Associations between Phosphate Concentrations and Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation
Abstract
:1. Introduction
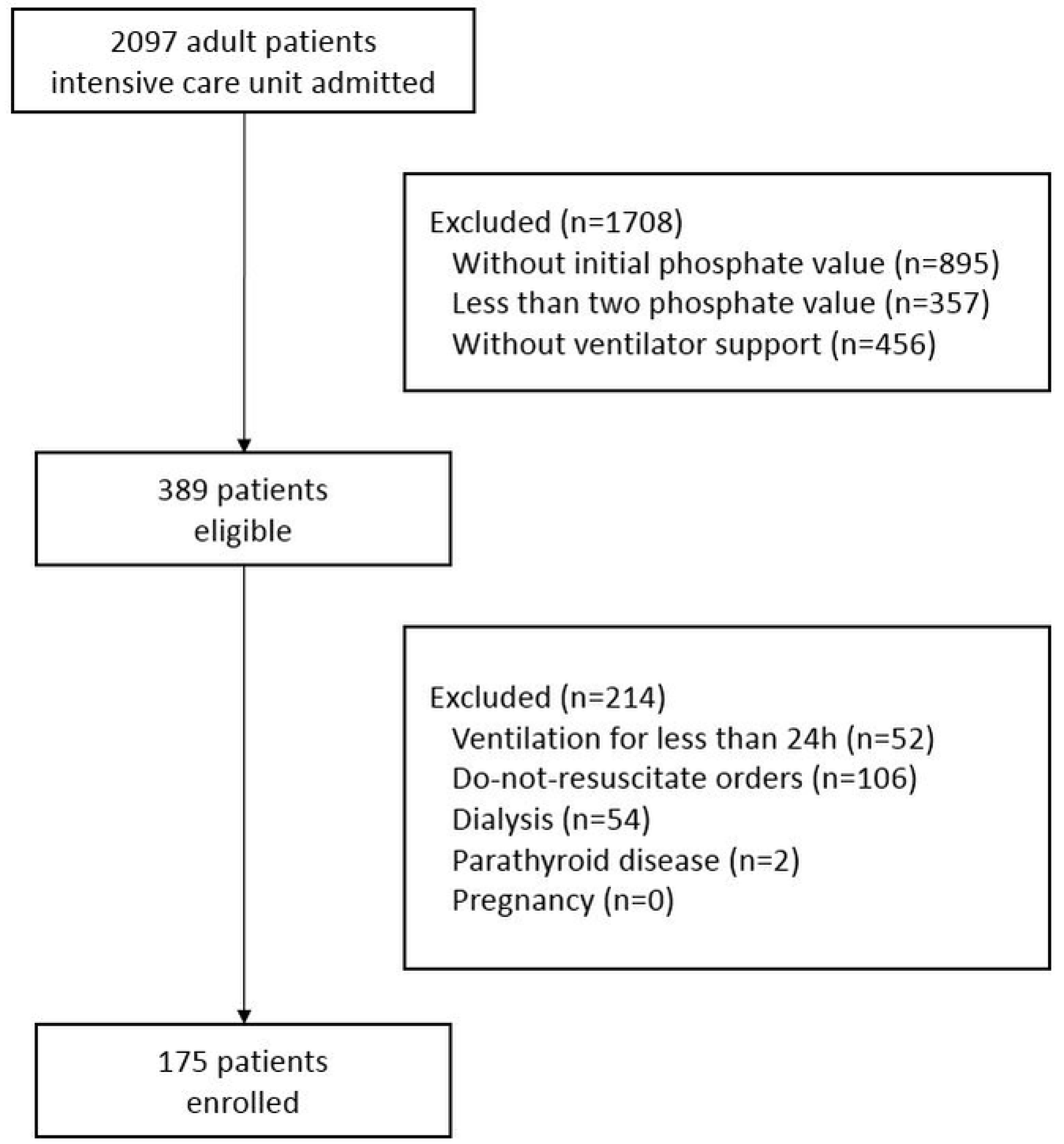
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Variable Definitions
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Clinical Course
3.2. Phosphate Concentrations and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, S.B.; Worthley, L.I. The essentials of calcium, magnesium and phosphate metabolism: Part I. Physiology. Crit. Care Resusc. 2002, 4, 301–306. [Google Scholar] [PubMed]
- Wadsworth, R.; Siddiqui, S. Phosphate homeostasis in critical care. BJA Educ. 2016, 16, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Sakhuja, A.; Erickson, S.B. Admission hyperphosphatemia increases the risk of acute kidney injury in hospitalized patients. J. Nephrol. 2018, 31, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Egi, M.; Schneider, A.G.; Bellomo, R.; Hart, G.K.; Hegarty, C. Hypophosphatemia in critically ill patients. J. Crit. Care 2013, 28, 536.e9–536.e19. [Google Scholar] [CrossRef] [PubMed]
- Fiaccadori, E.; Coffrini, E.; Fracchia, C.; Rampulla, C.; Montagna, T.; Borghetti, A. Hypophosphatemia and phosphorus depletion in respiratory and peripheral muscles of patients with respiratory failure due to COPD. Chest 1994, 105, 1392–1398. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.; Gurman, G.; Cohen, G.; Gilutz, H.; Brill, S.; Schily, M.; Gurevitch, B.; Shoenfeld, Y. Association between hypophosphatemia and cardiac arrhythmias in the early stages of sepsis. Eur. J. Intern. Med. 2002, 13, 434. [Google Scholar] [CrossRef]
- Han, S.A.; Park, H.Y.; Kim, H.W.; Choi, J.I.; Kang, D.Y.; Kim, H.L.; Chung, J.H.; Shin, B.C. Severe hypophosphatemia-induced acute toxic-metabolic encephalopathy in continuous renal replacement therapy. Electrolyte Blood Press. 2019, 17, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Berner, Y.N.; Shike, M. Consequences of phosphate imbalance. Annu. Rev. Nutr. 1988, 8, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Gravelyn, T.R.; Brophy, N.; Siegert, C.; Peters-Golden, M. Hypophosphatemia-associated respiratory muscle weakness in a general inpatient population. Am. J. Med. 1988, 84, 870–876. [Google Scholar] [CrossRef]
- Aubier, M.; Murciano, D.; Lecocguic, Y.; Viires, N.; Jacquens, Y.; Squara, P.; Pariente, R. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N. Engl. J. Med. 1985, 313, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Alsumrain, M.H.; Jawad, S.A.; Imran, N.B.; Riar, S.; DeBari, V.A.; Adelman, M. Association of hypophosphatemia with failure-to-wean from mechanical ventilation. Ann. Clin. Lab. Sci. 2010, 40, 144–148. [Google Scholar] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Thirunavukkarasu, S.; Kashani, K.B. Admission serum phosphate levels and the risk of respiratory failure. Int. J. Clin. Pract. 2020, 74, e13461. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, H.; Dos Santos Rocha, A.; Beckmann, T.S.; Larpin, C.; Buetti, N.; Quintard, H.; Pugin, J.; Heidegger, C.P. Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation. J. Clin. Med. 2022, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, S.; Teo, B.W.; Guzman, J.A.; Heyka, R.J.; Paganini, E.P.; Fissell, W.H.; Schold, J.D.; Schreiber, M.J. Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol. Dial. Transplant. 2011, 26, 3508–3514. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.K.; Irshad, M.; Gupta, N.; Kabra, S.K.; Lodha, R. Hypophosphatemia in critically ill children: Risk factors, outcome and mechanism. Indian J. Pediatr. 2016, 83, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Lindner, G.; Wolzt, M.; Ahmad, S.S.; Sauter, T.; Leichtle, A.B.; Fiedler, G.M.; Fuhrmann, V.; Exadaktylos, A.K. Hyperphosphatemia is an independent risk factor for mortality in critically ill patients: Results from a cross-sectional study. PLoS ONE 2015, 10, e0133426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xiao, C.; Chen, L.; Zhang, X.; Kou, Q. Impact of hypophosphatemia on outcome of patients in intensive care unit: A retrospective cohort study. BMC Anesthesiol. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Doepker, B.A.; Springer, A.N.; Exline, M.C.; Phillips, G.; Murphy, C.V. Impact of serum phosphate in mechanically ventilated patients with severe sepsis and septic shock. J. Intensive Care Med. 2020, 35, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Kittanamongkolchai, W.; Sakhuja, A.; Erickson, S.B. Admission serum phosphate levels predict hospital mortality. Hosp. Pract. 2018, 46, 121–127. [Google Scholar] [CrossRef]
- Hedjoudje, A.; Farha, J.; Cheurfa, C.; Grabar, S.; Weiss, E.; Badurdeen, D.; Kumbhari, V.; Prat, F.; Levy, P.; Piton, G. Serum phosphate is associated with mortality among patients admitted to ICU for acute pancreatitis. United Eur. Gastroenterol. J. 2021, 9, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.C.K.; Laupland, K.B.; Ramanan, M.; Tabah, A. Phosphate abnormalities and outcomes among admissions to the intensive care unit: A retrospective multicentre cohort study. J. Crit. Care 2021, 64, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Hansrivijit, P.; Thirunavukkarasu, S.; Chewcharat, A.; Medaura, J.; Mao, M.A.; Kashani, K.B. Impact of serum phosphate changes on in-hospital mortality. BMC Nephrol. 2020, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Eddington, H.; Hoefield, R.; Sinha, S.; Chrysochou, C.; Lane, B.; Foley, R.N.; Hegarty, J.; New, J.; O’Donoghue, D.J.; Middleton, R.J.; et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 2251–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Hamano, T. Serum phosphate level and the prognosis of dialysis patients. Clin. Calcium 2009, 19, 174–179. [Google Scholar]
- Ess, M.; Heitmair-Wietzorrek, K.; Frick, M.; Umlauf, N.; Ulmer, H.; Poelzl, G. Serum phosphate and long-term outcome among patients with stable heart failure. J. Card. Fail. 2013, 19, 25–30. [Google Scholar] [CrossRef]
- Sato, M.; Kataoka, H.; Ushio, Y.; Manabe, S.; Watanabe, S.; Akihisa, T.; Makabe, S.; Yoshida, R.; Iwasa, N.; Mitobe, M.; et al. High serum phosphate level as a risk factor to determine renal prognosis in autosomal dominant polycystic kidney disease: A retrospective study. Medicines 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.G.; Zhao, J.S.; Wang, J.Q.; Liu, B.; Cheng, S.H.; Fan, X.Q. Effect of the concentration of serum phosphate on the prognosis of patients with sepsis. Chin. J. Clin. Health 2008, 4, 371–373. [Google Scholar]
- Geerse, D.A.; Bindels, A.J.; Kuiper, M.A.; Roos, A.N.; Spronk, P.E.; Schultz, M.J. Approach to hypophosphataemia in intensive care units—A nationwide survey. Neth. J. Med. 2012, 70, 425–430. [Google Scholar]
- Charron, T.; Bernard, F.; Skrobik, Y.; Simoneau, N.; Gagnon, N.; Leblanc, M. Intravenous phosphate in the intensive care unit: More aggressive repletion regimens for moderate and severe hypophosphatemia. Intensive Care Med. 2003, 29, 1273–1278. [Google Scholar] [CrossRef]
- Bech, A.; Blans, M.; Raaijmakers, M.; Mulkens, C.; Telting, D.; de Boer, H. Hypophosphatemia on the intensive care unit: Individualized phosphate replacement based on serum levels and distribution volume. J. Crit. Care 2013, 28, 838–843. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristic | Value |
|---|---|
| Sex (male) | 108 (61.7%) |
| Age (years) | 66.68 ± 16.36 |
| Main diagnosis | |
| Respiratory failure | 49 (28.0%) |
| Septic shock | 46 (26.3%) |
| Aspiration | 25 (14.3%) |
| Post-cardiac arrest syndrome | 21 (12.0%) |
| Hypovolemic shock | 9 (5.1%) |
| Neurologic disease | 9 (5.1%) |
| Cardiovascular disease | 5 (2.9%) |
| Others | 11 (6.3%) |
| Body mass index (kg/m2) | 22.05 ± 4.50 |
| Phosphate categories (mg/dL) | |
| Initial phosphate levels * | 3.23 ± 1.67 |
| Delta phosphate levels ** | 2.50 ± 1.58 |
| Mean phosphate levels † | 2.75 ± 0.57 |
| Phosphate intravenous supplementation | |
| Total number of supplies | 2.86 ± 3.55 |
| Proportion of phosphate supply | 107 (61.1%) |
| Creatinine (mg/dL) | 1.03 ± 0.54 |
| Creatinine clearance (mL/min/1.73 m2) | 67.68 ± 43.98 |
| Chronic kidney disease without dialysis | 10 (5.7%) |
| Potassium (mmol/L) | 3.83 ± 0.70 |
| Ionized calcium (mg/dL) | 4.40 ± 0.45 |
| Magnesium (mg/dL) | 2.04 ± 0.37 |
| Albumin (g/dL) | 3.03 ± 0.54 |
| APACHE II score | 28.35 ± 7.06 |
| Charlson comorbidity index | 4.46 ± 2.48 |
| PaO2/FiO2 ratio (mmHg) | 223.57 ± 145.82 |
| Patient Outcome | Value |
|---|---|
| Duration of mechanical ventilation (days) | 11.97 ± 11.37 |
| LOS in the ICU (days) | 15.82 ± 12.60 |
| LOS in the hospital (days) | 29.83 ± 20.01 |
| 28-day mortality | 49 (28.0%) |
| ICU mortality | 51 (29.1%) |
| Hospital mortality | 57 (32.6%) |
| Univariable | Multivariable * | |||||
|---|---|---|---|---|---|---|
| Phosphate | Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value |
| Initial phosphate ** | 1.31 | 1.07–1.59 | 0.01 | 1.15 | 0.92–1.42 | 0.06 |
| Delta phosphate † | 1.65 | 1.35–2.01 | <0.0001 | 1.56 | 1.28–1.91 | <0.0001 |
| Mean phosphate †† | 2.24 | 1.63–3.06 | <0.0001 | 2.13 | 1.52–2.99 | <0.0001 |
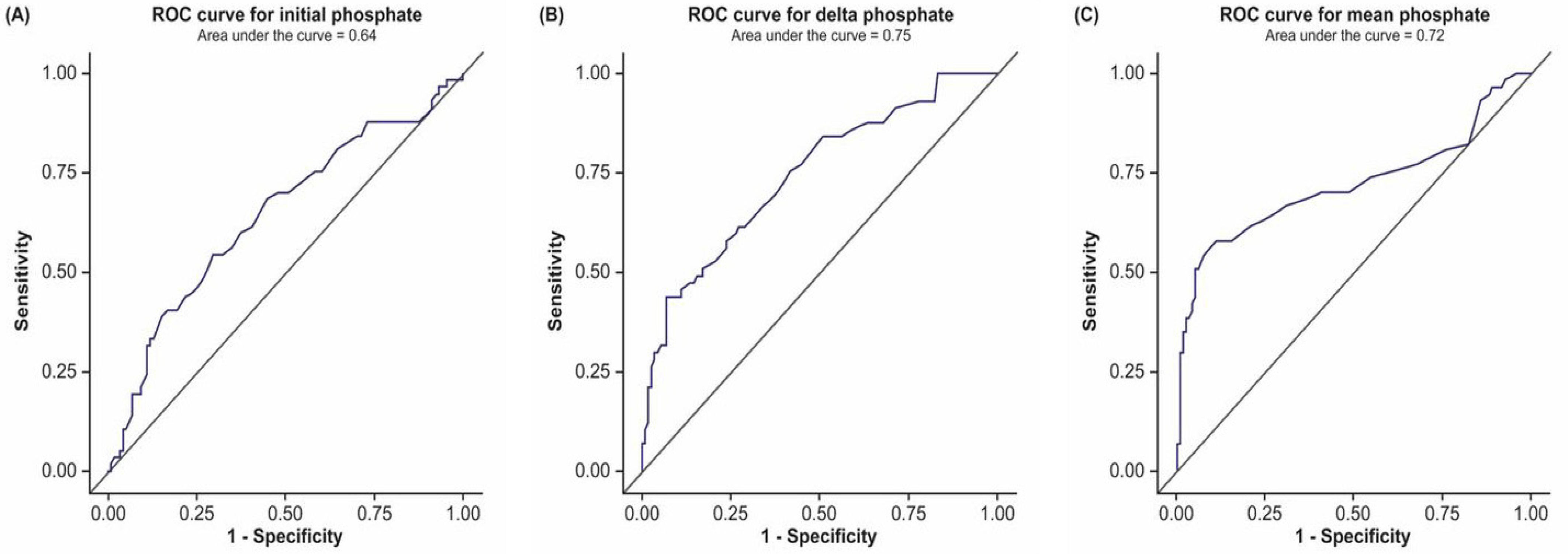
| Phosphate | AUC | 95% CI | Criterion (mg/dL) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Initial phosphate * | 0.64 | 0.55–0.73 | >3.80 | 49.1% | 72.9% | 46.7% | 74.8% |
| Delta phosphate ** | 0.75 | 0.67–0.82 | >5.58 | 24.6% | 97.5% | 82.4% | 72.8% |
| Mean phosphate † | 0.72 | 0.63–0.82 | >3.70 | 57.9% | 89.0% | 71.7% | 81.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.K.; Kim, C.Y.; Kim, S.; Kim, Y.J.; Lee, S.H.; Kim, J.H. Associations between Phosphate Concentrations and Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation. J. Clin. Med. 2022, 11, 1897. https://doi.org/10.3390/jcm11071897
Kim BK, Kim CY, Kim S, Kim YJ, Lee SH, Kim JH. Associations between Phosphate Concentrations and Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation. Journal of Clinical Medicine. 2022; 11(7):1897. https://doi.org/10.3390/jcm11071897
Chicago/Turabian StyleKim, Beong Ki, Chi Young Kim, Sua Kim, Yu Jin Kim, Seung Heon Lee, and Je Hyeong Kim. 2022. "Associations between Phosphate Concentrations and Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation" Journal of Clinical Medicine 11, no. 7: 1897. https://doi.org/10.3390/jcm11071897







