Prognosis of Atrial Fibrillation with or without Comorbidities: Analysis of Younger Adults from a Nationwide Database
Abstract
:1. Introduction
2. Methods
2.1. Study Design
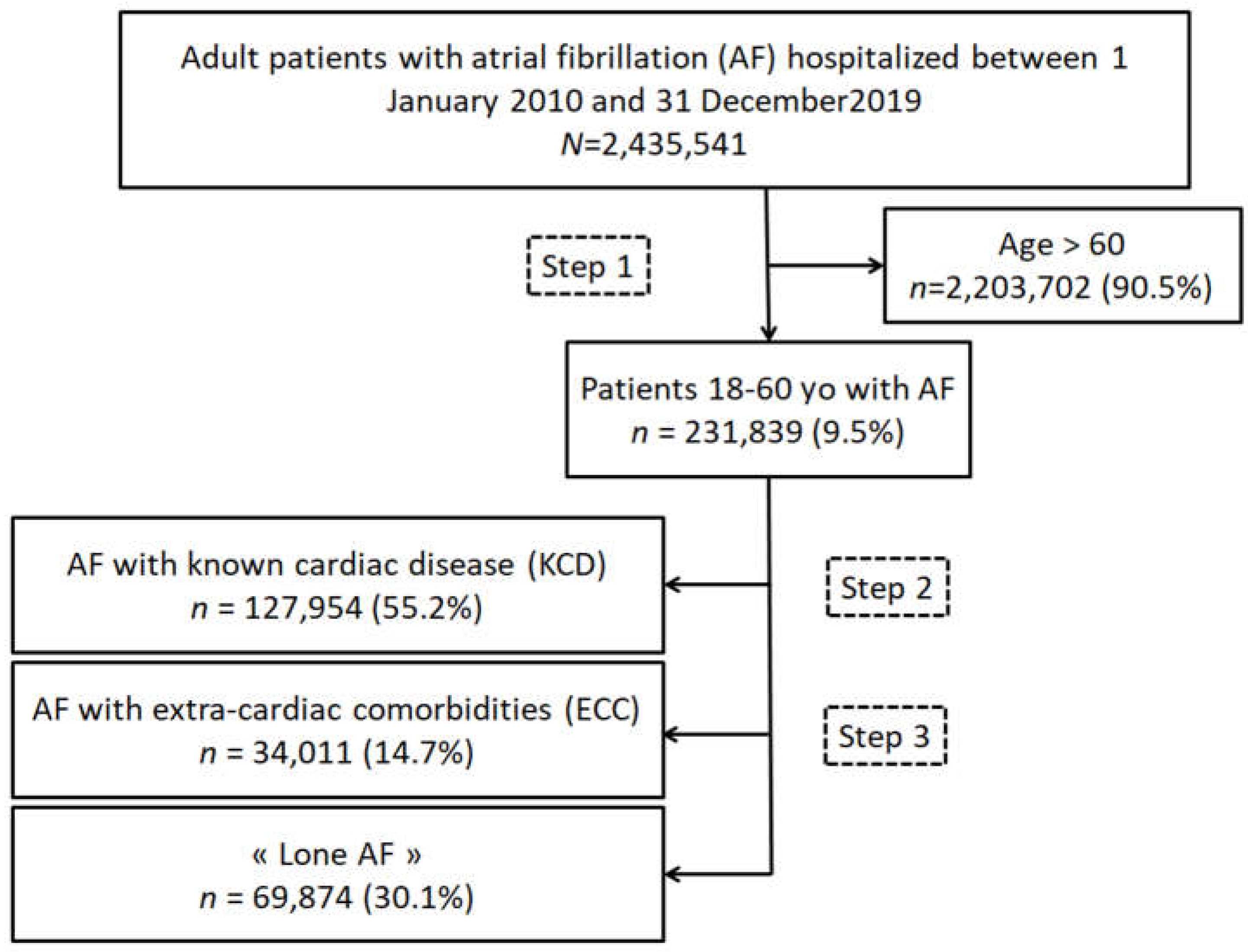
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients Aged under 60 Years
3.2. Clinical Outcomes in Patients with AF of Patients Aged under 60 Years
3.3. Clinical Outcomes in the Cohort Aged under 60 Years
3.4. Clinical Outcomes of Patients Aged below or above 60 Years
4. Discussion
4.1. Comorbidities in Clinical Trials of AF
4.2. Known Heart Disease in Young Patients (≤60 Years Old) with AF
4.3. Cardiovascular Outcomes: Higher Incidence of Ischemic Stroke and Rehospitalization in Patients of the KCD Group Compared to the ECC Group
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J.N. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allessie, M.A.; Bonke, F.I.; Schopman, F.J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ. Res. 1976, 39, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyse, D.G.; Van Gelder, I.C.; Ellinor, P.T.; Go, A.S.; Kalman, J.M.; Narayan, S.M.; Nattel, S.; Schotten, U.; Rienstra, M. Lone atrial fibrillation: Does it exist? J. Am. Coll. Cardiol. 2014, 63, 1715–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 473–498. [Google Scholar]
- Osranek, M.; Bursi, F.; Bailey, K.R.; Grossardt, B.R.; Brown RDJr Kopecky, S.L.; Tsang, T.S.; Seward, J.B. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: Three-decade follow-up. Eur. Heart J. 2005, 26, 2556–2561. [Google Scholar] [CrossRef]
- Potpara, T.; Stankovic, G.; Beleslin, B.; Polovina, M.; Marinkovic, J.; Ostojic, M.; Lip, G. A 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation: Implications of arrhythmia progression on prognosis: The Belgrade Atrial Fibrillation study. Chest 2012, 141, 339–347. [Google Scholar] [CrossRef]
- Chantry, A.A.; Deneux-Tharaux, C.; Cans, C.; Ego, A.; Quantin, C.; Bouvier-Colle, M.-H.; GRACE study group. Hospital discharge data can be used for monitoring procedures and intensive care related to severe maternal morbidity. J. Clin. Epidemiol. 2011, 64, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Djennaoui, M.; Ficheur, G.; Beuscart, R.; Chazard, E. Improvement of the quality of medical databases: Data-mining-based prediction of diagnostic codes from previous patient codes. Stud. Health Technol. Inform. 2015, 210, 419–423. [Google Scholar]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y.H. Patients With Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef] [Green Version]
- Fauchier, L.; Bodin, A.; Bisson, A.; Herbert, J.; Spiesser, P.; Clementy, N.; Babuty, D.; Chao, T.-F.; Lip, G.Y.H. Incident Comorbidities, Aging and the Risk of Stroke in 608,108 Patients with Atrial Fibrillation: A Nationwide Analysis. J. Clin. Med. 2020, 9, 1234. [Google Scholar] [CrossRef]
- Evans, W.; Swann, P. Lone auricular fibrillation. Br. Heart J. 1954, 16, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Jones, D.M.; Wang, T.J.; Leip, E.P.; Larson, M.G.; Levy, D.; Vasan, R.S.; D’Agostino, R.B.; Massaro, J.M.; Beiser, A.; Wolf, P.A.; et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation 2004, 110, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Khan Su Khan, M.Z.; Lone, A.N.; Khan, M.S.; Subramanian, C.R.; Michiis, E.D.; Alkhouli, M. Trends of Comorbidities in Clinical Trials of Atrial Fibrillation. Am. J. Cardiol. 2020, 131, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, M.; Oldgren, J.; Conen, D.; Conen, J.A.; Connolly, S.J.; Avezum, A.; Yusuf, S.; Ezekowitz, M.D.; Wallentin Ntep-Gweth, M.; Joseph, P.; et al. Characteristics and outcomes of atrial fibrillation in patients without traditional risk factors: An RE-LY AF registry analysis. Europace 2020, 22, 870–877. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Alonso, A.; Gersh, B.J.; Manemann, S.M.; Killian, J.M.; Weston, S.A.; Roger, V.L. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am. Heart J. 2017, 185, 74–84. [Google Scholar] [CrossRef] [Green Version]
- De With, R.R.; Marcos, E.G.; Van Gelder, I.C.; Rienstra, M. Atrial fibrillation progres-sion and outcome in patients with young-onset atrial fibrillation. Europace 2018, 20, 1750–1757. [Google Scholar] [CrossRef]
- Kapur, S.; Kumar, S.; John, R.M.; Stevenson, W.G.; Tedrow, U.B.; Koplan, B.A.; Michaud, G.F. Family history of atrial fibrillation as a predictor of atrial substrate and arrhythmia recurrence in patients undergoing atrial fibrillation catheter ablation. Europace 2018, 20, 921–928. [Google Scholar] [CrossRef]
- Dawood, D.; Kathleen, H.; Jeffrey, B.; Arshad, J.; Bernard, G.; Win, S.; Stephen, H.; Douglas, P.; Timothy, O. Familial atrial fibrillation is a genetically heterogeneous disorder. J. Am. Coll Cardiol. 2003, 41, 2185–2192. [Google Scholar]
- Jahangir, A.; Lee, V.; Friedman, P.; Trusty, J.; Hodge, D.; Kopecky, S.; Packer, D.; Hammill, S.; Shen, W.K.; Gersh, B. Long-term progression and outcomes with aging in patients with «Lone» atrial fibrillation:a 30-year follow-up study. Circulation 2007, 115, 3050–3056. [Google Scholar] [CrossRef] [Green Version]
- Weijs, B.; Pisters, R.; Nieuwlaat, R.; Breithardt, G.; Le Heuzey, J.Y.; Vardas, P.; Limantoro, I.; Schotten, U.; Lip, G.Y.H.; Crijns, H. Idiopathic atrial fibrillation revisited in a large longitudinal clinical cohort. Europace 2012, 14, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Jouven, X.; Desnos, M.; Guerot, C.; Ducimetiere, P. Idiopathic atrial fibrillation as a risk factor for mortality. The Paris Prospective Study I. Eur. Heart J. 1999, 20, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Weijs, B.; De Vos, C.B.; Tieleman, R.G.; Peeters, F.E.; Limantoro, I.; Kroon, A.A. The occurrence of cardiovascular disease during 5-year follow-up in patients with idiopathic atrial fibrillation. Europace 2013, 15, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Van Gefer, I.C.; Hagnes, V.E.; Veeger, N.J.; van Veldhuisen, D.J.; Crijns, H.J.; Kroon, A.; Cheriex, E.; Pisters, R. Mending the rhythm does not improve prognosis in patients with persistent atrial fibrillation: A subanalysis of the RACE study. Eur. Heart J. 2006, 27, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Dong, J.; Ma, C. Is Atrial Fibrillation a Preventable Disease? J. Am. Coll. Cardiol. 2017, 69, 1968–1982. [Google Scholar] [CrossRef]
- Frost, L.; Vestergaard, P. Alcohol and risk of atrial fibrillation or flutter: A cohort study. Arch. Intern. Med. 2004, 164, 1993–1998. [Google Scholar] [CrossRef] [Green Version]
- Perticone, M.; Sciacqua, A.; Tripepi, G.; Miceli, S.; Corrao, S.; Sest, G.; Perticone, F. Competitive interaction between chronic obstructive pulmonary disease and CHA 2 DS 2-VASc score in predicting incident atrial fibrillation. Int. J. Cardiol. 2018, 255, 74–79. [Google Scholar] [CrossRef]
- Proietti, M.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.A.; Boriani, G.; et al. Long-Term General Registry Investigators. Impact of clinical phenotypes on management and outcomes in European atrial fibrillation patients: A report from the ESC-EHRA EURObservational Research Programme in AF (EORP-AF) General Long-Term Registry. BMC Med. 2021, 19, 256. [Google Scholar] [CrossRef]
- Proietti, M.; Marzona, I.; Vannini, T.; Tettamanti, M.; Fortino, I.; Merlino, L.; Basili, S.; Mannucci, P.M.; Boriani, G.; Lip, G.Y.H.; et al. Long-Term Relationship Between Atrial Fibrillation, Multimorbidity and Oral Anticoagulant Drug Use. Mayo Clin. Proc. 2019, 94, 2427–2436. [Google Scholar] [CrossRef]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Kozieł, M.; Yang, P.S.; Guo, Y.; et al. Adherence to the 'Atrial Fibrillation Better Care’ Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes-A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb. Haemost. 2022, 122, 406–414. [Google Scholar] [CrossRef]
- Yao, Y.; Guo, Y.; Lip, G.Y.H.; mAF-App II Trial investigators. The Effects of Implementing a Mobile Health-Technology Supported Pathway on Atrial Fibrillation-Related Adverse Events Among Patients with Multimorbidity: The mAFA-II Randomized Clinical Trial. JAMA Netw. Open. 2021, 4, e2140071. [Google Scholar] [CrossRef] [PubMed]




| Total | Lone AF | AF with KCD | p vs. Lone AF | AF with ECC | p vs. Lone AF | |
|---|---|---|---|---|---|---|
| (n = 231,839) | (n = 69,874) | (n = 127,954) | (n = 34,011) | |||
| Age (years), mean ± SD | 51.8 ± 8.4 | 49.1 ± 9.9 | 53.5 ± 6.8 | <0.0001 | 51.1 ± 8.6 | <0.0001 |
| Sex (male), n (%) | 167,893 (72.4) | 50,489 (72.3) | 94,014 (73.5) | <0.0001 | 23,390 (68.8) | <0.0001 |
| Hypertension, n (%) | 79,398 (34.2) | 0 (0.0) | 79,398 (62.1) | <0.0001 | 0 (0.0) | - |
| Diabetes mellitus, n (%) | 33,565 (14.5) | 0 (0.0) | 28,114 (22.0) | <0.0001 | 5451 (16.0) | <0.0001 |
| Smoker, n (%) | 42,942 (18.5) | 4672 (6.7) | 30,622 (23.9) | <0.0001 | 7648 (22.5) | <0.0001 |
| Dyslipidemia, n (%) | 38,179 (16.5) | 2583 (3.7) | 32,518 (25.4) | <0.0001 | 3078 (9.1) | <0.0001 |
| Obesity, n (%) | 44,952 (19.4) | 0 (0.0) | 35,124 (27.5) | <0.0001 | 9828 (28.9) | <0.0001 |
| Heart failure with congestion, n (%) | 59,249 (25.6) | 0 (0.0) | 59,249 (46.3) | <0.0001 | 0 (0.0) | - |
| History of pulmonary edema, n (%) | 8188 (3.5) | 0 (0.0) | 8188 (6.4) | <0.0001 | 0 (0.0) | - |
| Mitral regurgitation, n (%) | 13,036 (5.6) | 0 (0.0) | 13,036 (10.2) | <0.0001 | 0 (0.0) | - |
| Mitral stenosis, n (%) | 1463 (0.6) | 0 (0.0) | 1463 (1.1) | <0.0001 | 0 (0.0) | - |
| Aortic regurgitation, n (%) | 5399 (2.3) | 0 (0.0) | 5399 (4.2) | <0.0001 | 0 (0.0) | - |
| Aortic stenosis, n (%) | 6352 (2.7) | 0 (0.0) | 6352 (5.0) | <0.0001 | 0 (0.0) | - |
| Previous endocarditis, n (%) | 2067 (0.9) | 0 (0.0) | 2067 (1.6) | <0.0001 | 0 (0.0) | - |
| Dilated cardiomyopathy, n (%) | 23,809 (10.3) | 0 (0.0) | 23,809 (18.6) | <0.0001 | 0 (0.0) | - |
| Coronary artery disease, n (%) | 42,025 (18.1) | 0 (0.0) | 42,025 (32.8) | <0.0001 | 0 (0.0) | - |
| Previous MI, n (%) | 12,445 (5.4) | 0 (0.0) | 12,445 (9.7) | <0.0001 | 0 (0.0) | - |
| Previous PCI, n (%) | 10,238 (4.4) | 0 (0.0) | 10,238 (8.0) | <0.0001 | 0 (0.0) | - |
| Previous CABG, n (%) | 4853 (2.1) | 0 (0.0) | 4853 (3.8) | <0.0001 | 0 (0.0) | - |
| Vascular disease, n (%) | 30,992 (13.4) | 0 (0.0) | 28,952 (22.6) | <0.0001 | 2040 (6.0) | <0.0001 |
| Left BBB, n (%) | 2820 (1.2) | 204 (0.3) | 2498 (2.0) | <0.0001 | 118 (0.3) | 0.13 |
| Right BBB, n (%) | 3241 (1.4) | 536 (0.8) | 2375 (1.9) | <0.0001 | 330 (1.0) | 0.001 |
| Previous pacemaker or ICD, n (%) | 5009 (2.2) | 307 (0.4) | 4470 (3.5) | <0.0001 | 232 (0.7) | <0.0001 |
| Ischemic stroke, n (%) | 9186 (4.0) | 1657 (2.4) | 6300 (4.9) | <0.0001 | 1229 (3.6) | <0.0001 |
| Intracranial bleeding, n (%) | 3178 (1.4) | 480 (0.7) | 2107 (1.6) | <0.0001 | 591 (1.7) | <0.0001 |
| Alcohol related diagnoses, n (%) | 28,451 (12.3) | 0 (0.0) | 19,240 (15.0) | <0.0001 | 9211 (27.1) | <0.0001 |
| Abnormal renal function, n (%) | 7151 (3.1) | 0 (0.0) | 6517 (5.1) | <0.0001 | 634 (1.9) | <0.0001 |
| Lung disease, n (%) | 28,981 (12.5) | 0 (0.0) | 20,735 (16.2) | <0.0001 | 8246 (24.2) | <0.0001 |
| Sleep apnea syndrome, n (%) | 16,188 (7.0) | 0 (0.0) | 12,551 (9.8) | <0.0001 | 3637 (10.7) | <0.0001 |
| COPD, n (%) | 15,804 (6.8) | 0 (0.0) | 11,750 (9.2) | <0.0001 | 4054 (11.9) | <0.0001 |
| Liver disease, n (%) | 14,342 (6.2) | 613 (0.9) | 10,454 (8.2) | <0.0001 | 3275 (9.6) | <0.0001 |
| Thyroid diseases, n (%) | 14,411 (6.2) | 0 (0.0) | 9101 (7.1) | <0.0001 | 5310 (15.6) | <0.0001 |
| Inflammatory disease, n (%) | 9179 (4.0) | 0 (0.0) | 6187 (4.8) | <0.0001 | 2992 (8.8) | <0.0001 |
| Anemia, n (%) | 24,523 (10.6) | 2725 (3.9) | 17,754 (13.9) | <0.0001 | 4044 (11.9) | <0.0001 |
| Previous cancer, n (%) | 24,336 (10.5) | 5557 (8.0) | 13,438 (10.5) | <0.0001 | 5341 (15.7) | <0.0001 |
| CHA2DS2VASc score, mean ± SD | 1.2 ± 1.2 | 0.3 ± 0.5 | 1.9 ± 1.1 | <0.0001 | 0.6 ± 0.7 | <0.0001 |
| HASBLED score, mean ± SD | 1.1 ± 1.2 | 0.3 ± 0.7 | 1.6 ± 1.2 | <0.0001 | 1.0 ± 1.1 | <0.0001 |
| Charlson index, mean ± SD | 2.3 ± 2.8 | 0.8 ± 1.8 | 3.0 ± 3.0 | <0.0001 | 2.4 ± 2.7 | <0.0001 |
| Frailty index, mean ± SD | 4.1 ± 6.0 | 1.9 ± 3.6 | 5.1 ± 6.6 | <0.0001 | 4.9 ± 6.3 | <0.0001 |
| Frailty index, mean ± SD | 4.1 ± 6.0 | 1.9 ± 3.6 | 5.1 ± 6.6 | <0.0001 | 4.9 ± 6.3 | <0.0001 |
| Lone AF ≤ 60 yo | AF ≤ 60 yo with Known Cardiac Disease | AF ≤ 60 yo with Extra-Cardiac Comorbidities | AF > 60 yo | |
|---|---|---|---|---|
| All-cause death | 3518 (2.3) | 17,332 (5.7) | 4697 (6.2) | 592,190 (13.7) |
| Cardiovascular death | 486 (0.3) | 5095 (1.7) | 616 (0.8) | 180,989 (4.2) |
| Non-cardiovascular death | 3032 (2.0) | 12,237 (4.0) | 4081 (5.4) | 411,201 (9.5) |
| Ischemic stroke | 1030 (0.7) | 3866 (1.3) | 653 (0.9) | 106,118 (2.5) |
| Rehospitalization for HF | 1349 (0.9) | 15,400 (5.6) | 1165 (1.6) | 381,871 (9.9) |
| Hazard Ratio | p-Value | Adjusted * Hazard Ratio | p-Value | |
|---|---|---|---|---|
| All-cause death | ||||
| AF ≤ 60 yo with known cardiac disease (vs Lone AF) | 2.38 (2.30–2.47) | <0.0001 | 2.13 (2.05–2.21) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs Lone AF) | 2.57 (2.46–2.68) | <0.0001 | 2.46 (2.36–2.57) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs AF with known cardiac disease) | 1.08 (1.04–1.11) | <0.0001 | 1.15 (1.12–1.19) | <0.0001 |
| AF > 60 yo (vs Lone AF) | 5.46 (5.29–5.65) | <0.0001 | 5.52 (5.34–5.70) | <0.0001 |
| Cardiovascular death | ||||
| AF ≤ 60 yo with known cardiac disease (vs Lone AF) | 5.08 (4.63–5.58) | <0.0001 | 4.80 (4.37–5.27) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs Lone AF) | 2.44 (2.17–2.75) | <0.0001 | 2.39 (2.12–2.69) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs AF with known cardiac disease) | 0.48 (0.44–0.52) | <0.0001 | 0.50 (0.46–0.54) | <0.0001 |
| AF > 60 yo (vs Lone AF) | 11.99 (10.97–13.11) | <0.0001 | 11.64 (10.65–12.73) | <0.0001 |
| Non-cardiovascular death | ||||
| AF ≤ 60 yo with known cardiac disease (vs Lone AF) | 1.95 (1.88–2.03) | <0.0001 | 1.72 (1.65–1.79) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs Lone AF) | 2.59 (2.47–2.71) | <0.0001 | 2.47 (2.35–2.59) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs AF with known cardiac disease) | 1.33 (1.28–1.37) | <0.0001 | 1.44 (1.39–1.49) | <0.0001 |
| AF > 60 yo (vs Lone AF) | 4.42 (4.26–4.58) | <0.0001 | 4.54 (4.38–4.70) | <0.0001 |
| Ischemic stroke | ||||
| AF ≤ 60 yo with known cardiac disease (vs Lone AF) | 1.85 (1.73–1.98) | <0.0001 | 1.72 (1.61–1.85) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs Lone AF) | 1.24 (1.13–1.37) | <0.0001 | 1.21 (1.09–1.33) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs AF with known cardiac disease) | 0.67 (0.62–0.73) | <0.0001 | 0.69 (0.64–0.75) | <0.0001 |
| AF > 60 yo (vs Lone AF) | 3.54 (3.33–3.76) | <0.0001 | 3.35 (3.15–3.56) | <0.0001 |
| Rehospitalization for HF | ||||
| AF ≤ 60 yo with known cardiac disease (vs Lone AF) | 5.97 (5.65–6.31) | <0.0001 | 5.76 (5.44–6.09) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs Lone AF) | 1.69 (1.56–1.83) | <0.0001 | 1.67 (1.55–1.81) | <0.0001 |
| AF ≤ 60 yo with extra-cardiac comorbidities (vs AF with known cardiac disease) | 0.28 (0.27–0.30) | <0.0001 | 0.29 (0.27–0.31) | <0.0001 |
| AF > 60 yo (vs Lone AF) | 10.30 (9.77–10.87) | <0.0001 | 9.93 (9.41–10.47) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertz, V.; Cottin, Y.; Bentounes, S.A.; Pastier-Debeaumarché, J.; Didier, R.; Herbert, J.; Zeller, M.; Lip, G.Y.H.; Fauchier, L. Prognosis of Atrial Fibrillation with or without Comorbidities: Analysis of Younger Adults from a Nationwide Database. J. Clin. Med. 2022, 11, 1981. https://doi.org/10.3390/jcm11071981
Mertz V, Cottin Y, Bentounes SA, Pastier-Debeaumarché J, Didier R, Herbert J, Zeller M, Lip GYH, Fauchier L. Prognosis of Atrial Fibrillation with or without Comorbidities: Analysis of Younger Adults from a Nationwide Database. Journal of Clinical Medicine. 2022; 11(7):1981. https://doi.org/10.3390/jcm11071981
Chicago/Turabian StyleMertz, Valentin, Yves Cottin, Sid Ahmed Bentounes, Julie Pastier-Debeaumarché, Romain Didier, Julien Herbert, Marianne Zeller, Gregory Y. H. Lip, and Laurent Fauchier. 2022. "Prognosis of Atrial Fibrillation with or without Comorbidities: Analysis of Younger Adults from a Nationwide Database" Journal of Clinical Medicine 11, no. 7: 1981. https://doi.org/10.3390/jcm11071981
APA StyleMertz, V., Cottin, Y., Bentounes, S. A., Pastier-Debeaumarché, J., Didier, R., Herbert, J., Zeller, M., Lip, G. Y. H., & Fauchier, L. (2022). Prognosis of Atrial Fibrillation with or without Comorbidities: Analysis of Younger Adults from a Nationwide Database. Journal of Clinical Medicine, 11(7), 1981. https://doi.org/10.3390/jcm11071981







