Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Ethics Statement
2.3. Data Collection
2.4. Laboratory Testing
2.5. Definitions
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Biomarkers on ICU Admission
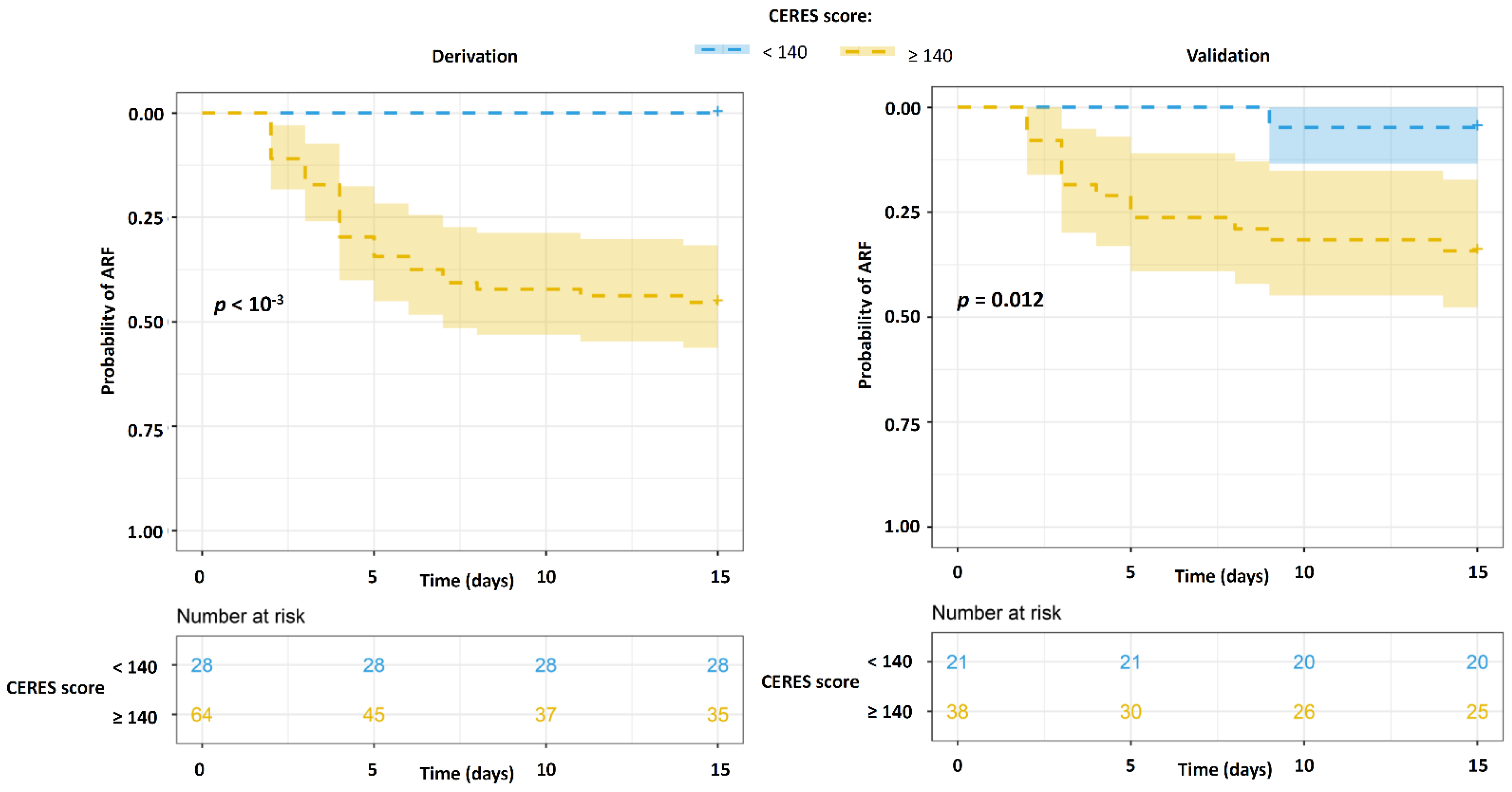
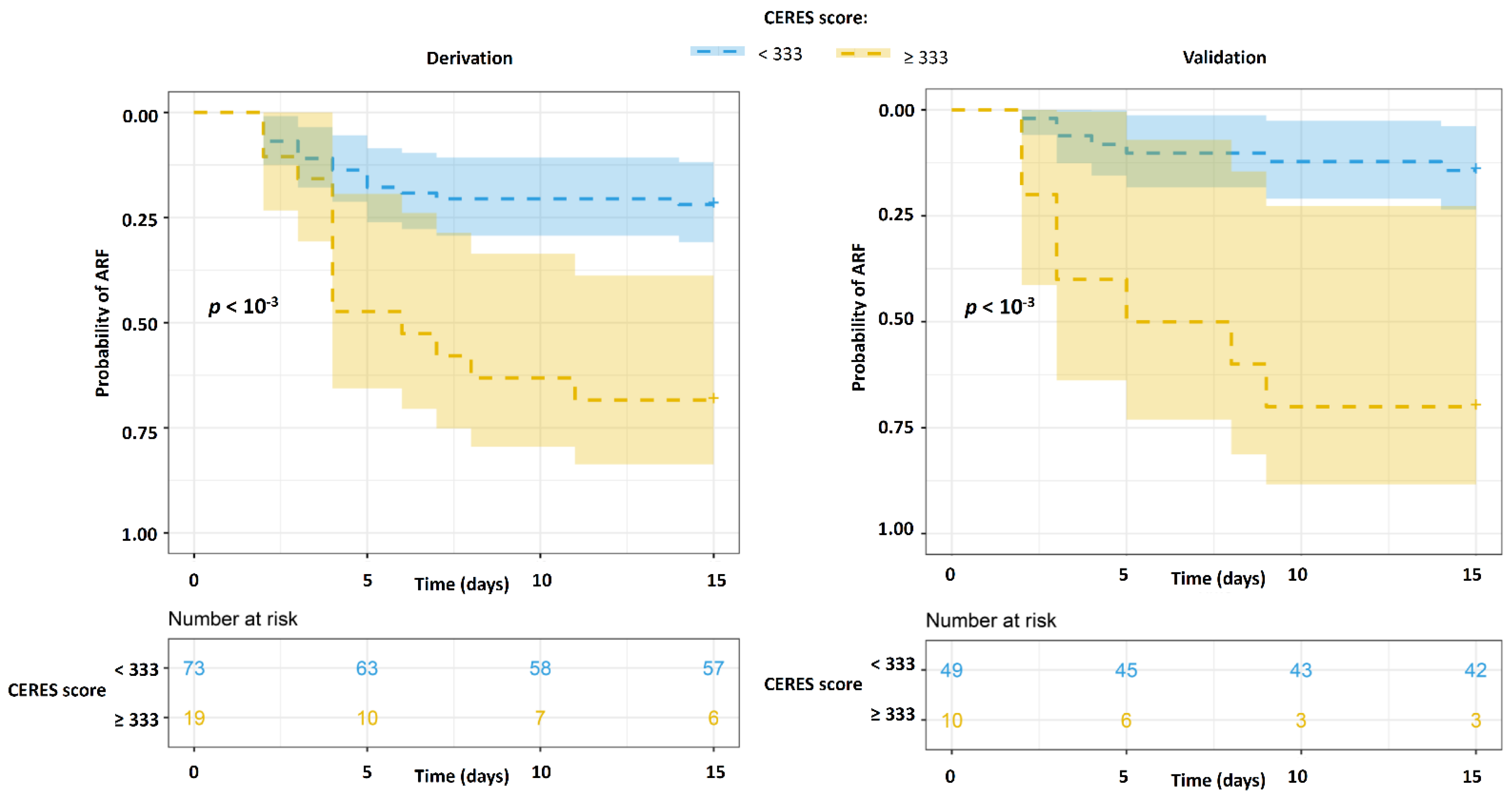
3.3. Derivation and Validation of the CERES Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhtar, A.; Lotfy, A.; Hasanin, A.; El-Hefnawy, I.; El Adawy, A. Outcome of non-invasive ventilation in COVID-19 critically ill patients: A Retrospective observational Study. Anaesth. Crit. Care Pain Med. 2020, 39, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, G.L.; Lalla, U.; Audley, G.; Gina, P.; Miller, M.G.; Mendelson, M.; Dlamini, S.; Wasserman, S.; Meintjes, G.; Peter, J.; et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine 2020, 28, 100570. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; De Soyza, A.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA 2022, 327, 546. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods–United States, December 2020-January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Koh, Y.; Lim, C.-M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.-S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef]
- Boscolo, A.; Pasin, L.; Sella, N.; Pretto, C.; Tocco, M.; Tamburini, E.; Rosi, P.; Polati, E.; Donadello, K.; Gottin, L.; et al. Outcomes of COVID-19 patients intubated after failure of non-invasive ventilation: A multicenter observational study. Sci. Rep. 2021, 11, 17730. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; de Gonzalo-Calvo, D.; Torres, G.; de Batlle, J.; Gómez, S.; Moncusí-Moix, A.; Carmona, P.; Santisteve, S.; Monge, A.; et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: A prospective cohort study. Crit. Care Lond. Engl. 2022, 26, 18. [Google Scholar] [CrossRef]
- Prakash, J.; Bhattacharya, P.K.; Yadav, A.K.; Kumar, A.; Tudu, L.C.; Prasad, K. ROX index as a good predictor of high flow nasal cannula failure in COVID-19 patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis. J. Crit. Care 2021, 66, 102–108. [Google Scholar] [CrossRef]
- Dupont, A.; Rauch, A.; Staessens, S.; Moussa, M.; Rosa, M.; Corseaux, D.; Jeanpierre, E.; Goutay, J.; Caplan, M.; Varlet, P.; et al. Vascular Endothelial Damage in the Pathogenesis of Organ Injury in Severe COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1760–1773. [Google Scholar] [CrossRef]
- Medetalibeyoglu, A.; Emet, S.; Kose, M.; Akpinar, T.S.; Senkal, N.; Catma, Y.; Kaytaz, A.M.; Genc, S.; Omer, B.; Tukek, T. Serum Endocan Levels on Admission Are Associated With Worse Clinical Outcomes in COVID-19 Patients: A Pilot Study. Angiology 2020, 72, 187–193. [Google Scholar] [CrossRef]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. Prothrombotic Phenotype in COVID-19: Focus on Platelets. Int. J. Mol. Sci. 2021, 22, 13638. [Google Scholar] [CrossRef] [PubMed]
- Görgün, S.; Cindoruk, Ş.; Özgen, E.; Yadigaroğlu, M.; Demir, M.T.; Yücel, M.; Akpınar, Ç.K.; Güzel, M. Diagnostic and Prognostic Value of Serum Endocan Levels in Patients With COVID-19. Angiology 2021, 72, 942–946. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Caires, N.; Gaudet, A.; Portier, L.; Tsicopoulos, A.; Mathieu, D.; Lassalle, P. Endocan, sepsis, pneumonia, and acute respiratory distress syndrome. Crit. Care Lond. Engl. 2018, 22, 280. [Google Scholar] [CrossRef] [Green Version]
- Ioakeimidou, A.; Pagalou, E.; Kontogiorgi, M.; Antoniadou, E.; Kaziani, K.; Psaroulis, K.; Giamarellos-Bourboulis, E.J.; Prekates, A.; Antonakos, N.; Lassale, P.; et al. Increase of circulating endocan over sepsis follow-up is associated with progression into organ dysfunction. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2017, 36, 1749–1756. [Google Scholar] [CrossRef]
- Perrotti, A.; Chenevier-Gobeaux, C.; Ecarnot, F.; Bardonnet, K.; Barrucand, B.; Flicoteaux, G.; Lassalle, P.; Chocron, S. Is Endocan a Diagnostic Marker for Pneumonia After Cardiac Surgery? The ENDOLUNG Study. Ann. Thorac. Surg. 2018, 105, 535–541. [Google Scholar] [CrossRef]
- Perrotti, A.; Chenevier-Gobeaux, C.; Ecarnot, F.; Barrucand, B.; Lassalle, P.; Dorigo, E.; Chocron, S. Relevance of Endothelial Cell-Specific Molecule 1 (Endocan) Plasma Levels for Predicting Pulmonary Infection after Cardiac Surgery in Chronic Kidney Disease Patients: The Endolung Pilot Study. Cardiorenal Med. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Alberdi-Iglesias, A.; Martín-Rodríguez, F.; Ortega Rabbione, G.; Rubio-Babiano, A.I.; Núñez-Toste, M.G.; Sanz-García, A.; Del Pozo Vegas, C.; Castro Villamor, M.A.; Martín-Conty, J.L.; Jorge-Soto, C.; et al. Role of SpO2/FiO2 Ratio and ROX Index in Predicting Early Invasive Mechanical Ventilation in COVID-19. A Pragmatic, Retrospective, Multi-Center Study. Biomedicines 2021, 9, 1036. [Google Scholar] [CrossRef]
- Valencia, C.F.; Lucero, O.D.; Castro, O.C.; Sanko, A.A.; Olejua, P.A. Comparison of ROX and HACOR scales to predict high-flow nasal cannula failure in patients with SARS-CoV-2 pneumonia. Sci. Rep. 2021, 11, 22559. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, S.; Sancho, J.; Bocigas, I.; Bures, E.; Mora, H.; Monclou, E.; Mulet, A.; Quezada, A.; Royo, P.; Signes-Costa, J. ROX index as predictor of high flow nasal cannula therapy success in acute respiratory failure due to SARS-CoV-2. Respir. Med. 2021, 189, 106638. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Chowdhury, J.; Mills, N.; Marron, R.; Gangemi, A.; Dorey-Stein, Z.; Yousef, I.; Zheng, M.; Tragesser, L.; Giurintano, J.; et al. Utility of the ROX Index in Predicting Intubation for Patients With COVID-19-Related Hypoxemic Respiratory Failure Receiving High-Flow Nasal Therapy: Retrospective Cohort Study. JMIRx Med. 2021, 2, e29062. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, J.; Wu, W.; Chen, H.; Li, S.; He, H.; Yu, Y.; Hu, M.; Li, J.; Zheng, R.; et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: A retrospective multicentre study. Lancet Digit. Health 2021, 3, e166–e174. [Google Scholar] [CrossRef]
- Aljouie, A.F.; Almazroa, A.; Bokhari, Y.; Alawad, M.; Mahmoud, E.; Alawad, E.; Alsehawi, A.; Rashid, M.; Alomair, L.; Almozaai, S.; et al. Early Prediction of COVID-19 Ventilation Requirement and Mortality from Routinely Collected Baseline Chest Radiographs, Laboratory, and Clinical Data with Machine Learning. J. Multidiscip. Healthc. 2021, 14, 2017–2033. [Google Scholar] [CrossRef]
- Mauer, E.; Lee, J.; Choi, J.; Zhang, H.; Hoffman, K.L.; Easthausen, I.J.; Rajan, M.; Weiner, M.G.; Kaushal, R.; Safford, M.M.; et al. A predictive model of clinical deterioration among hospitalized COVID-19 patients by harnessing hospital course trajectories. J. Biomed. Inform. 2021, 118, 103794. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Hentschker, C.; Westhoff, M.; Weber-Carstens, S.; Janssens, U.; Kluge, S.; Pfeifer, M.; Spies, C.; Welte, T.; Rossaint, R.; et al. Observational study of changes in utilization and outcomes in mechanical ventilation in COVID-19. PLoS ONE 2022, 17, e0262315. [Google Scholar] [CrossRef]
- Yu, M.; Xu, D.; Lan, L.; Tu, M.; Liao, R.; Cai, S.; Cao, Y.; Xu, L.; Liao, M.; Zhang, X.; et al. Thin-Section Chest CT Imaging of COVID-19 Pneumonia: A Comparison Between Patients with Mild and Severe Disease. Radiol. Cardiothorac. Imaging 2020, 2, e200126. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhong, Z.; Xie, X.; Yu, Q.; Liu, J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am. J. Roentgenol. 2020, 214, 1072–1077. [Google Scholar] [CrossRef]
- Grillet, F.; Behr, J.; Calame, P.; Aubry, S.; Delabrousse, E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology 2020, 296, E186–E188. [Google Scholar] [CrossRef] [Green Version]


| Variable | Derivation Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| ARF Worsening at D15 | p | ARF Worsening at D15 | p | |||
| No (n = 63) | Yes (n = 29) | No (n = 45) | Yes (n = 14) | |||
| Demographics | ||||||
| Age, years | 63 (13) | 69 (8) | 0.02 | 63 (14) | 64 (13) | 0.73 |
| BMI, kg/m2 | 31 (6) | 29 (5) | 0.26 | 31 (7) | 32 (6) | 0.65 |
| Gender, female | 16 (25) | 5 (17) | 0.55 | 13 (29) | 2 (14) | 0.48 |
| No comorbidities α | 17 (27) | 10 (34) | 0.46 | 10 (22) | 4 (29) | 0.72 |
| BMI > 30 | 29 (46) | 9 (31) | 0.17 | 21 (47) | 7 (50) | 0.83 |
| Diabetes | 20 (32) | 9 (31) | 1 | 14 (31) | 4 (29) | 1 |
| Chronic respiratory failure | 4 (6) | 6 (21) | 0.09 | 0 (0) | 1 (7) | 0.24 |
| COPD | 10 (16) | 6 (21) | 0.79 | 5 (11) | 6 (43) | 0.01 |
| Chronic heart failure | 6 (9) | 4 (14) | 0.8 | 5 (11) | 3 (21) | 0.38 |
| Cirrhosis Child B or C | 1 (2) | 0 (0) | 1 | 0 (0) | 0 (0) | 1 |
| End stage kidney disease β | 4 (6) | 4 (14) | 0.44 | 3 (7) | 2 (14) | 0.58 |
| Immunocompromised γ | 4 (6) | 6 (21) | 0.09 | 6 (13) | 2 (14) | 1 |
| Characteristics of disease on ICU admission | ||||||
| SAPS2 | 35 (8) | 38 (8) | 0.2 | 32 (11) | 37 (7) | 0.14 |
| SOFA | 2.5 (0.9) | 2.8 (1.5) | 0.2 | 2.8 (1.4) | 2.9 (1.3) | 0.84 |
| FiO2, % | 77 (20) | 88 (16) | 0.01 | 70 (20) | 84 (16) | 0.01 |
| CT-scan extension, % | 48 (19) | 45 (20) | 0.62 | 47 (21) | 60 (21) | 0.08 |
| Predominant findings on CT-scanGround-glass opacities | 39 (62) | 17 (59) | 0.76 | 26 (58) | 10 (71) | 0.55 |
| Consolidation | 13 (21) | 3 (10) | 0.36 | 8 (18) | 3 (21) | 0.71 |
| Pulmonary embolism | 10 (16) | 6 (21) | 0.79 | 3 (7) | 1 (7) | 1 |
| Purulent sputum | 8 (13) | 2 (7) | 0.5 | 5 (11) | 2 (14) | 0.67 |
| Microbiologically confirmed bacterial co-infection δ | 4 (6) | 2 (7) | 1 | 5 (11) | 0 (0) | 0.33 |
| Use of antibiotics prior to collection of microbiological specimens | 20 (32) | 10 (34) | 0.98 | 12 (27) | 7 (50) | 0.12 |
| Treatments on ICU admission | ||||||
| CPAP H0–H48 ϕ | 20 (32) | 13 (45) | 0.33 | 19 (42) | 5 (36) | 0.9 |
| NIV H0–H48 χ | 31 (49) | 21 (72) | 0.06 | 18 (40) | 10 (71) | 0.08 |
| Prone positioning H0–H48 | 11 (17) | 3 (10) | 0.57 | 9 (20) | 2 (14) | 1 |
| Antibiotics | 43 (68) | 20 (69) | 1 | 40 (89) | 13 (93) | 1 |
| Tocilizumab | 2 (3) | 4 (14) | 0.14 | 1 (2) | 0 (0) | 1 |
| Remdesivir | 5 (8) | 4 (4) | 0.62 | 4 (9) | 0 (0) | 0.56 |
| Outcomes | ||||||
| ICU mortality | 1 (2) | 22 (76) | <10−3 | 1 (2) | 11 (79) | <10−3 |
| ICU length of stay, days | 9 (12) | 21 (15) | <10−3 | 8 (5) | 27 (17) | <10−3 |
| Variable | Derivation Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| ARF Worsening at D15 | p | ARF Worsening at D15 | p | |||
| No (n = 63) | Yes (n = 29) | No (n = 45) | Yes (n = 14) | |||
| VWF:Ag, % | 458 (129) | 466 (125) | 0.79 | 422 (101) | 418 (120) | 0.91 |
| Angiopoietin 2, pg/mL | 2311(1312) | 3042 (2306) | 0.06 | -- | -- | -- |
| VEGF, pg/mL | 161 (127) | 133 (92) | 0.29 | -- | -- | -- |
| Syndecan, ng/mL | 209 (239) | 297 (515) | 0.27 | -- | -- | -- |
| Endocan, ng/mL | 3.39 (3.08) | 9.13 (15.34) | <10−2 | 4.04 (3.73) | 7.93 (17.3) | 0.16 |
| suPAR, ng/mL | 6.22 (2.21) | 7.52 (4.01) | 0.048 | -- | -- | -- |
| PAI-1, ng/mL | 84.9 (63.7) | 81.5 (36.5) | 0.79 | -- | -- | -- |
| TFPI, ng/mL | 112 (43) | 123 (65) | 0.33 | -- | -- | -- |
| CRP, mg/L | 144 (89) | 166 (99) | 0.29 | 155 (92) | 128 (74) | 0.33 |
| PCT, ng/mL | 0.57 (0.74) | 6.45 (21.88) | 0.03 | 3.34 (11.21) | 1.28 (2.59) | 0.52 |
| LDH, UI/L | 510 (190) | 570 (188) | 0.16 | 507 (153) | 640 (459) | 0.16 |
| ALAT, UI/L | 49.4 (34.1) | 45.6 (25.8) | 0.6 | 78.5 (107.2) | 83 (111.9) | 0.89 |
| ASAT, UI/L | 60.8 (31.7) | 71.1 (38.3) | 0.18 | 92.8 (110) | 147 (220) | 0.22 |
| Total bilirubin, mg/L | 4.86 (2.17) | 5.45 (2.34) | 0.24 | 5.44 (3.09) | 6.29 (5.06) | 0.45 |
| Creatinine, mg/L | 11.6 (17.3) | 14.8 (18.1) | 0.42 | 15.2 (20.7) | 11.5 (5.2) | 0.51 |
| Ferritin, µg/L | 1952 (1590) | 2153 (1821) | 0.59 | 2246 (2496) | 1848 (1159) | 0.63 |
| TQ ratio | 1.27 (0.69) | 1.16 (0.42) | 0.45 | 1.24 (0.40) | 1.15 (0.17) | 0.42 |
| Fibrinogen, g/L | 7.24 (1.58) | 6.8 (1.21) | 0.19 | 7.06 (1.65) | 6.05 (1.62) | 0.049 |
| DDimers, µg/mL | 2.13 (3.39) | 6.85 (15.53) | 0.02 | 2.41 (1.94) | 2.78 (4.41) | 0.68 |
| Hemoglobin, g/dL | 12.9 (1.6) | 12.9 (2.5) | 0.86 | 12.9 (2) | 13.1 (2) | 0.76 |
| Leucocytes, (G/L) | 9.84 (4.3) | 9.65 (5.13) | 0.86 | 7.84 (3.89) | 5.57 (2.28) | 0.04 |
| Neutrophiles, (G/L) | 8.52 (3.84) | 8.80 (4.52) | 0.76 | -- | -- | -- |
| Lymphocytes, (G/L) | 0.82 (0.54) | 0.99 (1.54) | 0.44 | -- | -- | -- |
| Platelets (G/L) | 274 (109) | 200 (65) | <10−2 | 237 (92) | 187 (68) | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudet, A.; Ghozlan, B.; Dupont, A.; Parmentier-Decrucq, E.; Rosa, M.; Jeanpierre, E.; Bayon, C.; Tsicopoulos, A.; Duburcq, T.; Susen, S.; et al. Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score. J. Clin. Med. 2022, 11, 2172. https://doi.org/10.3390/jcm11082172
Gaudet A, Ghozlan B, Dupont A, Parmentier-Decrucq E, Rosa M, Jeanpierre E, Bayon C, Tsicopoulos A, Duburcq T, Susen S, et al. Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score. Journal of Clinical Medicine. 2022; 11(8):2172. https://doi.org/10.3390/jcm11082172
Chicago/Turabian StyleGaudet, Alexandre, Benoit Ghozlan, Annabelle Dupont, Erika Parmentier-Decrucq, Mickael Rosa, Emmanuelle Jeanpierre, Constance Bayon, Anne Tsicopoulos, Thibault Duburcq, Sophie Susen, and et al. 2022. "Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score" Journal of Clinical Medicine 11, no. 8: 2172. https://doi.org/10.3390/jcm11082172
APA StyleGaudet, A., Ghozlan, B., Dupont, A., Parmentier-Decrucq, E., Rosa, M., Jeanpierre, E., Bayon, C., Tsicopoulos, A., Duburcq, T., Susen, S., & Poissy, J. (2022). Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score. Journal of Clinical Medicine, 11(8), 2172. https://doi.org/10.3390/jcm11082172






