Rendt-Zagar, K.E.; Merkel, P.A.; Weyand, C.M.; Stone, J.H.; Salvarani, C.; Xu, W.; Visvanathan, S.; Rahman, M.U.; et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: A randomized trial. Ann. Intern. Med. 2007, 146, 621–630. [Google Scholar]
- Langford, C.A.; Cuthbertson, D.; Ytterberg, S.R.; Khalidi, N.; Monach, P.A.; Carette, S.; Seo, P.; Moreland, L.W.; Weisman, M.; Koening, C.L.; et al. A Randomized, double-blind trial of abatacept (CTLA4-IG) for the treatment of giant cell arteritis. Arthritis Rheumatol. 2017, 69, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Cecchi, I.; Sciascia, S.; Naretto, C.; Alpa, M.; Roccatello, D. An agent-to-agent real life comparison study of tocilizumab versus abatacept in giant cell arteritis. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 129), 125–128. [Google Scholar] [PubMed]
- Conway, R.; O’Neill, L.; O’Flynn, E.; Gallagher, P.; McCarthy, G.M.; Murphy, C.C.; Veale, D.J.; Fearon, U.; Molloy, E.S. Ustekinumab for the treatment of refractory giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1578–1579. [Google Scholar] [CrossRef] [PubMed]
- Matza, M.A.; Fernandes, A.D.; Stone, J.H.; Unizony, S.H. Ustekinumab for the treatment of giant cell arteritis. Arthritis Care Res. 2021, 73, 893–897. [Google Scholar] [CrossRef]
- Ly, K.-H.; Stirnemann, J.; Liozon, E.; Michel, M.; Fain, O.; Fauchais, A.-L. Interleukin-1 blockade in refractory giant cell arteritis. Jt. Bone Spine 2014, 81, 76–78. [Google Scholar] [CrossRef]
- Deshayes, S.; Ly, K.-H.; Rieu, V.; Maigné, G.; Martin Silva, N.; Manrique, A.; Monteil, J.; de Boysson, H.; Aouba, A. French study group for large vessel vasculitis (GEFA) steroid-sparing effect of anakinra in giant-cell arteritis: A case series with clinical, biological and iconographic long-term assessments. Rheumatology 2021, 61, 400–406. [Google Scholar] [CrossRef]
- Roche, N.E.; Fulbright, J.W.; Wagner, A.D.; Hunder, G.G.; Goronzy, J.J.; Weyand, C.M. Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum. 1993, 36, 1286–1294. [Google Scholar]
- Régent, A.; Redeker, S.; Deroux, A.; Kieffer, P.; Ly, K.H.; Dougados, M.; Liozon, E.; Larroche, C.; Guillevin, L.; Bouillet, L.; et al. Tocilizumab in giant cell arteritis: A multicentre retrospective study of 34 patients. J. Rheumatol. 2016, 43, 1547–1552. [Google Scholar] [CrossRef]
- Villiger, P.M.; Adler, S.; Kuchen, S.; Wermelinger, F.; Dan, D.; Fiege, V.; Bütikofer, L.; Seitz, M.; Reichenbach, S. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 1921–1927. [Google Scholar] [CrossRef]
- Stone, J.H.; Spotswood, H.; Unizony, S.H.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; Salvarani, C.; et al. New-onset versus relapsing giant cell arteritis treated with tocilizumab: 3-year results from a randomized controlled trial and extension. Rheumatology 2021, keab780. [Google Scholar] [CrossRef]
- Adler, S.; Reichenbach, S.; Gloor, A.; Yerly, D.; Cullmann, J.L.; Villiger, P.M. Risk of relapse after discontinuation of tocilizumab therapy in giant cell arteritis. Rheumatology 2019, 58, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Goercke, M.; Castañeda, S.; Aldasoro, V.; Villa, I.; Moriano, C.; Romero-Yuste, S.; Narváez, J.; Gómez-Arango, C.; Pérez-Pampín, E.; Melero, R.; et al. Tocilizumab in refractory giant cell arteritis. monotherapy versus combined therapy with conventional immunosuppressive drugs. Observational multicenter study of 134 patients. Semin. Arthritis Rheum. 2021, 51, 387–394. [Google Scholar] [CrossRef] [PubMed]
- De Boysson, H.; Le Besnerais, M.; Blaison, F.; Daumas, A.; Jarrot, P.-A.; Perrin, F.; Tieulié, N.; Maria, A.; Duffau, P.; Gombert, B.; et al. Assessment of the efficacy and safety of tocilizumab in patients over 80 years old with giant cell arteritis. Arthritis Res. Ther. 2021, 23, 143. [Google Scholar] [CrossRef]
- Strand, V.; Dimonaco, S.; Tuckwell, K.; Klearman, M.; Collinson, N.; Stone, J.H. Health-related quality of life in patients with giant cell arteritis treated with tocilizumab in a phase 3 randomised controlled trial. Arthritis Res. Ther. 2019, 21, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Régent, A.; Terrier, B.; Legendre, P.; Wartski, M.; Cohen, P.; Mouthon, L.; Le Jeunne, C. Efficacy of baricitinib for refractory large-vessel vasculitis. Rheumatology 2021, 60, e389–e391. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.J.; Crowson, C.S.; Giblon, R.E.; Jaquith, J.M.; Duarte-García, A.; Matteson, E.L.; Weyand, C.M.; Warrington, K.J. Baricitinib for relapsing giant cell arteritis: A prospective open-label 52-week pilot study. Ann. Rheum. Dis. 2022; ahead of print. [Google Scholar] [CrossRef]
- Rotar, Ž.; Tomšic, M.; Hocevar, A. Secukinumab for the maintenance of glucocorticoid-free remission in a patient with giant cell arteritis and psoriatic arthritis. Rheumatology 2018, 57, 934–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaval, L.; Daumas, A.; Samson, M.; Ebbo, M.; De Boysson, H.; Liozon, E.; Dupuy, H.; Puyade, M.; Blockmans, D.; Benhamou, Y.; et al. Large-vessel vasculitis diagnosed between 50 and 60 years: Case-control study based on 183 cases and 183 controls aged over 60 years. Autoimmun. Rev. 2019, 18, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Devilliers, H.; Ly, K.H.; Maurier, F.; Bienvenu, B.; Terrier, B.; Charles, P.; Besancenot, J.F.; Faucher, A.L.; Binquet, C.; et al. Tocilizumab as an add-on therapy to glucocorticoids during the first 3 months of treatment of giant cell arteritis: Results of a French multicenter prospective open-label study. Arthritis Rheumatol. 2016, 68 (Suppl. S10). [Google Scholar]
- Wilson, J.C.; Sarsour, K.; Collinson, N.; Tuckwell, K.; Musselman, D.; Klearman, M.; Napalkov, P.; Jick, S.S.; Stone, J.H.; Meier, C.R. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): A nested case-control analysis. Semin. Arthritis Rheum. 2016, 46, 819–827. [Google Scholar] [CrossRef]
- Nesher, G.; Sonnenblick, M.; Friedlander, Y. Analysis of steroid related complications and mortality in temporal arteritis: A 15-year survey of 43 patients. J. Rheumatol. 1994, 21, 1283–1286. [Google Scholar] [PubMed]
- Aussedat, M.; Lobbes, H.; Samson, M.; Euvrard, R.; Lega, J.-C.; Mainbourg, S. Epidemiology of major relapse in giant cell arteritis: A study-level meta-analysis. Autoimmun. Rev. 2022, 21, 102930. [Google Scholar] [CrossRef]
- Saleem, B.; Keen, H.; Goeb, V.; Parmar, R.; Nizam, S.; Hensor, E.M.A.; Churchman, S.M.; Quinn, M.; Wakefield, R.; Conaghan, P.G.; et al. Patients with RA in remission on TNF blockers: When and in whom can TNF blocker therapy be stopped? Ann. Rheum. Dis. 2010, 69, 1636–1642. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesher, G.; Berkun, Y.; Mates, M.; Baras, M.; Rubinow, A.; Sonnenblick, M. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum. 2004, 50, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Smith, S.D.; Galor, A.; Hoffman, G.S. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum. 2006, 54, 3306–3309. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Taboada, V.M.; López-Hoyos, M.; Narvaez, J.; Muñoz-Cacho, P. Effect of antiplatelet/anticoagulant therapy on severe ischemic complications in patients with giant cell arteritis: A cumulative meta-analysis. Autoimmun. Rev. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- García-Martínez, A.; Hernández-Rodríguez, J.; Grau, J.M.; Cid, M.C. Treatment with statins does not exhibit a clinically relevant corticosteroid-sparing effect in patients with giant cell arteritis. Arthritis Rheum. 2004, 51, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Narváez, J.; Bernad, B.; Gómez-Vaquero, C.; García-Gómez, C.; Roig-Vilaseca, D.; Juanola, X.; Rodriguez-Moreno, J.; Nolla, J.M.; Valverde, J. Impact of antiplatelet therapy in the development of severe ischemic complications and in the outcome of patients with giant cell arteritis. Clin. Exp. Rheumatol. 2008, 26, S57–S62. [Google Scholar] [PubMed]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Sadun, R.E.; et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: Version 1. Arthritis Rheumatol. 2021, 73, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Cadiou, S.; Perdriger, A.; Ardois, S.; Albert, J.-D.; Berthoud, O.; Lescoat, A.; Guggenbuhl, P.; Robin, F. SARS-CoV-2, Polymyalgia rheumatica and giant cell arteritis: COVID-19 vaccine shot as a trigger? Comment on: “Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica?” By Manzo et al. Jt. Bone Spine 2022, 89, 105282. [Google Scholar] [CrossRef]
| Author, Year | Number of Patients | Disease Activity | Drug Evaluated | GC Therapy | Main Primary Outcome | Result |
|---|---|---|---|---|---|---|
| Azathioprine | ||||||
| De Silva et al., 1986 | 31 | GCA in remission | Azathioprine 150 mg/day | GCs ≥ 5 mg/day Tapering 1 mg/month | Not specified (studied GCs daily dose) | Positive at month 12 |
| Dapsone | ||||||
| Liozon et al., 1993 | 48 | New-onset GCA | Dapsone 50–100 mg/day | 0.7–1.0 mg/kg/day Tapering ≈ 14 months | Relapses | Positive but dapsone-related side effects |
| Hydroxychloroquine | ||||||
| Sailler et al., 2009 | 74 | New-onset GCA | Hydroxychloroquine 400 mg/day | 0.7 mg/kg/day Tapering ≈ 16 months | Remission > 3 month at the end of follow-up | Negative and hydroxychloroquine-related side effects |
| Methotrexate | ||||||
| Spiera et al., 2001 | 21 | New-onset GCA | MTX 7.5–20 mg/week | >40 mg/day. Suggested tapering ≈ 4 months | Cumulative dose of GCs at year 2 | Negative |
| Jover et al., 2001 | 42 | New-onset GCA | MTX 10 mg/week | 60 mg/day. Tapering ≈ 6 months | Cumulative dose of GCs and relapses | Positive |
| Hoffman et al., 2002 | 98 | New-onset GCA | MTX 0.15–0.25 mg/kg/week; max 15 mg/week | 1 mg/kg/day and <60 mg/day; alternate day tapering ≈ 6 months | Relapses | Negative |
| Cyclosporine A | ||||||
| Schaufelberger et al., 2006 | 60 | New-onset GCA | CsA 2 mg/kg/day reduced or increased up to 3.5 mg/kg/day | Not specified | Cumulative dose of GCs and relapses | Negative. Numerous side effects |
| Author, Year | Number of Patients | Disease Activity | Drug Evaluated | GC Therapy | Main Primary Outcome | Result |
|---|---|---|---|---|---|---|
| Anti-TNF therapy | ||||||
| Hoffman et al., 2007 | 44 | New-onset GCA | Infliximab 5 mg/kg W0, 2 and 6 and every 8 weeks | Tapering < 6 months | Relapses at W22 | Negative |
| Martinez-Taboadda et al., 2008 | 17 | GCA in remission under GC > 10 mg/day. GC-related side effects | Etanercept 25 mg × 2/week | Tapering < 4 months (depending on initial daily dose) | GC-free remission at M12 | Negative |
| Seror et al., 2013 | 70 | New-onset GCA | Adalimumab 40 mg at W2, 4, 6, 8, 10 | 0.7 mg/kg. Tapering ≈ 10 months | Remission at W26 with GC < 0.1 mg/kg | Negative |
| Abatacept (CTLA4–Ig) | ||||||
| Langford et al., 2017 | 49 | New-onset or relapsing GCA Abatacept 10 mg/kg D1, 15, 29, 56 and GCs 40–60 mg/day for remission induction | 41 patients randomised at W12, abatacept 10 mg/kg/4 weeks | 20 mg/day at randomisation. Tapering until W28 | Relapse-free survival | Positive |
| Tocilizumab (IL-6 receptor inhibitor) | ||||||
| Villiger et al., 2016 | 30 | New-onset or relapsing | Tocilizumab 8 mg/kg/month | 1 mg/kg/day Tapering ≈ 9 months | Remission at W12 with GCs 0.1 mg/kg. Normal ESR and CRP | Positive |
| Stone et al., 2017 | 251 | New-onset or relapsing | Tocilizumab 162 mg/week or 162 mg every other week | 20–60 mg/day. Tapering 26 or 52 weeks | Prednisone-free remission at W52 | Positive |
| Mavrilimumab (GM-CSF receptor-α inhibitor) | ||||||
| Cid et al., 2020 | 70 | New-onset or relapsing | Mavrilimumab 150 mg every other week | 20–60 mg/day. Tapering 26 weeks | Relapse at W26 | Positive |
| Secukinumab (IL-17A inhibitor) | ||||||
| Venhoff et al., 2021 | 52 | New-onset or relapsing | Secukinumab 300 mg/week (5 doses) then 300 mg/4 week until W48 | 25–60 mg/day. Tapering 26 weeks | Sustained remission at W28 | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régent, A.; Mouthon, L. Treatment of Giant Cell Arteritis (GCA). J. Clin. Med. 2022, 11, 1799. https://doi.org/10.3390/jcm11071799
Régent A, Mouthon L. Treatment of Giant Cell Arteritis (GCA). Journal of Clinical Medicine. 2022; 11(7):1799. https://doi.org/10.3390/jcm11071799
Chicago/Turabian StyleRégent, Alexis, and Luc Mouthon. 2022. "Treatment of Giant Cell Arteritis (GCA)" Journal of Clinical Medicine 11, no. 7: 1799. https://doi.org/10.3390/jcm11071799
APA StyleRégent, A., & Mouthon, L. (2022). Treatment of Giant Cell Arteritis (GCA). Journal of Clinical Medicine, 11(7), 1799. https://doi.org/10.3390/jcm11071799






