Oncological Outcomes of Robotic Lobectomy and Radical Lymphadenectomy for Early-Stage Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preoperative Staging
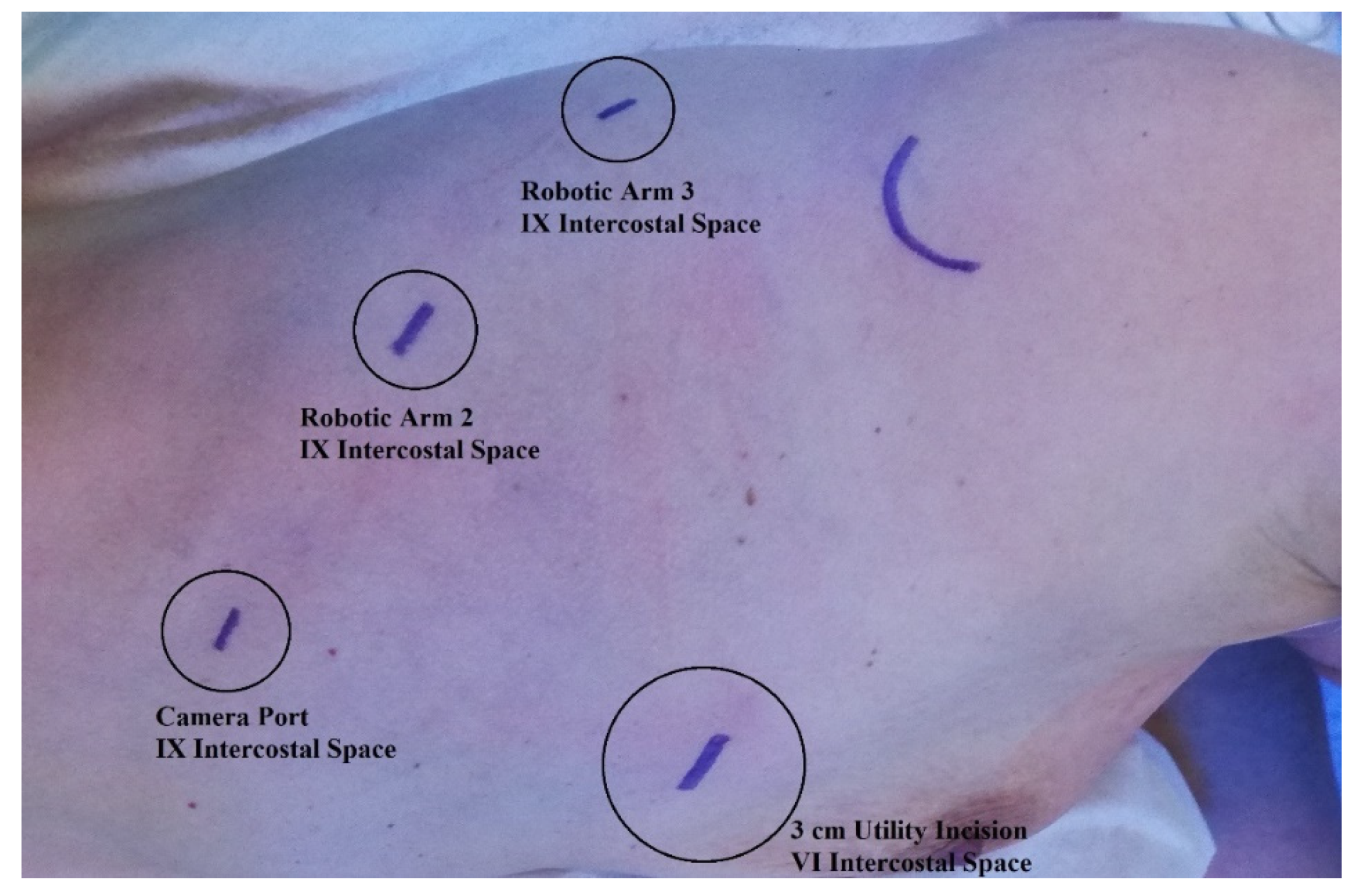
2.3. Surgical Technique
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Comparison between Upstaging and Non-Upstaging Groups
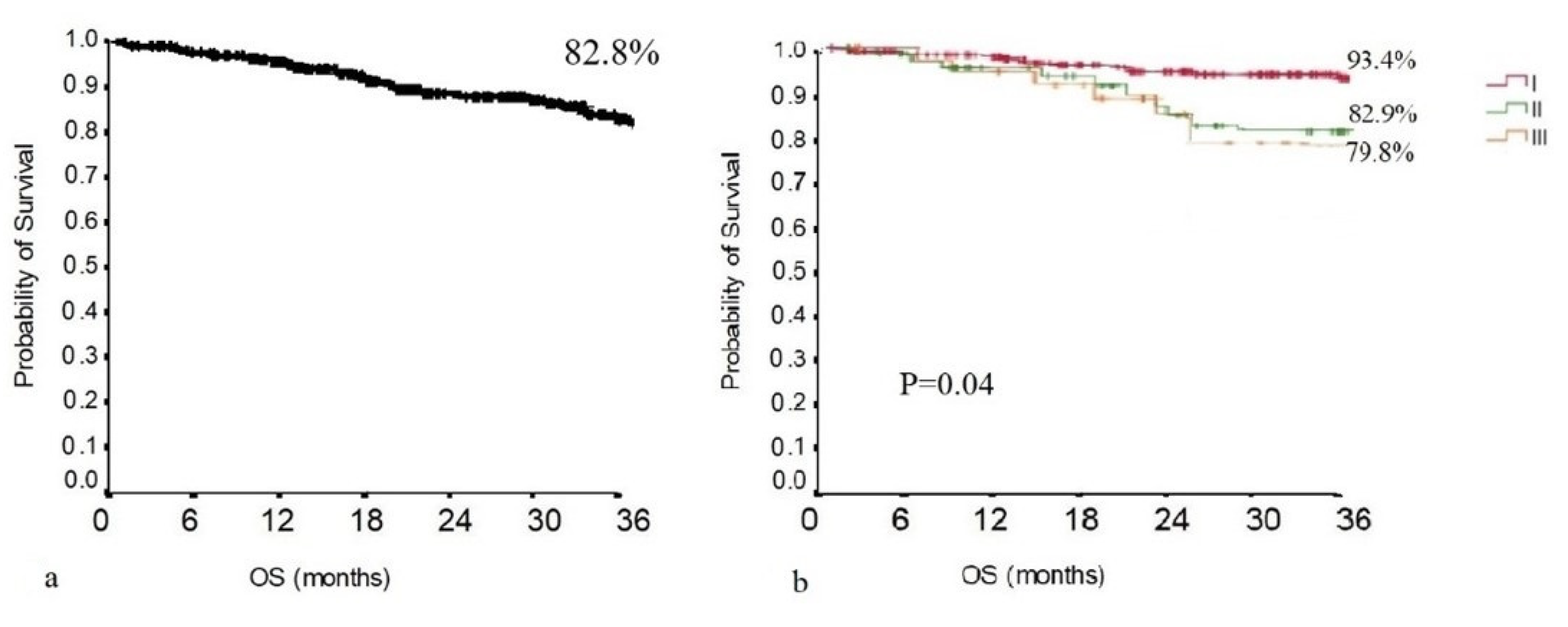
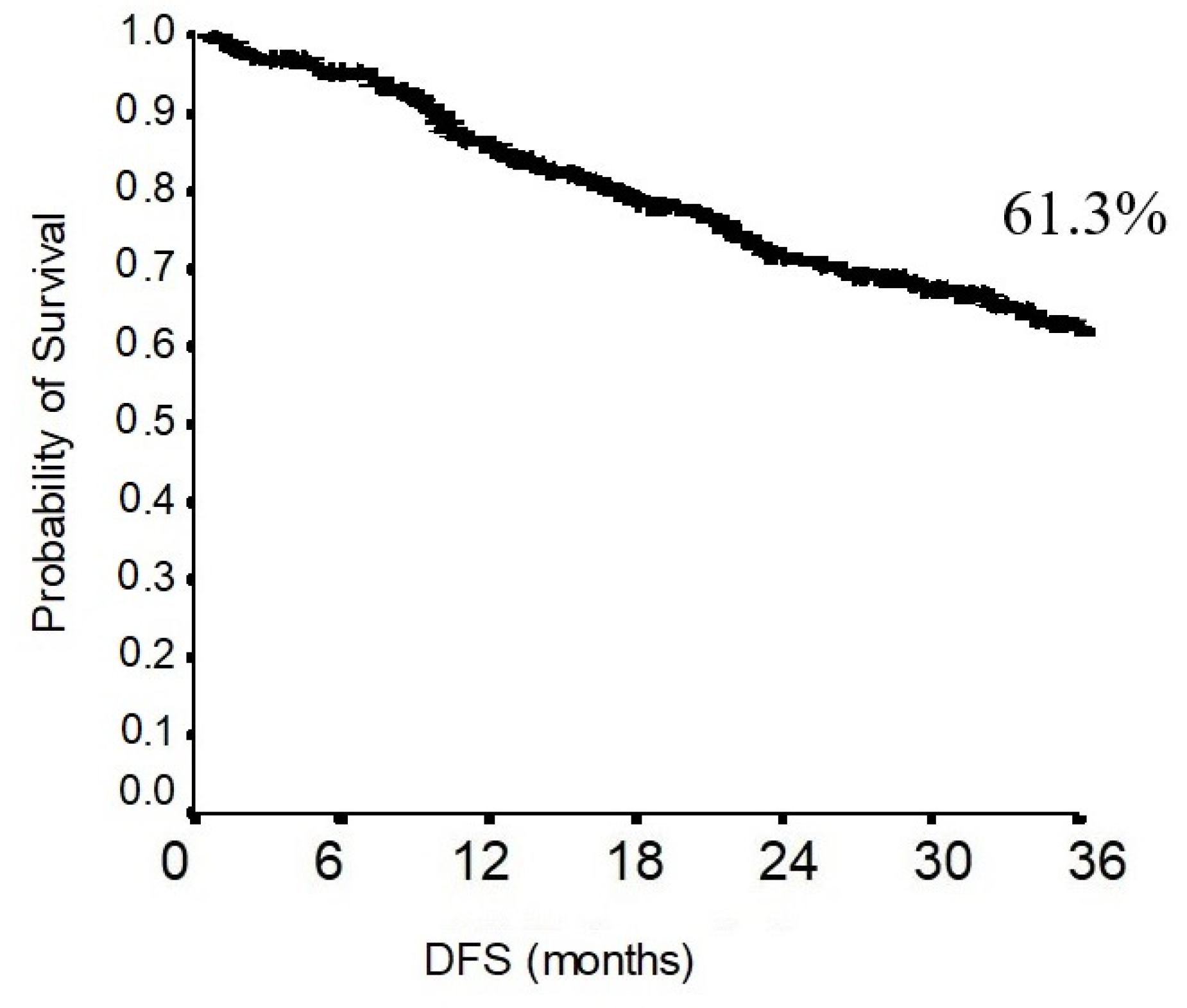
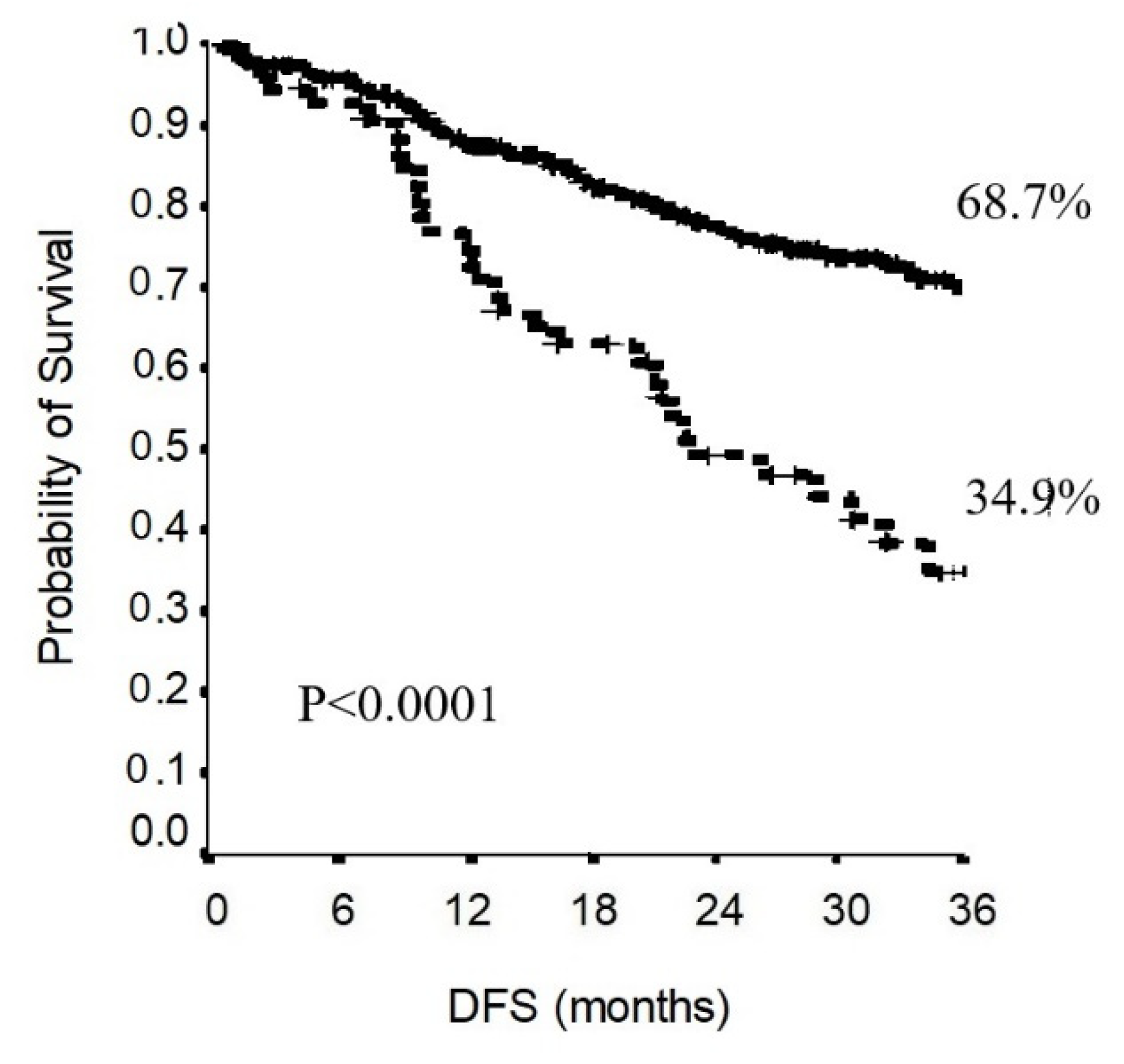
3.2. Survival Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for Staging Non-small Cell Lung Cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e211S–e250S. [Google Scholar] [CrossRef]
- Swanson, S.J.; Herndon, J.E.; D’Amico, T.A.; Demmy, T.L.; McKenna, R.J.; Green, M.R.; Sugarbaker, D.J. Video-Assisted Thoracic Surgery Lobectomy: Report of CALGB 39802—A Prospective, Multi-Institution Feasibility Study. J. Clin. Oncol. 2007, 25, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, Y.; Jiang, H.; Xu, J.; Yu, D. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, D.M.; Sarkaria, I.S.; Song, C.; Reddy, R.M.; Villamizar, N.; Herrera, L.J.; Shi, L.; Liu, E.; Rice, D.; Oh, D.S. Clinical and economic comparative effectiveness of robotic-assisted, video-assisted thoracoscopic, and open lobectomy. J. Thorac. Dis. 2020, 12, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.S.; Skylizard, L.; Minnich, D.J. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J. Thorac. Cardiovasc. Surg. 2011, 142, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: A meta-analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Yang, S.; Guo, W.; Chen, X.; Wu, H.; Li, H. Early outcomes of robotic versus uniportal video-assisted thoracic surgery for lung cancer: A propensity score-matched study. Eur. J. Cardio-Thoracic Surg. 2018, 53, 348–352. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv1–iv21. [Google Scholar] [CrossRef]
- Al-Sarraf, N.; Aziz, R.; Gately, K.; Lucey, J.; Wilson, L.; McGovern, E.; Young, V. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur. J. Cardio-Thoracic Surg. 2008, 33, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, J.R.; Passlick, B.; Karg, O.; Bloechle, C.; Pantel, K.; Knoefel, W.T.; Thetter, O. Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann. Thorac. Surg. 1995, 59, 209–214. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 615–623; discussion 622–623. [Google Scholar] [CrossRef] [PubMed]
- Kuritzky, A.M.; Ryder, B.A.; Ng, T. Long-Term Survival Outcomes of Video-assisted Thoracic Surgery (VATS) Lobectomy after Transitioning from Open Lobectomy. Ann. Surg. Oncol. 2013, 20, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, L.; Wouters, M.W.; Hartemink, K.J.; Heineman, D.J.; Verhagen, A.F. Completeness of lymph node dissection in patients undergoing minimally invasive- or open surgery for non-small cell lung cancer: A nationwide study. Eur. J. Surg. Oncol. 2020, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Melfi, F.; Mussi, A.; Maisonneuve, P.; Spaggiari, L.; Da Silva, R.K.C.; Veronesi, G. Robotic lobectomy for non–small cell lung cancer (NSCLC): Long-term oncologic results. J. Thorac. Cardiovasc. Surg. 2012, 143, 383–389. [Google Scholar] [CrossRef]
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracoscopic surgery. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2002, 21, 864–868. [Google Scholar] [CrossRef]
- Veronesi, G.; Abbas, A.E.-S.; Muriana, P.; Lembo, R.; Bottoni, E.; Perroni, G.; Testori, A.; Dieci, E.; Bakhos, C.T.; Car, S.; et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front. Oncol. 2021, 11, 726408. [Google Scholar] [CrossRef]
- Zirafa, C.; Aprile, V.; Ricciardi, S.; Romano, G.; Davini, F.; Cavaliere, I.; Alì, G.; Fontanini, G.; Melfi, F. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg. Endosc. 2019, 33, 153–158. [Google Scholar] [CrossRef]
- Toosi, K.; Velez-Cubian, F.O.; Glover, J.; Ng, E.P.; Moodie, C.C.; Garrett, J.R.; Fontaine, J.P.; Toloza, E.M. Upstaging and survival after robotic-assisted thoracoscopic lobectomy for non-small cell lung cancer. Surgery 2016, 160, 1211–1218. [Google Scholar] [CrossRef]
- Keller, S.M.; Adak, S.; Wagner, H.; Johnson, D.H. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann. Thorac. Surg. 2000, 70, 356–358. [Google Scholar] [CrossRef]
- Bille, A.; Woo, K.M.; Ahmad, U.; Rizk, N.P.; Jones, D.R. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2017, 51, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, M.; Leuzzi, G.; Sperduti, I.; Bria, E.; Mucilli, F.; Lococo, F.; Spaggiari, L.; Ratto, G.B.; Filosso, P.L.; Facciolo, F. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: A multicentre analysis. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2019, 55, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gallina, F.T.; Melis, E.; Forcella, D.; Mercadante, E.; Marinelli, D.; Ceddia, S.; Cappuzzo, F.; Vari, S.; Cecere, F.L.; Caterino, M.; et al. Nodal Upstaging Evaluation After Robotic-Assisted Lobectomy for Early-Stage Non-small Cell Lung Cancer Compared to Video-Assisted Thoracic Surgery and Thoracotomy: A Retrospective Single Center Analysis. Front. Surg. 2021, 8, 666158. [Google Scholar] [CrossRef]
- Wilson, J.L.; Louie, B.E.; Cerfolio, R.J.; Park, B.J.; Vallières, E.; Aye, R.W.; Abdel-Razek, A.; Bryant, A.; Farivar, A.S. The Prevalence of Nodal Upstaging During Robotic Lung Resection in Early Stage Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2014, 97, 1901–1907. [Google Scholar] [CrossRef]
- Riquet, M.; Legras, A.; Mordant, P.; Rivera, C.; Arame, A.; Gibault, L.; Foucault, C.; Dujon, A.; Barthes, F.L.P. Number of Mediastinal Lymph Nodes in Non-Small Cell Lung Cancer: A Gaussian Curve, Not a Prognostic Factor. Ann. Thorac. Surg. 2014, 98, 224–231. [Google Scholar] [CrossRef]
- Saji, H.; Tsuboi, M.; Yoshida, K.; Kato, Y.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; Kajiwara, N.; et al. Prognostic Impact of Number of Resected and Involved Lymph Nodes at Complete Resection on Survival in Non-small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1865–1871. [Google Scholar] [CrossRef]
- Darling, G.E.; Allen, M.S.; Decker, P.A.; Ballman, K.; Malthaner, R.A.; Inculet, R.I.; Jones, D.R.; McKenna, R.J.; Landreneau, R.J.; Rusch, V.; et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J. Thorac. Cardiovasc. Surg. 2011, 141, 662–670. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; A Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Samayoa, A.; Pezzi, T.A.; Pezzi, C.M.; Gay, E.G.; Asai, M.; Kulkarni, N.; Carp, N.; Chun, S.; Putnam, J.B., Jr. Rationale for a Minimum Number of Lymph Nodes Removed with Non-Small Cell Lung Cancer Resection: Correlating the Number of Nodes Removed with Survival in 98,970 Patients. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 1005–1011. [Google Scholar] [CrossRef]
- Spaggiari, L.; Sedda, G.; Maisonneuve, P.; Tessitore, A.; Casiraghi, M.; Petrella, F.; Galetta, D. A Brief Report on Survival After Robotic Lobectomy for Early-Stage Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.J.; Wherley, E.M.; Agyabeng-Dadzie, K.; Ramsuchit, B.; Johnston, M.A.; Escalon, J.C. 500 Consecutive Robotic Lobectomies for Non-Small Cell Lung Cancer: Perioperative and Oncologic Outcomes. Innovations 2021, 16, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Shahin, G.M.; Topal, B.; Pouwels, S.; Markou, T.L.; Boon, R.; Stigt, J.A. Quality assessment of robot assisted thoracic surgical resection of non-small cell lung cancer: Nodal upstaging and mediastinal recurrence. J. Thorac. Dis. 2021, 13, 592–599. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 299) | |
|---|---|
| Age (years) | 67.4 ± 8.4 |
| Gender (M/F) | 154/145 |
| Active or former smoker (n, %) | 221 (73.9) |
| Preoperative T (n, %) | |
| I | 149 (50.2) |
| II | 123 (40.8) |
| III | 27 (6.3) |
| Side (R/L) | 160/139 |
| Lobectomy (n, %) | |
| RUL | 92 (30.8) |
| ML | 16 (5.3) |
| RLL | 52 (17.4) |
| LUL | 86 (28.8) |
| LLL | 53 (17.7) |
| Dissected lymph node (mean ± SD) | 13.5 ± 7.7 |
| Dissected nodal stations (mean ± SD) | 4.4 ± 1.3 |
| Dissected lymph node/nodal stations (mean ± SD) | 3.1 ± 1.7 |
| In-hospital stay (days) | 6.2 ± 2.3 |
| Histology (n, %) | |
| Adenocarcinoma | 262 (87.6) |
| Squamous Cell Carcinoma | 37 (12.4) |
| Pathologic T (n, %) | |
| I | 133 (44.5) |
| II | 139 (46.5) |
| III | 21 (7) |
| IV | 6 (2) |
| Follow-up duration (months) | 27.6 ± 16.5 |
| Nodal Upstaging (n = 55) | Hilar Upstaging (n = 29) | Mediastinal Upstaging (n = 26) | p-Value | |
|---|---|---|---|---|
| N of resected lymph node | 17.2 ± 8.5 | 16.1 ± 9.9 | 18.3 ± 6.7 | 0.36 |
| N of resected nodal station | 4.7 ± 1.2 | 4.4 ± 1.2 | 5.0 ± 1.2 | 0.08 |
| N of metastatic lymph node | 2.3 ± 2.1 | 1.4 ± 0.7 | 3.3 ± 2.6 | 0.001 |
| N of metastatic nodal stations | 1.5 ± 0.8 | 1 ± 0.2 | 1.9 ± 1.0 | <0.001 |
| Upstaging Group (n = 55) | Non-Upstaging Group (n = 244) | p-Value | |
|---|---|---|---|
| Age (years) | 67.7 ± 7.5 | 68.0 ± 8.3 | 0.76 |
| Gender (M/F) | 33/22 | 119/121 | 0.16 |
| Active or former smoker (n, %) | 42 (76.4) | 179 (73.4) | 0.59 |
| Preoperative T (n, %) | 0.10 | ||
| I | 20 (36.4) | 129 (52.9) | |
| II | 29 (52.7) | 94 (38.5) | |
| III | 6 (10.9) | 21 (8.6) | |
| Side (R/L) | 27/28 | 133/111 | 0.47 |
| Lobectomy (n, %) | 0.44 | ||
| RUL | 13 | 79 | |
| ML | 5 | 11 | |
| RLL | 9 | 43 | |
| LUL | 19 | 67 | |
| LLL | 9 | 44 | |
| Dissected lymph node (mean ± SD) | 17.2 ± 8.5 | 12.7 ± 7.3 | <0.0001 |
| Dissected nodal stations (mean ± SD) | 4.7 ± 1.2 | 4.3 ± 1.3 | 0.2 |
| Dissected lymph nodes/nodal stations (mean ± SD) | 3.7 ± 1.6 | 2.9 ± 1.7 | 0.003 |
| In-hospital stay (days) | 6.0 ± 1.8 | 6.2 ± 2.5 | 0.76 |
| Histology (n, %) | 0.34 | ||
| Adenocarcinoma | 46 (83.6) | 216 (88.5) | |
| Squamous Cell Carcinoma | 9 (16.4) | 28 (11.5) | |
| Pathologic T (n, %) | |||
| I | 18 (32.7) | 115 (47.1) | |
| II | 31 (56.4) | 108 (44.3) | |
| III | 5 (9.1) | 16 (6.6) | |
| IV | 1 (1.8) | 5 (2) | |
| Follow-up duration (months) | 26.3 ± 15.3 | 27.9 ± 16.8 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallina, F.T.; Tajè, R.; Forcella, D.; Corzani, F.; Cerasoli, V.; Visca, P.; Coccia, C.; Pierconti, F.; Sperduti, I.; Cecere, F.L.; et al. Oncological Outcomes of Robotic Lobectomy and Radical Lymphadenectomy for Early-Stage Non-Small Cell Lung Cancer. J. Clin. Med. 2022, 11, 2173. https://doi.org/10.3390/jcm11082173
Gallina FT, Tajè R, Forcella D, Corzani F, Cerasoli V, Visca P, Coccia C, Pierconti F, Sperduti I, Cecere FL, et al. Oncological Outcomes of Robotic Lobectomy and Radical Lymphadenectomy for Early-Stage Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2022; 11(8):2173. https://doi.org/10.3390/jcm11082173
Chicago/Turabian StyleGallina, Filippo Tommaso, Riccardo Tajè, Daniele Forcella, Felicita Corzani, Virna Cerasoli, Paolo Visca, Cecilia Coccia, Federico Pierconti, Isabella Sperduti, Fabiana Letizia Cecere, and et al. 2022. "Oncological Outcomes of Robotic Lobectomy and Radical Lymphadenectomy for Early-Stage Non-Small Cell Lung Cancer" Journal of Clinical Medicine 11, no. 8: 2173. https://doi.org/10.3390/jcm11082173
APA StyleGallina, F. T., Tajè, R., Forcella, D., Corzani, F., Cerasoli, V., Visca, P., Coccia, C., Pierconti, F., Sperduti, I., Cecere, F. L., Cappuzzo, F., Melis, E., & Facciolo, F. (2022). Oncological Outcomes of Robotic Lobectomy and Radical Lymphadenectomy for Early-Stage Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 11(8), 2173. https://doi.org/10.3390/jcm11082173







