Radiomics-Based Image Phenotyping of Kidney Apparent Diffusion Coefficient Maps: Preliminary Feasibility & Efficacy
Abstract
:1. Introduction
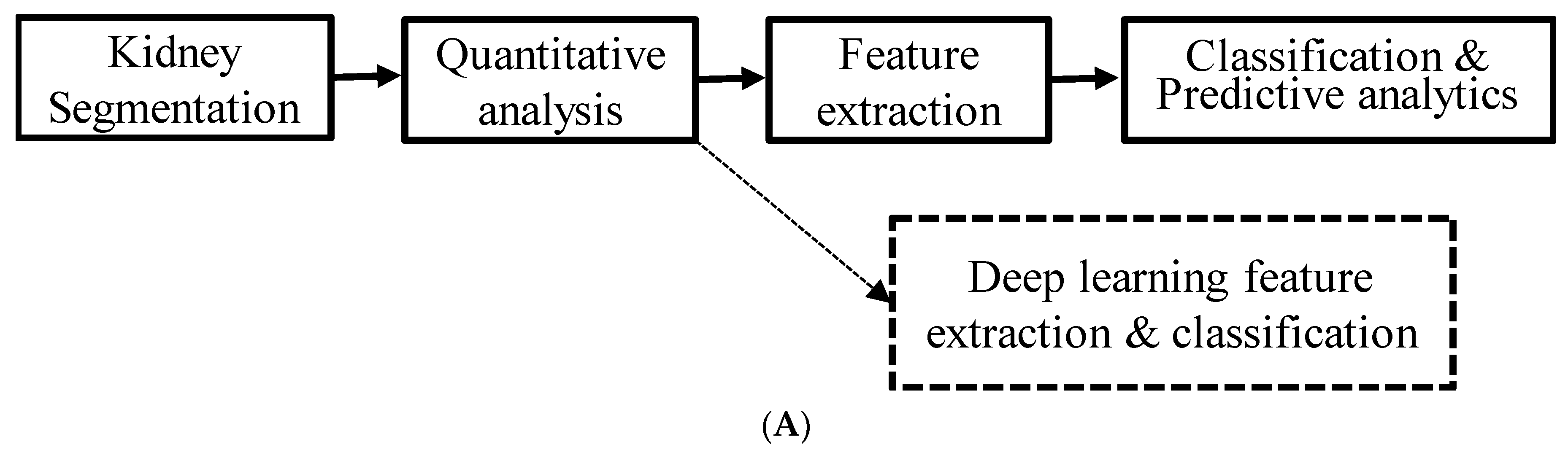
2. Materials and Methods
2.1. Study Population
2.2. MRI Acquisition
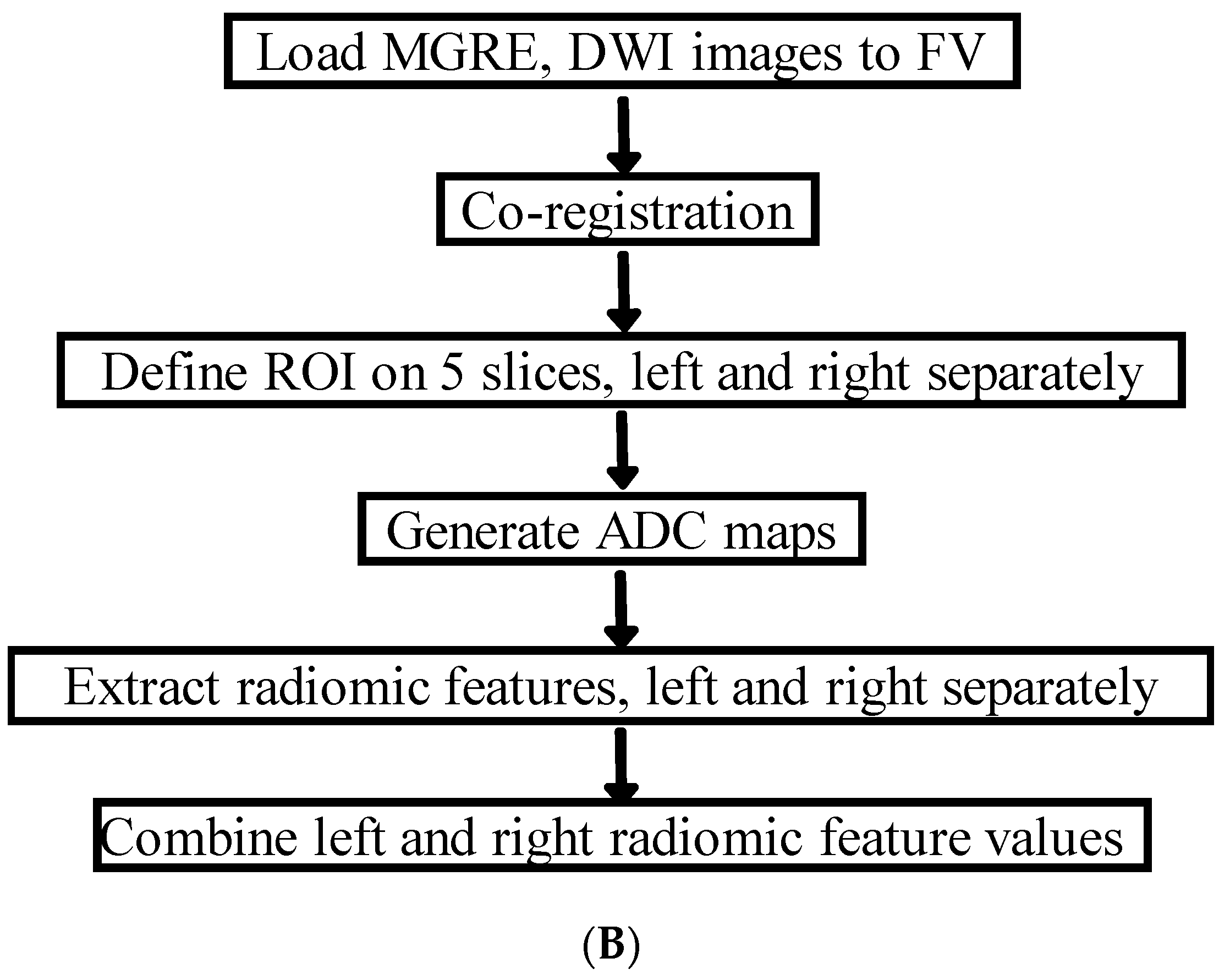
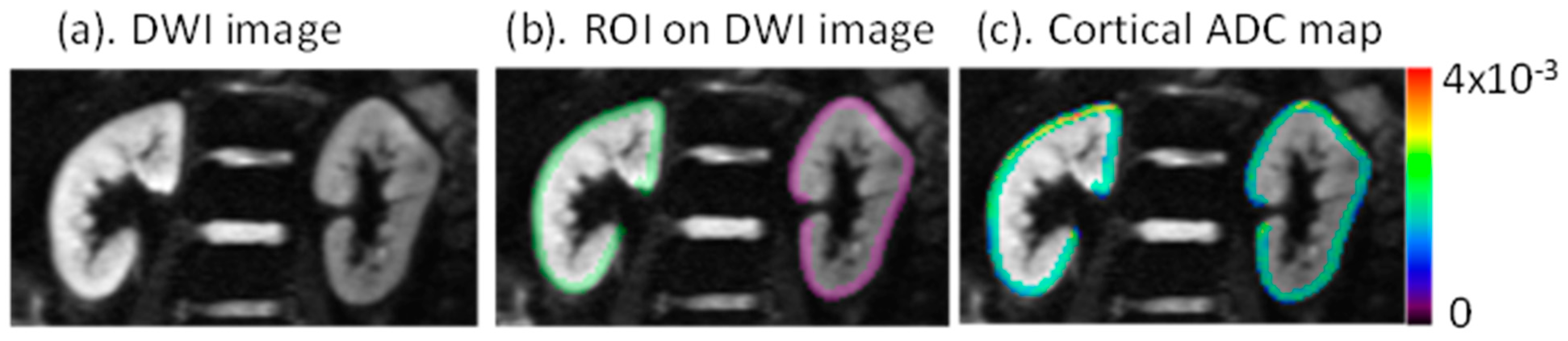
2.3. MRI Analysis
2.4. Exposures and Outcomes
2.5. Assessment of Clinical Information
2.6. Statistical Analysis
3. Results
3.1. Study Participants
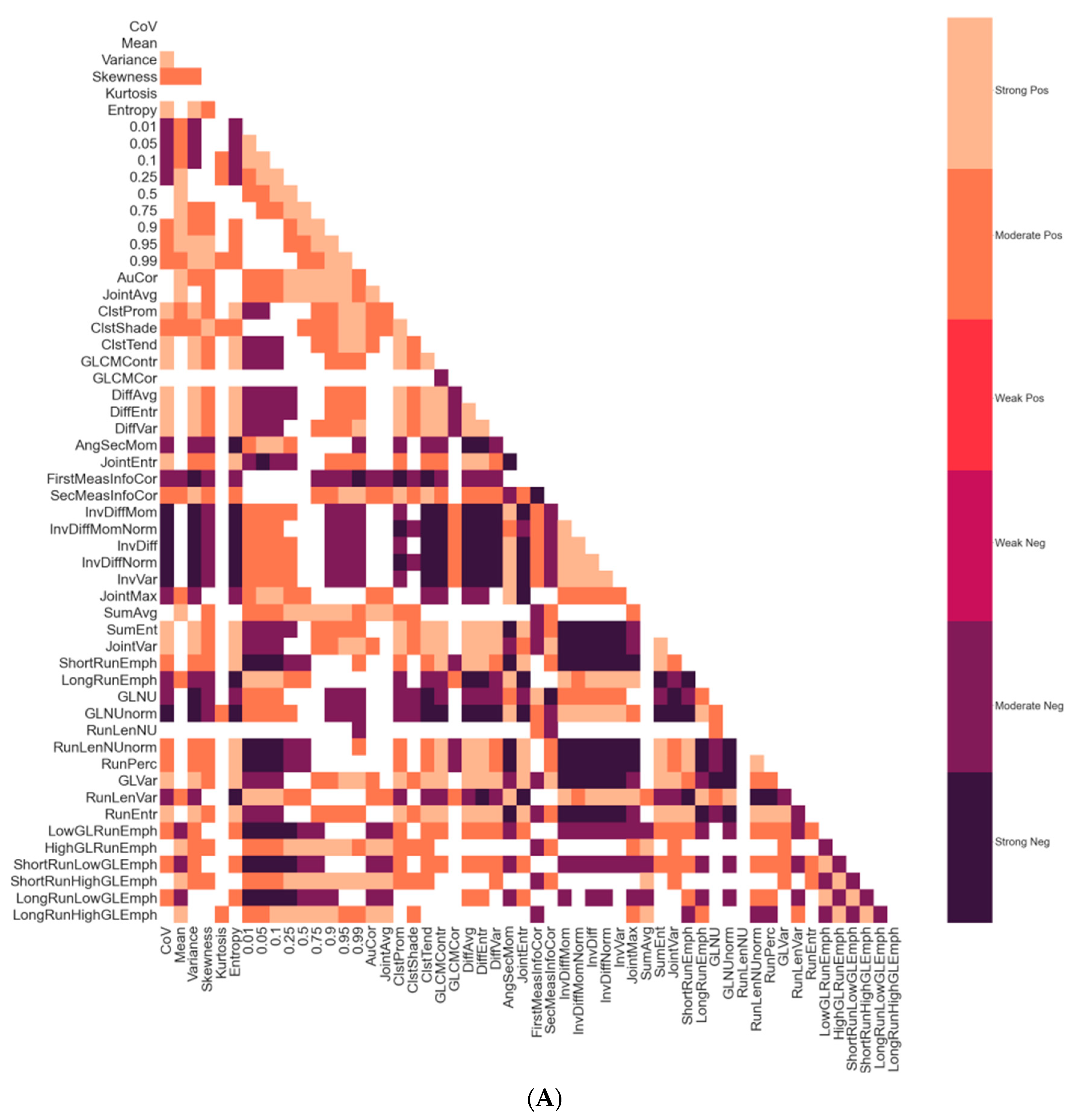
3.2. Correlations between Radiomic Features
3.3. Radiomic Features in Individuals with CKD vs. Healthy Participants
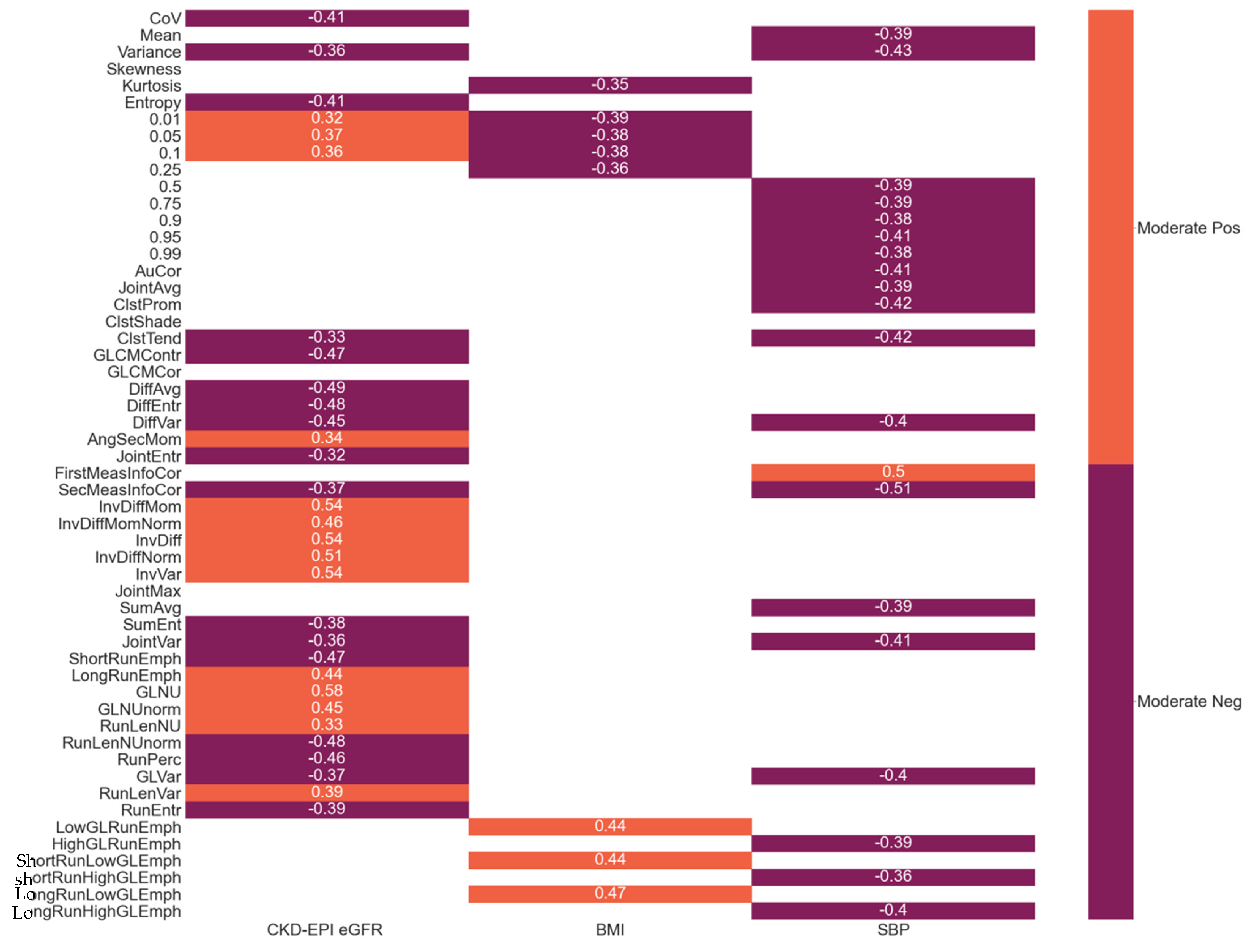
3.4. Correlations between Radiomic Features and Clinical Parameters
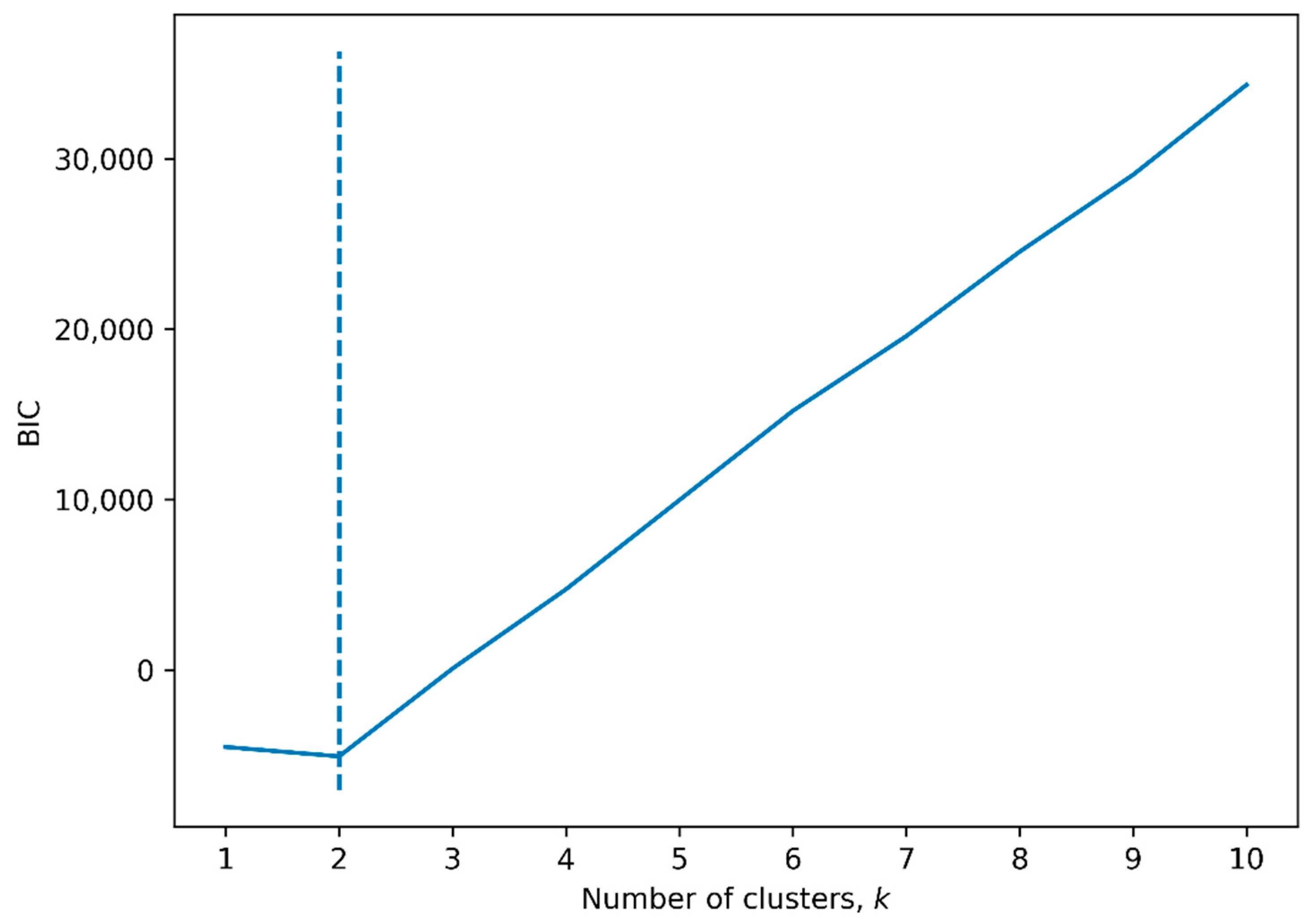
3.5. Hierarchical Clustering by Radiomic Features
3.6. Radiomics-Based Prediction of CKD and CKD Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. Chronic Kidney Disease in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. [Google Scholar]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Palsson, R.; Kaze, A.D.; Chen, M.E.; Palacios, P.; Sabbisetti, V.; Betensky, R.A.; Steinman, T.I.; Thadhani, R.I.; McMahon, G.M.; et al. The Prognostic Value of Histopathologic Lesions in Native Kidney Biopsy Specimens: Results from the Boston Kidney Biopsy Cohort Study. J. Am. Soc. Nephrol. 2018, 29, 2213–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, Y.N.; Himmelfarb, J. The CKD Classification System in the Precision Medicine Era. Clin. J. Am. Soc. Nephrol. 2017, 12, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Schneider, M.; Friedli, I.; Ljimani, A.; De Seigneux, S.; Boor, P.; Gullapudi, L.; Kazmi, I.; Mendichovszky, I.A.; Notohamiprodjo, M.; et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: A systematic review and statement paper. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 2), ii29–ii40. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.V.; Li, L.-P.; Thacker, J.M.; Li, W.; Hack, B.; Kohn, O.; Sprague, S.M. Cortical Perfusion and Tubular Function as Evaluated by Magnetic Resonance Imaging Correlates with Annual Loss in Renal Function in Moderate Chronic Kidney Disease. Am. J. Nephrol. 2019, 49, 114–124. [Google Scholar] [CrossRef]
- Li, L.-P.; Thacker, J.M.; Li, W.; Hack, B.; Wang, C.; Kohn, O.; Sprague, S.M.; Prasad, P.V. Medullary Blood Oxygen Level-Dependent MRI Index (R2*) is Associated with Annual Loss of Kidney Function in Moderate CKD. Am. J. Nephrol. 2020, 51, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Pruijm, M.; Milani, B.; Pivin, E.; Podhajska, A.; Vogt, B.; Stuber, M.; Burnier, M. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018, 93, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, K.; Inoue, T.; Kozawa, E.; Ishikawa, M.; Shimada, A.; Kobayashi, N.; Tanaka, J.; Okada, H. Reduced oxygenation but not fibrosis defined by functional magnetic resonance imaging predicts the long-term progression of chronic kidney disease. Nephrol. Dial. Transplant. 2018, 35, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, M.; Jiang, Z.; Ding, J.; Di, J.; Cui, L. Renal Hypoxia: An Important Prognostic Marker in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2018, 48, 46–55. [Google Scholar] [CrossRef]
- Lafata, K.J.; Wang, Y.; Konkel, B.; Yin, F.-F.; Bashir, M.R. Radiomics: A primer on high-throughput image phenotyping. Abdom. Radiol. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, H.; Li, R.; Lin, H.; Grimm, R.; Fu, C.; Yan, F. Whole-liver histogram and texture analysis on T1 maps improves the risk stratification of advanced fibrosis in NAFLD. Eur. Radiol. 2021, 31, 1748–1759. [Google Scholar] [CrossRef]
- De Leon, A.D.; Kapur, P.; Pedrosa, I. Radiomics in Kidney Cancer: MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Monelli, F.; Piccinini, A.; Besutti, G.; Trojani, V.; Ligabue, G.; Alfano, G.; Cappell, G. Magnetic Resonance Imaging Texture Analysis Predicts Interstitial Fibrosis/Tubular Atrophy in Transplanted Kidneys: A Single Center Crosssectional Study. In Proceedings of the 58th ERA-EDTA Congress, online, 5–8 June 2021; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Wilt, E.; Sprague, S.; Kohn, O.; Mikheev, A.; Rusinek, R.; Prasad, P.; Li, L.P. Spatial Heterogeneity vs. Spatial Average in the Analysis of ADC Maps to Evaluate Renal Fibrosis. In Proceedings of the ISMRM Workshop on Kidney MRI Biomarkers: The Route to Clinical Adoption, Philadelphia, PA, USA, 10–12 September 2021. [Google Scholar]
- Wei, Q.; Hu, Y. A study on using texture analysis methods for identifying lobar fissure regions in isotropic CT images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 3537–3540. [Google Scholar] [CrossRef]
- Doshi, A.M.; Tong, A.; Davenport, M.S.; Khalaf, A.M.; Mresh, R.; Rusinek, H.; Schieda, N.; Shinagare, A.B.; Smith, A.D.; Thornhill, R.; et al. Assessment of Renal Cell Carcinoma by Texture Analysis in Clinical Practice: A Six-Site, Six-Platform Analysis of Reliability. Am. J. Roentgenol. 2021, 217, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A. Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0 Contributors. SciPy 1.0 Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraley, C.; Raftery, A. Model based clustering, discrimination analysis, and density estimation. J. Am. Stat. Assoc. 2002, 97, 611–631. [Google Scholar] [CrossRef]
- Levey, A.S.; Cattran, D.; Friedman, A.; Miller, W.G.; Sedor, J.; Tuttle, K.; Kasiske, B.; Hostetter, T. Proteinuria as a Surrogate Outcome in CKD: Report of a Scientific Workshop Sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2009, 54, 205–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hysi, E.; Yuen, D.A. Imaging of renal fibrosis. Curr. Opin. Nephrol. Hypertens. 2020, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.; Buch, K.; Soto, J.A.; Jara, H.; Nakhmani, A.; Anderson, S.W. Quantifying liver fibrosis through the application of texture analysis to diffusion weighted imaging. Magn. Reson. Imaging 2014, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.; Ganeshan, B.; Nathan, P.; Juttla, J.K.; Vinayan, A.; Miles, K.A. Assessment of Response to Tyrosine Kinase Inhibitors in Metastatic Renal Cell Cancer: CT Texture as a Predictive Biomarker. Radiology 2011, 261, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Braman, N.M.; Etesami, M.; Prasanna, P.; Dubchuk, C.; Gilmore, H.; Tiwari, P.; Plecha, D.; Madabhushi, A. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017, 19, 57, Correction in 2017, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Fornacon-Wood, I.; Mistry, H.; Ackermann, C.J.; Blackhall, F.; McPartlin, A.; Faivre-Finn, C.; Price, G.; O’Connor, J.P.B. Reliability and prognostic value of radiomic features are highly dependent on choice of feature extraction platform. Eur. Radiol. 2020, 30, 6241–6250. [Google Scholar] [CrossRef]
- Kart, T.; Fischer, M.; Küstner, T.; Hepp, T.; Bamberg, F.; Winzeck, S.; Glocker, B.; Rueckert, D.; Gatidis, S. Deep Learning-Based Automated Abdominal Organ Segmentation in the UK Biobank and German National Cohort Magnetic Resonance Imaging Studies. Investig. Radiol. 2021, 56, 401–408. [Google Scholar] [CrossRef] [PubMed]


| Healthy (n = 10) | CKD (n = 30) | p-Value | |
|---|---|---|---|
| Female or Male? | 0.4 | 0.5 | 0.86 |
| Age (years) | 58.1 ± 9.4 | 65.3 ± 9.6 | 0.05 |
| SBP (mmHg) | 133.87 ± 15.9 | ||
| DBP (mmHg) | 67.9 ± 10.6 | ||
| CKD-EPI eGFR (mL/min/1.73 m2) | 88.6 ± 12.6 | 51.5 ± 12.2 | <0.001 |
| BMI (kg/m2) | 25.8 ± 2.7 | 32.4 ± 7.5 | 0.01 |
| eGFR slope (mL/min/1.73 m2/year) | −0.53 ± 3.68 | ||
| 24 h urine protein excretion (gm) | 0.16 (0.018–0.253) | ||
| Blood glucose (mg/dL) | 149.6 ± 68.0 |
| Healthy (n = 10) | CKD (n = 30) | p-Value | ||
|---|---|---|---|---|
| CoV | 1.64 × 10−1 (1.58 × 10−1–1.94 × 10−1) | 2.41 × 10−1 (2.01 × 10−1–2.97 × 10−1) | 0.001 | 1st order |
| Mean | 1.83 × 10−3 (1.77 × 10−3–1.87 × 10−3) | 1.75 × 10−3 (1.65 × 10−3–1.82 × 10−3) | 0.092 | |
| Variance | 9.77 × 10−8 (8.09 × 10−8–1.19 × 10−7) | 1.68 × 10−7 (1.12 × 10−7–2.29 × 10−7) | 0.006 | |
| Skewness | −1.22 × 10−1 (−5.36 × 10−1–2.70 × 10−1) | −3.02 × 10−2 (−4.68 × 10−1–5.80 × 10−1) | 0.288 | |
| Kurtosis | 2.89 × 100 (1.77 × 100–3.77 × 100) | 1.65 × 100 (1.23 × 100–2.70 × 100) | 0.126 | |
| Entropy | 3.38 × 100 (3.27 × 100–3.46 × 100) | 3.59 × 100 (3.39 × 100–3.69 × 100) | 0.006 | |
| 0.01 | 9.75 × 10−4 (9.12 × 10−4–9.92 × 10−4) | 7.11 × 10−4 (5.77 × 10−4–8.09 × 10−4) | 0.000 | |
| 0.05 | 1.32 × 10−3 (1.25 × 10−3–1.37 × 10−3) | 1.10 × 10−3 (9.55 × 10−4–1.15 × 10−3) | 0.000 | |
| 0.1 | 1.47 × 10−3 (1.38 × 10−3–1.52 × 10−3) | 1.30 × 10−3 (1.17 × 10−3–1.36 × 10−3) | 0.001 | |
| 0.25 | 1.68 × 10−3 (1.60 × 10−3–1.74 × 10−3) | 1.54 × 10−3 (1.45 × 10−3–1.61 × 10−3) | 0.007 | |
| 0.5 | 1.83 × 10−3 (1.76 × 10−3–1.87 × 10−3) | 1.75 × 10−3 (1.67 × 10−3–1.82 × 10−3) | 0.027 | |
| 0.75 | 1.97 × 10−3 (1.95 × 10−3–2.03 × 10−3) | 1.94 × 10−3 (1.88 × 10−3–2.01 × 10−3) | 0.274 | |
| 0.9 | 2.16 × 10−3 (2.08 × 10−3–2.23 × 10−3) | 2.13 × 10−3 (2.03 × 10−3–2.26 × 10−3) | 1.000 | |
| 0.95 | 2.32 × 10−3 (2.20 × 10−3–2.37 × 10−3) | 2.30 × 10−3 (2.14 × 10−3–2.67 × 10−3) | 0.685 | |
| 0.99 | 2.59 × 10−3 (2.50 × 10−3–2.70 × 10−3) | 2.80 × 10−3 (2.41 × 10−3–3.30 × 10−3) | 0.235 | |
| AuCor | 2.17 × 103 (2.08 × 103–2.31 × 103) | 2.02 × 103 (1.84 × 103–2.21 × 103) | 0.179 | gray level co-occurrence matrix |
| JointAvg | 4.63 × 101 (4.49 × 101–4.76 × 101) | 4.44 × 101 (4.22 × 101–4.64 × 101) | 0.098 | |
| ClstProm | 2.29 × 105 (1.58 × 105–2.70 × 105) | 5.98 × 105 (1.95 × 105–1.09 × 106) | 0.053 | |
| ClstShade | −1.23 × 102 (−5.89 × 102–1.55 × 103) | 9.08 × 102 (−8.14 × 102–7.55 × 103) | 0.492 | |
| ClstTend | 1.98 × 102 (1.63 × 102–2.31 × 102) | 3.12 × 102 (2.10 × 102–4.64 × 102) | 0.008 | |
| GLCMContr | 3.42 × 101 (3.28 × 101–4.07 × 101) | 6.65 × 101 (4.95 × 101–9.84 × 101) | 0.001 | |
| GLCMCor | 7.00 × 10−1 (6.08 × 10−1–7.14 × 10−1) | 6.22 × 10−1 (5.92 × 10−1–6.75 × 10−1) | 0.190 | |
| DiffAvg | 4.22 × 100 (4.04 × 100–4.46 × 100) | 5.49 × 100 (4.93 × 100–6.81 × 100) | 0.001 | |
| DiffEntr | 3.55 × 100 (3.50 × 100–3.65 × 100) | 3.91 × 100 (3.72 × 100–4.22 × 100) | 0.001 | |
| DiffVar | 1.66 × 101 (1.52 × 101–1.96 × 101) | 3.34 × 101 (2.03 × 101–4.76 × 101) | 0.002 | |
| AngSecMom | 3.57 × 10−3 (3.36 × 10−3–4.18 × 10−3) | 2.59 × 10−3 (2.07 × 10−3–3.64 × 10−3) | 0.021 | |
| JointEntr | 8.69 × 100 (8.46 × 100–8.89 × 100) | 9.13 × 100 (8.63 × 100–9.36 × 100) | 0.025 | |
| FirstMeasInfoCor | −2.02 × 10−1 (−2.12 × 10−1–−1.93 × 10−1) | −2.27 × 10−1 (−2.70 × 10−1–−1.95 × 10−1) | 0.042 | |
| SecMeasInfoCor | 9.24 × 10−1 (9.21 × 10−1–9.26 × 10−1) | 9.49 × 10−1 (9.25 × 10−1–9.62 × 10−1) | 0.018 | |
| InvDiffMom | 2.33 × 10−1 (2.30 × 10−1–2.50 × 10−1) | 1.84 × 10−1 (1.66 × 10−1–2.18 × 10−1) | 0.001 | |
| InvDiffMomNorm | 9.97 × 10−1 (9.96 × 10−1–9.97 × 10−1) | 9.94 × 10−1 (9.91 × 10−1–9.95 × 10−1) | 0.001 | |
| InvDiff | 3.23 × 10−1 (3.20 × 10−1–3.38 × 10−1) | 2.73 × 10−1 (2.52 × 10−1–3.08 × 10−1) | 0.000 | |
| InvDiffNorm | 9.61 × 10−1 (9.59 × 10−1–9.63 × 10−1) | 9.50 × 10−1 (9.40 × 10−1–9.55 × 10−1) | 0.001 | |
| InvVar | 2.40 × 10−1 (2.38 × 10−1–2.58 × 10−1) | 1.90 × 10−1 (1.63 × 10−1–2.26 × 10−1) | 0.001 | |
| JointMax | 1.16 × 10−2 (1.09 × 10−2–1.33 × 10−2) | 9.03 × 10−3 (7.42 × 10−3–1.19 × 10−2) | 0.065 | |
| SumAvg | 9.27 × 101 (8.98 × 101–9.53 × 101) | 8.87 × 101 (8.44 × 101–9.27 × 101) | 0.098 | |
| SumEnt | 5.69 × 100 (5.49 × 100–5.76 × 100) | 5.96 × 100 (5.65 × 100–6.09 × 100) | 0.016 | |
| JointVar | 5.79 × 101 (4.79 × 101–7.20 × 101) | 9.90 × 101 (6.54 × 101–1.38 × 102) | 0.007 | |
| ShortRunEmph | 9.49 × 10−1 (9.45 × 10−1–9.51 × 10−1) | 9.62 × 10−1 (9.53 × 10−1–9.64 × 10−1) | 0.002 | gray level run length matrix |
| LongRunEmph | 1.23 × 100 (1.22 × 100–1.25 × 100) | 1.17 × 100 (1.15 × 100–1.21 × 100) | 0.002 | |
| GLNU | 6.67 × 101 (5.98 × 101–6.86 × 101) | 4.43 × 101 (3.61 × 101–5.25 × 101) | 0.000 | |
| GLNUnorm | 4.26 × 10−2 (4.16 × 10−2–4.79 × 10−2) | 3.51 × 10−2 (3.04 × 10−2–4.12 × 10−2) | 0.007 | |
| RunLenNU | 1.30 × 103 (1.17 × 103–1.41 × 103) | 1.21 × 103 (1.06 × 103–1.32 × 103) | 0.142 | |
| RunLenNUnorm | 8.76 × 10−1 (8.67 × 10−1–8.80 × 10−1) | 9.04 × 10−1 (8.83 × 10−1–9.10 × 10−1) | 0.001 | |
| RunPerc | 9.33 × 10−1 (9.27 × 10−1–9.36 × 10−1) | 9.49 × 10−1 (9.38 × 10−1–9.53 × 10−1) | 0.002 | |
| GLVar | 6.32 × 101 (5.21 × 101–7.59 × 101) | 1.07 × 102 (7.21 × 101–1.44 × 102) | 0.005 | |
| RunLenVar | 8.05 × 10−2 (7.40 × 10−2–8.95 × 10−2) | 6.20 × 10−2 (5.36 × 10−2–7.46 × 10−2) | 0.006 | |
| RunEntr | 5.25 × 100 (5.10 × 100–5.33 × 100) | 5.45 × 100 (5.23 × 100–5.60 × 100) | 0.014 | |
| LowGLRunEmph | 5.54 × 10−4 (5.17 × 10−4–6.23 × 10−4) | 7.54 × 10−4 (6.06 × 10−4–1.56 × 10−3) | 0.015 | |
| HighGLRunEmph | 2.17 × 103 (2.07 × 103–2.30 × 103) | 2.02 × 103 (1.83 × 103–2.23 × 103) | 0.179 | |
| ShortRunLowGLEmph | 5.29 × 10−4 (4.96 × 10−4–6.00 × 10−4) | 7.26 × 10−4 (5.82 × 10−4–1.50 × 10−3) | 0.014 | |
| ShortRunHighGLEmph | 2.03 × 103 (1.97 × 103–2.18 × 103) | 1.93 × 103 (1.75 × 103–2.10 × 103) | 0.235 | |
| LongRunLowGLEmph | 6.65 × 10−4 (6.46 × 10−4–7.26 × 10−4) | 8.87 × 10−4 (7.09 × 10−4–1.64 × 10−3) | 0.025 | |
| LongRunHighGLEmph | 2.71 × 103 (2.56 × 103–2.89 × 103) | 2.47 × 103 (2.18 × 103–2.66 × 103) | 0.049 |
| Cluster 1 (n = 17) | Cluster 2 (n = 23) | p-Value | ||
|---|---|---|---|---|
| CKD | 1.0 ± 0.0 | 0.6 ± 0.5 | 0.001 | clinical |
| Female or Male? | 0.4 ± 0.5 | 0.3 ± 0.5 | 0.689 | |
| Age (years) | 65.1 ± 10.3 | 62.3 ± 9.7 | 0.376 | |
| SBP (mmHg) | 135.4 ± 16.5 | 131.6 ± 15.5 | 0.527 | |
| DBP (mmHg) | 67.1 ± 12.6 | 69.1 ± 7.7 | 0.615 | |
| CKD-EPI eGFR (mL/min/1.73 m2) | 49.8 ± 11.5 | 68.9 ± 21.7 | 0.002 | |
| BMI (kg/m2) | 33.2 ± 8.1 | 29.0 ± 6.0 | 0.067 | |
| eGFR slope (mL/min/1.73 m2/year) | −0.3 ± 4.4 | −0.8 ± 2.6 | 0.745 | |
| 24 h urine protein excretion (gm) | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.194 | |
| Blood glucose (mg/dL) | 157.9 ± 74.4 | 138.8 ± 59.8 | 0.454 | |
| CoV | 2.63 × 10−1 (2.37 × 10−1–3.18 × 10−1) | 1.86 × 10−1 (1.59 × 10−1–2.05 × 10−1) | 0.000 | 1st order |
| Mean | 1.72 × 10−3 (1.61 × 10−3–1.82 × 10−3) | 1.79 × 10−3 (1.72 × 10−3–1.84 × 10−3) | 0.245 | |
| Variance | 1.99 × 10−7 (1.67 × 10−7–2.49 × 10−7) | 9.84 × 10−8 (8.25 × 10−8–1.23 × 10−7) | 0.000 | |
| Skewness | 5.78 × 10−1 (−2.90 × 10−1–8.08 × 10−1) | −1.34 × 10−1 (−5.31 × 10−1–2.35 × 10−1) | 0.014 | |
| Kurtosis | 1.93 × 100 (1.24 × 100–3.31 × 100) | 1.67 × 100 (1.30 × 100–3.26 × 100) | 0.989 | |
| Entropy | 3.66 × 100 (3.60 × 100–3.75 × 100) | 3.37 × 100 (3.31 × 100–3.45 × 100) | 0.000 | |
| 0.01 | 5.86 × 10−4 (4.48 × 10−4–7.17 × 10−4) | 9.24 × 10−4 (7.82 × 10−4–9.87 × 10−4) | 0.000 | |
| 0.05 | 9.77 × 10−4 (9.14 × 10−4–1.12 × 10−3) | 1.25 × 10−3 (1.12 × 10−3–1.33 × 10−3) | 0.000 | |
| 0.1 | 1.19 × 10−3 (1.14 × 10−3–1.31 × 10−3) | 1.37 × 10−3 (1.32 × 10−3–1.47 × 10−3) | 0.000 | |
| 0.25 | 1.52 × 10−3 (1.43 × 10−3–1.58 × 10−3) | 1.61 × 10−3 (1.54 × 10−3–1.68 × 10−3) | 0.003 | |
| 0.5 | 1.72 × 10−3 (1.65 × 10−3–1.80 × 10−3) | 1.80 × 10−3 (1.74 × 10−3–1.84 × 10−3) | 0.057 | |
| 0.75 | 1.94 × 10−3 (1.86 × 10−3–2.02 × 10−3) | 1.96 × 10−3 (1.90 × 10−3–2.00 × 10−3) | 0.613 | |
| 0.9 | 2.20 × 10−3 (2.06 × 10−3–2.33 × 10−3) | 2.13 × 10−3 (2.04 × 10−3–2.21 × 10−3) | 0.245 | |
| 0.95 | 2.40 × 10−3 (2.28 × 10−3–2.69 × 10−3) | 2.26 × 10−3 (2.13 × 10−3–2.36 × 10−3) | 0.109 | |
| 0.99 | 3.05 × 10−3 (2.65 × 10−3–3.56 × 10−3) | 2.56 × 10−3 (2.42 × 10−3–2.81 × 10−3) | 0.029 | |
| AuCor | 1.99 × 103 (1.73 × 103–2.22 × 103) | 2.14 × 103 (1.95 × 103–2.26 × 103) | 0.404 | gray level co-occurrence matrix |
| JointAvg | 4.40 × 101 (4.10 × 101–4.64 × 101) | 4.55 × 101 (4.38 × 101–4.68 × 101) | 0.256 | |
| ClstProm | 7.26 × 105 (4.97 × 105–1.77 × 106) | 2.05 × 105 (1.14 × 105–2.93 × 105) | 0.001 | |
| ClstShade | 5.03 × 103 (−1.49 × 103–9.33 × 103) | −2.36 × 101 (−7.35 × 102–1.93 × 103) | 0.318 | |
| ClstTend | 3.76 × 102 (3.05 × 102–4.71 × 102) | 2.01 × 102 (1.63 × 102–2.46 × 102) | 0.000 | |
| GLCMContr | 9.19 × 101 (7.35 × 101–1.04 × 102) | 3.74 × 101 (3.33 × 101–5.00 × 101) | 0.000 | |
| GLCMCor | 6.06 × 10−1 (5.75 × 10−1–6.36 × 10−1) | 6.80 × 10−1 (6.02 × 10−1–7.18 × 10−1) | 0.009 | |
| DiffAvg | 6.74 × 100 (5.86 × 100–7.44 × 100) | 4.39 × 100 (4.11 × 100–4.99 × 100) | 0.000 | |
| DiffEntr | 4.20 × 100 (3.99 × 100–4.32 × 100) | 3.61 × 100 (3.53 × 100–3.74 × 100) | 0.000 | |
| DiffVar | 4.29 × 101 (3.42 × 101–5.68 × 101) | 1.77 × 101 (1.52 × 101–2.25 × 101) | 0.000 | |
| AngSecMom | 2.13 × 10−3 (1.90 × 10−3–2.33 × 10−3) | 3.65 × 10−3 (3.23 × 10−3–4.52 × 10−3) | 0.000 | |
| JointEntr | 9.35 × 100 (9.22 × 100–9.52 × 100) | 8.62 × 100 (8.30 × 100–8.85 × 100) | 0.000 | |
| FirstMeasInfoCor | −2.27 × 10−1 (−2.44 × 10−1–−2.18 × 10−1) | −2.04 × 10−1 (−2.41 × 10−1–−1.86 × 10−1) | 0.151 | |
| SecMeasInfoCor | 9.50 × 10−1 (9.45 × 10−1–9.62 × 10−1) | 9.24 × 10−1 (9.11 × 10−1–9.54 × 10−1) | 0.024 | |
| InvDiffMom | 1.69 × 10−1 (1.43 × 10−1–1.79 × 10−1) | 2.31 × 10−1 (2.13 × 10−1–2.38 × 10−1) | 0.000 | |
| InvDiffMomNorm | 9.91 × 10−1 (9.90 × 10−1–9.93 × 10−1) | 9.96 × 10−1 (9.95 × 10−1–9.97 × 10−1) | 0.000 | |
| InvDiff | 2.59 × 10−1 (2.32 × 10−1–2.68 × 10−1) | 3.19 × 10−1 (3.02 × 10−1–3.25 × 10−1) | 0.000 | |
| InvDiffNorm | 9.40 × 10−1 (9.35 × 10−1–9.48 × 10−1) | 9.59 × 10−1 (9.55 × 10−1–9.62 × 10−1) | 0.000 | |
| InvVar | 1.69 × 10−1 (1.49 × 10−1–1.80 × 10−1) | 2.37 × 10−1 (2.19 × 10−1–2.49 × 10−1) | 0.000 | |
| JointMax | 7.47 × 10−3 (6.04 × 10−3–9.02 × 10−3) | 1.17 × 10−2 (1.03 × 10−2–1.45 × 10−2) | 0.000 | |
| SumAvg | 8.80 × 101 (8.20 × 101–9.28 × 101) | 9.11 × 101 (8.76 × 101–9.35 × 101) | 0.256 | |
| SumEnt | 6.06 × 100 (5.99 × 100–6.23 × 100) | 5.65 × 100 (5.52 × 100–5.76 × 100) | 0.000 | |
| JointVar | 1.17 × 102 (9.70 × 101–1.50 × 102) | 5.86 × 101 (4.86 × 101–7.32 × 101) | 0.000 | |
| ShortRunEmph | 9.64 × 10−1 (9.63 × 10−1–9.71 × 10−1) | 9.50 × 10−1 (9.48 × 10−1–9.54 × 10−1) | 0.000 | gray level run length matrix |
| LongRunEmph | 1.16 × 100 (1.14 × 100–1.16 × 100) | 1.23 × 100 (1.21 × 100–1.24 × 100) | 0.000 | |
| GLNU | 4.40 × 101 (3.80 × 101–4.60 × 101) | 5.88 × 101 (4.65 × 101–6.83 × 101) | 0.004 | |
| GLNUnorm | 3.19 × 10−2 (2.96 × 10−2–3.43 × 10−2) | 4.29 × 10−2 (3.97 × 10−2–4.60 × 10−2) | 0.000 | |
| RunLenNU | 1.24 × 103 (1.13 × 103–1.30 × 103) | 1.26 × 103 (1.09 × 103–1.41 × 103) | 0.774 | |
| RunLenNUnorm | 9.09 × 10−1 (9.06 × 10−1–9.27 × 10−1) | 8.77 × 10−1 (8.73 × 10−1–8.85 × 10−1) | 0.000 | |
| RunPerc | 9.52 × 10−1 (9.50 × 10−1–9.59 × 10−1) | 9.34 × 10−1 (9.30 × 10−1–9.38 × 10−1) | 0.000 | |
| GLVar | 1.24 × 102 (1.06 × 102–1.49 × 102) | 6.35 × 101 (5.31 × 101–7.94 × 101) | 0.000 | |
| RunLenVar | 5.38 × 10−2 (4.86 × 10−2–5.64 × 10−2) | 7.76 × 10−2 (7.16 × 10−2–8.55 × 10−2) | 0.000 | |
| RunEntr | 5.55 × 100 (5.45 × 100–5.71 × 100) | 5.21 × 100 (5.10 × 100–5.33 × 100) | 0.000 | |
| LowGLRunEmph | 1.40 × 10−3 (6.75 × 10−4–2.20 × 10−3) | 5.94 × 10−4 (5.31 × 10−4–7.09 × 10−4) | 0.000 | |
| HighGLRunEmph | 2.00 × 103 (1.75 × 103–2.25 × 103) | 2.13 × 103 (1.94 × 103–2.25 × 103) | 0.503 | |
| ShortRunLowGLEmph | 1.38 × 10−3 (6.55 × 10−4–2.17 × 10−3) | 5.69 × 10−4 (5.05 × 10−4–6.85 × 10−4) | 0.000 | |
| ShortRunHighGLEmph | 1.93 × 103 (1.70 × 103–2.17 × 103) | 2.01 × 103 (1.84 × 103–2.11 × 103) | 0.753 | |
| LongRunLowGLEmph | 1.50 × 10−3 (7.66 × 10−4–2.32 × 10−3) | 7.10 × 10−4 (6.53 × 10−4–8.17 × 10−4) | 0.002 | |
| LongRunHighGLEmph | 2.31 × 103 (2.09 × 103–2.61 × 103) | 2.64 × 103 (2.44 × 103–2.80 × 103) | 0.065 |
| Model Features | Features | Sensitivity | Specificity | AUC-ROC |
|---|---|---|---|---|
| Radiomics | GLCMContr, SumEnt, CoV, FirstMeasInfoCor | 71% | 43% | 0.75 |
| Clinical | 24 h urine protein excretion, sex | 57% | 91% | 0.94 |
| Combination * | 24 h urine protein excretion, sex, AuCor | 57% | 96% | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.-P.; Leidner, A.S.; Wilt, E.; Mikheev, A.; Rusinek, H.; Sprague, S.M.; Kohn, O.F.; Srivastava, A.; Prasad, P.V. Radiomics-Based Image Phenotyping of Kidney Apparent Diffusion Coefficient Maps: Preliminary Feasibility & Efficacy. J. Clin. Med. 2022, 11, 1972. https://doi.org/10.3390/jcm11071972
Li L-P, Leidner AS, Wilt E, Mikheev A, Rusinek H, Sprague SM, Kohn OF, Srivastava A, Prasad PV. Radiomics-Based Image Phenotyping of Kidney Apparent Diffusion Coefficient Maps: Preliminary Feasibility & Efficacy. Journal of Clinical Medicine. 2022; 11(7):1972. https://doi.org/10.3390/jcm11071972
Chicago/Turabian StyleLi, Lu-Ping, Alexander S. Leidner, Emily Wilt, Artem Mikheev, Henry Rusinek, Stuart M. Sprague, Orly F. Kohn, Anand Srivastava, and Pottumarthi V. Prasad. 2022. "Radiomics-Based Image Phenotyping of Kidney Apparent Diffusion Coefficient Maps: Preliminary Feasibility & Efficacy" Journal of Clinical Medicine 11, no. 7: 1972. https://doi.org/10.3390/jcm11071972







