Cerebral Tissue Oxygen Saturation Is Enhanced in Patients following Transcatheter Aortic Valve Implantation: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Perioperative Outcome
3.3. Cerebral Oximetry
3.4. Hemodynamic Confounder
3.5. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef]
- Chiam, P.T.; Ewe, S.H. The expanding indications of transcatheter aortic valve implantation. Futur. Cardiol. 2016, 12, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Hawken, N.; Brookes, E.; Kuehn, C.; Liden, B. Patient-centered benefit-risk analysis of transcatheter aortic valve replacement. F1000Research 2021, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Siemieniuk, R.A.; Agoritsas, T.; Manja, V.; Devji, T.; Chang, Y.; Bala, M.M.; Thabane, L.; Guyatt, G.H. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: Systematic review and meta-analysis. BMJ 2016, 354, i5130. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Wang, K.; House, J.A.; Magnuson, E.A.; Reynolds, M.R.; Makkar, R.; Herrmann, H.C.; Kodali, S.; Thourani, V.H.; Kapadia, S.; et al. Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Risk. Circulation 2019, 139, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Lone, A.N.; Saleem, M.A.; Kaluski, E. Transcatheter vs surgical aortic-valve replacement in low-to intermediate-surgical-risk candidates: A meta-analysis and systematic review. Clin. Cardiol. 2017, 40, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Makhdoum, A.; Koziarz, A.; Gupta, S.; Alsagheir, A.; Pandey, A.; Reza, S.; Um, K.; Teoh, K.; Alhazzani, W.; et al. Outcomes of sutureless aortic valve replacement versus conventional aortic valve replacement and transcatheter aortic valve replacement, updated systematic review, and meta-analysis. J. Card. Surg. 2021, 36, 4734–4742. [Google Scholar] [CrossRef]
- Lou, Y.; Gao, Y.; Yu, Y.; Li, Y.; Xi, Z.; Swe, K.N.C.; Zhou, Y.; Nie, X.; Liu, W. Efficacy and Safety of Transcatheter vs. Surgical Aortic Valve Replacement in Low-to-Intermediate-Risk Patients: A Meta-Analysis. Front. Cardiovasc. Med. 2020, 7, 590975. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e27–e227. [Google Scholar] [CrossRef]
- Lazkani, M.; Singh, N.; Howe, C.; Patel, N.; Colon, M.J.; Tasset, M.; Amabile, O.; Morris, M.; Fang, H.K.; Pershad, A. An updated meta-analysis of TAVR in patients at intermediate risk for SAVR. Cardiovasc. Revasc. Med. 2019, 20, 57–69. [Google Scholar] [CrossRef]
- McInerney, A.; Vera-Urquiza, R.; Tirado-Conte, G.; Marroquin, L.; Jimenez-Quevedo, P.; Nuñez-Gil, I.; Pozo, E.; Gonzalo, N.; de Agustín, J.A.; Escaned, J.; et al. Pre-dilation and Post-dilation in Transcatheter Aortic Valve Replacement: Indications, Benefits and Risks. Interv. Cardiol. Rev. Res. Resour. 2021, 16, e28. [Google Scholar] [CrossRef]
- Vigelius-Rauch, U.; Zajonz, T.; Sander, M. Anesthesiological implications of minimally invasive valve interventions: Transcatheter aortic valve implantation, clip reconstruction on the mitral and tricuspid valve. Der Anaesth. 2021, 70, 97–111. [Google Scholar] [CrossRef]
- Rivera-Lara, L.; Vaca, A.Z.; Geocadin, R.G.; Healy, R.J.; Ziai, W.; Mirski, M.A. Cerebral autoregulation-oriented therapy at the bedside a comprehensive review. Anesthesiology 2017, 126, 1187–1199. [Google Scholar] [CrossRef]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, Techniques, and Limitations of Near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef]
- Lei, L.; Katznelson, R.; Fedorko, L.; Carroll, J.; Poonawala, H.; Machina, M.; Styra, R.; Rao, V.; Djaiani, G. Cerebral oximetry and postoperative delirium after cardiac surgery: A randomised, controlled trial. Anaesthesia 2017, 72, 1456–1466. [Google Scholar] [CrossRef]
- Heringlake, M.; Garbers, C.; Käbler, J.-H.; Anderson, I.; Heinze, H.; Schön, J.; Berger, K.-U.; Dibbelt, L.; Sievers, H.-H.; Hanke, T. Preoperative Cerebral Oxygen Saturation and Clinical Outcomes in Cardiac Surgery. Anesthesiology 2011, 114, 58–69. [Google Scholar] [CrossRef]
- Stannard, B.; Levin, M.A.; Lin, H.-M.; Weiner, M.M. Regional cerebral oximetry is consistent across self-reported racial groups and predicts 30-day mortality in cardiac surgery: A retrospective analysis. Int. J. Clin. Monit. Comput. 2021, 35, 413–421. [Google Scholar] [CrossRef]
- Ortega-Loubon, C.; Herrera-Gómez, F.; Bernuy-Guevara, C.; Jorge-Monjas, P.; Ochoa-Sangrador, C.; Bustamante-Munguira, J.; Tamayo, E.; Álvarez, F.J. Near-Infrared Spectroscopy Monitoring in Cardiac and Noncardiac Surgery: Pairwise and Network Meta-Analyses. J. Clin. Med. 2019, 8, 2208. [Google Scholar] [CrossRef]
- Mailhot, T.; Cossette, S.; Lambert, J.; Cournoyer, A.; Denault, A.Y. Cerebral oximetry as a biomarker of postoperative delirium in cardiac surgery patients. J. Crit. Care 2016, 34, 17–23. [Google Scholar] [CrossRef]
- Zorrilla-Vaca, A.; Healy, R.; Grant, M.; Joshi, B.; Rivera-Lara, L.; Brown, C.; Mirski, M.A. Intraoperative cerebral oximetry-based management for optimizing perioperative outcomes: A meta-analysis of randomized controlled trials. Can. J. Anaesth. 2018, 65, 529–542. [Google Scholar] [CrossRef]
- Gusmao-Flores, D.; Salluh, J.I.F.; Chalhub, R.Á.; Quarantini, L.C. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: A systematic review and meta-analysis of clinical studies. Crit. Care 2012, 16, R115. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef]
- Mayr, N.P.; Hapfelmeier, A.; Martin, K.; Kurz, A.; van der Starre, P.; Babik, B.; Mazzitelli, D.; Lange, R.; Wiesner, G.; Tassani-Prell, P. Comparison of sedation and general anaesthesia for transcatheter aortic valve implantation on cerebral oxygen saturation and neurocognitive outcome. Br. J. Anaesth. 2016, 116, 90–99. [Google Scholar] [CrossRef]
- Seppelt, P.C.; Mas-Peiro, S.; De Rosa, R.; Murray, I.M.; Arsalan, M.; Holzer, L.; Lotz, G.; Meybohm, P.; Zacharowski, K.; Walther, T.; et al. Dynamics of cerebral oxygenation during rapid ventricular pacing and its impact on outcome in transfemoral transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2021, 97, E146–E153. [Google Scholar] [CrossRef]
- Fanning, J.P.; Walters, D.L.; Wesley, A.J.; Anstey, C.; Huth, S.; Bellapart, J.; Collard, C.; Rapchuk, I.L.; Natani, S.; Savage, M.; et al. Intraoperative Cerebral Perfusion Disturbances During Transcatheter Aortic Valve Replacement. Ann. Thorac. Surg. 2017, 104, 1564–1568. [Google Scholar] [CrossRef]
- Pennekamp, C.; Bots, M.; Kappelle, L.; Moll, F.; de Borst, G. The Value of Near-Infrared Spectroscopy Measured Cerebral Oximetry During Carotid Endarterectomy in Perioperative Stroke Prevention. A Review. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 539–545. [Google Scholar] [CrossRef]
- Suppan, M.; Barcelos, G.; Luise, S.; Diaper, J.; Frei, A.; Ellenberger, C.; Adamopoulos, D.; Noble, S.; Licker, M. Improved Exercise Tolerance, Oxygen Delivery, and Oxygen Utilization After Transcatheter Aortic Valve Implantation for Severe Aortic Stenosis. CJC Open 2020, 2, 490–496. [Google Scholar] [CrossRef]
- Chan, M.J.; Chung, T.; Glassford, N.; Bellomo, R. Near-Infrared Spectroscopy in Adult Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1155–1165. [Google Scholar] [CrossRef]
- Mayr, N.P.; Wiesner, G.; Van Der Starre, P.; Hapfelmeier, A.; Goppel, G.; Kasel, A.M.; Hengstenberg, C.; Husser, O.; Schunkert, H.; Tassani-Prell, P. Dexmedetomidine versus propofol-opioid for sedation in transcatheter aortic valve implantation patients: A retrospective analysis of periprocedural gas exchange and hemodynamic support. Can. J. Anaesth. 2018, 65, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kitamura, T.; Kohira, S.; Torii, S.; Horai, T.; Hirata, M.; Mishima, T.; Sughimoto, K.; Ohkubo, H.; Irisawa, Y.; et al. Factors associated with a low initial cerebral oxygen saturation value in patients undergoing cardiac surgery. J. Artif. Organs 2017, 20, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Vretzakis, G.; Georgopoulou, S.; Stamoulis, K.; Stamatiou, G.; Tsakiridis, K.; Zarogoulidis, P.; Katsikogianis, N.; Kougioumtzi, I.; Machairiotis, N.; Tsiouda, T.; et al. Cerebral oximetry in cardiac anesthesia. J. Thorac. Dis. 2014, 6, S60–S69. [Google Scholar] [CrossRef] [PubMed]
- Battisti-Charbonney, A.; Fisher, J.; Duffin, J. The cerebrovascular response to carbon dioxide in humans. J. Physiol. 2011, 589, 3039–3048. [Google Scholar] [CrossRef]
- Madsen, P.L.; Secher, N.H. Near-infrared oximetry of the brain. Prog. Neurobiol. 1999, 58, 541–560. [Google Scholar] [CrossRef]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Shear, T.D.; Vender, J.S.; Levin, S.D.; Koh, J.; Parikh, K.N.; Patel, S.S. Effect of ventilation on cerebral oxygenation in patients undergoing surgery in the beach chair position: A randomized controlled trial. Br. J. Anaesth. 2014, 113, 618–627. [Google Scholar] [CrossRef]
- Wong, C.; Churilov, L.; Cowie, D.; Tan, C.O.; Hu, R.; Tremewen, D.; Pearce, B.; Pillai, P.; Karalapillai, D.; Bellomo, R.; et al. Randomised controlled trial to investigate the relationship between mild hypercapnia and cerebral oxygen saturation in patients undergoing major surgery. BMJ Open 2020, 10, e029159. [Google Scholar] [CrossRef]
- Sørensen, H.; Nielsen, H.B.; Secher, N.H. Near-infrared spectroscopy assessed cerebral oxygenation during open abdominal aortic aneurysm repair: Relation to end-tidal CO2 tension. Int. J. Clin. Monit. Comput. 2015, 30, 409–415. [Google Scholar] [CrossRef]
- Akça, O.; Sessler, D.; Delong, D.; Keijner, R.; Ganzel, B.; Doufas, A. Tissue oxygenation response to mild hypercapnia during cardiopulmonary bypass with constant pump output. Br. J. Anaesth. 2006, 96, 708–714. [Google Scholar] [CrossRef][Green Version]
- Eertmans, W.; Genbrugge, C.; Fret, T.; Beran, M.; Engelen, K.; Gutermann, H.; Laenen, M.V.; Boer, W.; Ferdinande, B.; Jans, F.; et al. Influence of continuously evolving transcatheter aortic valve implantation technology on cerebral oxygenation. Int. J. Clin. Monit. Comput. 2016, 31, 1133–1141. [Google Scholar] [CrossRef]
- Schoen, J.; Meyerrose, J.; Paarmann, H.; Heringlake, M.; Hueppe, M.; Berger, K.-U. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: A prospective observational trial. Crit. Care 2011, 15, R218. [Google Scholar] [CrossRef]
- Goudzwaard, J.A.; Ronde-Tillmans, M.J.A.G.D.; Jager, T.A.J.D.; Lenzen, M.J.; Nuis, R.-J.; van Mieghem, N.M.; Daemen, J.; de Jaegere, P.P.T.; Mattace-Raso, F.U.S. Incidence, determinants and consequences of delirium in older patients after transcatheter aortic valve implantation. Age Ageing 2020, 49, 389–394. [Google Scholar] [CrossRef]
| Characteristics | n = 275 |
|---|---|
| Patient characteristics | |
| male-no. (%) | 139 (50.5) |
| median age (Q1–Q3)-years | 81 (77–84) |
| mean Body-Mass-Index (SD)-kg/m2 | 27 (±4) |
| arterial hypertension-no. (%) | 230 (83.6) |
| pulmonary hypertension-no. (%) | 14 (5.1) |
| coronary artery disease-no. (%) | 190 (69.1) |
| peripheral vessel disease-no. (%) | 34 (12.4) |
| carotid artery stenosis-no. (%) | 45 (16.4) |
| prior stroke or transient ischemic attack-no. (%) | 29 (10.5) |
| chronic kidney disease-no. (%) | 69 (25.1) |
| chronic obstructive pulmonary disease-no. (%) | 35 (12.7) |
| current smoker-no. (%) | 15 (5.5) |
| family history of CAD-no. (%) | 24 (8.7) |
| pacemaker-no. (%) | 31 (11.3) |
| pleural effusion present-no. (%) | 57 (22.2) |
| EuroScore II (SD)-% | 6.43 (±5.7) |
| Anesthetic management | |
| conscious sedation-no. (%) | 239 (86.9) |
| general anesthesia-no. (%) | 22 (8.0) |
| switch to general anesthesia-no. (%) | 14 (5.1) |
| amount of crystalloids (Q1–Q3)-mL | 1000 (647–1071) |
| amount of colloids (Q1–Q3)-mL | 0 (0–0) |
| amount of red blood cell concentrate (Q1–Q3)-mL | 0 (0–0) |
| amount of fresh frozen plasma (Q1–Q3)-mL | 0 (0–0) |
| catecholamine administration-no. (%) | 206 (74.9) |
| cumulative dosage of noradrenaline (Q1–Q3)-µg | 59 (2–169) |
| cumulative dosage of adrenaline (Q1–Q3)-µg | 0 (0–0) |
| duration of anesthesia (SD)-min | 131 (±36) |
| Procedural details | |
| duration of intervention (Q1–Q3)-min | 55 (45–69) |
| transfemoral approach-no. (%) | 267 (97.1) |
| transapical approach-no. (%) | 5 (1.8) |
| subclavian approach-no. (%) | 3 (1.1) |
| balloon-expandable valve-no. (%) | 52 (18.9) |
| self-expandable valve-no. (%) | 223 (81.1) |
| valvuloplasty-no. (%) | 196 (71.3) |
| postdilatation-no. (%) | 38 (13.8) |
| Second valve implantation-no. (%) | 4 (1.5) |
| number of rapid pacing (Q1–Q3)-no. | 1 (1–2) |
| number of fast pacing (Q1–Q3)-no. | 1 (0–1) |
| Cardioversion-no. (%) | 2 (0.7) |
| Characteristics | n = 275 |
|---|---|
| Postprocedural ECG | |
| new high-grade AVB-no. (%) | 30 (10.9) |
| new LBBB-no. (%) | 45 (16.9) |
| new pacemaker dependency, permanent-no. (%) | 33 (12.0) |
| new pacemaker dependency, temporary-no. (%) | 10 (3.6) |
| Outcome | |
| Any paravalvular leak-no. (%) | 113 (42.5) |
| Median length of ICU stay (Q1–Q3)-days | 4 (2.2–6.7) |
| Median length of hospital stay (Q1–Q3)-days | 11 (8.5–17.0) |
| POCD or delirium-no. (%) | 42 (15.3) |
| Adverse events | |
| Conversion to open surgery-no. (%) | 0 (0.0) |
| CPR-no. (%) | 10 (3.6) |
| Intraoperative mortality-no. (%) | 0 (0.0) |
| In-hospital mortality-no. (%) | 9 (3.3) |
| Characteristics | n | Preoperative | Postoperative | n | p |
|---|---|---|---|---|---|
| Cerebral oximetry | |||||
| ΔrSO2 (Q1–Q3)-% | 275 | 4.0 (−1.0–8.0) | 275 | ||
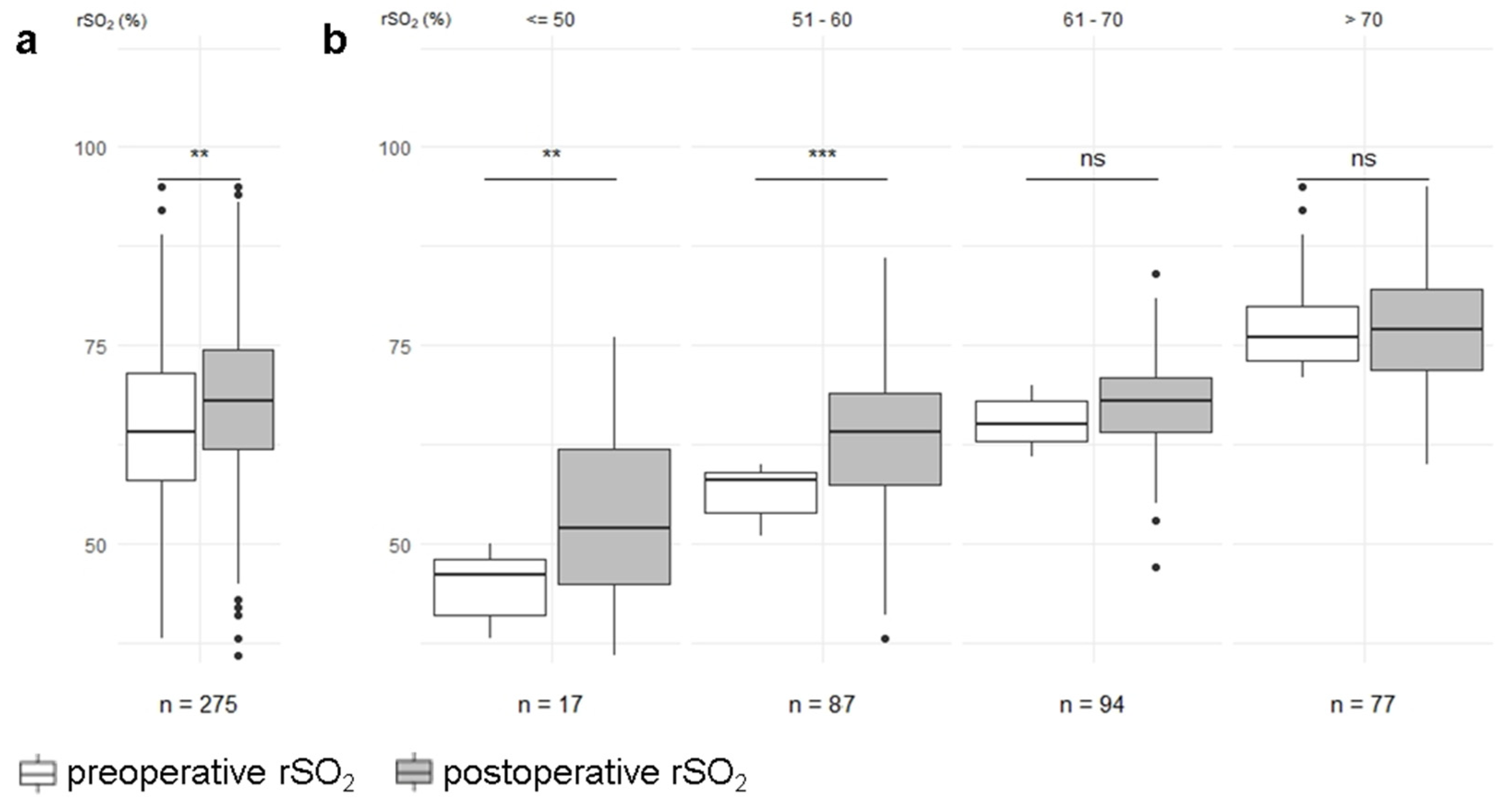
| rSO2 (SD)-% | 275 | 64.6 (±10) | 68.1 (±10) | 275 | <0.01 |
| Confounding parameters | |||||
| Hb (SD)-mg/dL | 262 | 11.6 (±1.8) | 10.3 (±1.7) | 213 | <0.01 |
| MAP (SD)-mmHg | 275 | 91 (±17) | 76 (±18) | 275 | <0.01 |
| CVP (SD)-mmHg | 205 | 11 (±7) | 13 (±7) | 255 | <0.01 |
| pH (Q1–Q3)-no. | 101 | 7.41 (7.3–7.4) | 7.36 (7.3–7.4) | 121 | <0.01 |
| PaCO2 (Q1–Q3)-mmHg | 101 | 39 (36–43) | 42 (37–47) | 121 | 0.03 |
| PaO2 (Q1–Q3)-mmHg | 101 | 128 (98–169) | 125 (90–160) | 119 | 0.49 |
| SaO2 (Q1–Q3)-% | 101 | 99 (98–99) | 99 (97–99) | 119 | 0.18 |
| Echocardiography | |||||
| LVEF (SD)-% | 237 | 54 (±11) | 55 (±9) | 176 | 0.38 |
| AV-PPG (SD)-mmHg | 255 | 66 (±22) | 16 (±8) | 237 | <0.01 |
| AV-MPG (SD)-mmHg | 259 | 40 (±14) | 9 (±4) | 225 | <0.01 |
| Variable | Estimate | Standard Error | p |
|---|---|---|---|
| ΔrSO2 | |||
| Intercerpt | 5.06 | 1.51 | <0.01 |
| ΔHb | 4.21 | 1.12 | <0.01 |
| ΔMAP | −0.03 | 0.04 | 0.48 |
| ΔCVP | −0.06 | 0.15 | 0.70 |
| ΔpH | −23.98 | 31.71 | 0.45 |
| ΔPaCO2 | 0.10 | 0.24 | 0.66 |
| ΔPaO2 | 0.00 | 0.02 | 0.78 |
| ΔSaO2 | 0.27 | 0.35 | 0.44 |
| n = 60 | Adjusted R2: 0.17; p = 0.029 | ||
| Characteristics | p | ||
|---|---|---|---|
| Valve Type | Self-Expanding n = 223 | Balloon-Expandable n = 52 | |
| ΔrSO2 (Q1–Q3)-% | 3.0 (−1.0–8.0) | 4.0 (−1.0–8.0) | 0.98 |
| preoperative rSO2 (SD)-% | 64.7 (±10.3) | 64.2 (±8.8) | 0.71 |
| catecholamine administration-no. (%) | 18 (8.1) | 6 (11.5) | 0.42 |
| postoperative rSO2 (SD)-% | 68.2 (±10.2) | 67.7 (±9.8) | 0.73 |
| catecholamine administration-no. (%) | 66 (29.6) | 23 (44.2) | 0.05 |
| POCD or Delirium | no n = 233 | yes n = 42 | |
| ΔrSO2 (Q1–Q3)-% | 3.0 (−1.0–8.0) | 4.0 (0–7.75) | 0.97 |
| preoperative rSO2 (SD)-% | 64.9 (±10.3) | 63.2 (±8.0) | 0.33 |
| catecholamine administration-no. (%) | 21 (9.0) | 3 (7.1) | 1.00 |
| postoperative rSO2 (SD)-% | 68.3 (±10.5) | 66.7 (±7.5) | 0.34 |
| catecholamine administration-no. (%) | 71 (30.5) | 18 (42.9) | 0.15 |
| EuroScore II | ≤4% n = 116 | >4% n = 151 | |
| ΔrSO2 (Q1–Q3)-% | 4.0 (−1.0–8.0) | 3.0 (−1.0–8.0) | 0.72 |
| preoperative rSO2 (SD)-% | 66.2 (±9.7) | 63.5 (±10.1) | 0.03 |
| catecholamine administration-no. (%) | 9 (7.8) | 15 (9.4) | 0.67 |
| postoperative rSO2 (SD)-% | 69.8 (±8.5) | 66.8 (±10.9) | 0.01 |
| catecholamine administration-no. (%) | 29 (25.0) | 60 (37.7) | 0.03 |
| length of ICU stay | short (≤4 d) n = 146 | long (>4 d) n = 129 | |
| ΔrSO2 (Q1–Q3)-% | 3.0 (−1.0–7.0) | 4.0 (−1.0–8.3) | 0.38 |
| preoperative rSO2 (SD)-% | 66.2 (±9.7) | 62.6 (±10.0) | <0.01 |
| catecholamine administration-no. (%) | 9 (6.5) | 15 (11.0) | 0.20 |
| postoperative rSO2 (SD)-% | 69.7 (±9.9) | 66.5 (±10.0) | <0.01 |
| catecholamine administration-no. (%) | 34 (24.5) | 55 (40.4) | <0.01 |
| length of hospital stay | short (≤11 d) n = 150 | long (>11 d) n = 125 | |
| ΔrSO2 (Q1–Q3)-% | 3.5 (−1.0–7.75) | 4.0 (−1.0–8.0) | 0.77 |
| preoperative rSO2 (SD)-% | 67.5 (±9.8) | 61.2 (±9.2) | <0.01 |
| catecholamine administration-no. (%) | 10 (6.7) | 14 (11.2) | 0.20 |
| postoperative rSO2 (SD)-% | 70.9 (±8.9) | 64.8 (±10.4) | <0.01 |
| catecholamine administration-no. (%) | 38 (25.3) | 51 (40.8) | <0.01 |
| Adverse event | no n = 260 | yes n = 15 | |
| ΔrSO2 (Q1–Q3)-% | 4 (−1.0–8.0) | 2 (−3.5–6.0) | 0.32 |
| preoperative rSO2 (SD)-% | 64.9 (±10.1) | 60.9 (±7.2) | 0.14 |
| catecholamine administration-no. (%) | 20 (7.7) | 4 (26.7) | 0.03 |
| postoperative rSO2 (SD)-% | 68.4 (±10.1) | 62.5 (±8.4) | 0.03 |
| catecholamine administration-no. (%) | 76 (29.2) | 13 (86.7) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, G.; Kreissl, H.; Vigelius-Rauch, U.; Schneck, E.; Edinger, F.; Nef, H.; Böning, A.; Sander, M.; Koch, C. Cerebral Tissue Oxygen Saturation Is Enhanced in Patients following Transcatheter Aortic Valve Implantation: A Retrospective Study. J. Clin. Med. 2022, 11, 1930. https://doi.org/10.3390/jcm11071930
Schmidt G, Kreissl H, Vigelius-Rauch U, Schneck E, Edinger F, Nef H, Böning A, Sander M, Koch C. Cerebral Tissue Oxygen Saturation Is Enhanced in Patients following Transcatheter Aortic Valve Implantation: A Retrospective Study. Journal of Clinical Medicine. 2022; 11(7):1930. https://doi.org/10.3390/jcm11071930
Chicago/Turabian StyleSchmidt, Götz, Hannes Kreissl, Ursula Vigelius-Rauch, Emmanuel Schneck, Fabian Edinger, Holger Nef, Andreas Böning, Michael Sander, and Christian Koch. 2022. "Cerebral Tissue Oxygen Saturation Is Enhanced in Patients following Transcatheter Aortic Valve Implantation: A Retrospective Study" Journal of Clinical Medicine 11, no. 7: 1930. https://doi.org/10.3390/jcm11071930
APA StyleSchmidt, G., Kreissl, H., Vigelius-Rauch, U., Schneck, E., Edinger, F., Nef, H., Böning, A., Sander, M., & Koch, C. (2022). Cerebral Tissue Oxygen Saturation Is Enhanced in Patients following Transcatheter Aortic Valve Implantation: A Retrospective Study. Journal of Clinical Medicine, 11(7), 1930. https://doi.org/10.3390/jcm11071930








