The Impact of Exosomes/Microvesicles Derived from Myeloid Dendritic Cells Cultured in the Presence of Calcitriol and Tacalcitol on Acute B-Cell Precursor Cell Lines with MLL Fusion Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Compounds
2.3. Anti-Proliferative In Vitro Test
2.4. Preparation of Immature Myeloid Dendritic Cells (iDCs)
2.5. Isolation of EVs
2.6. Total Protein Concentration
2.7. Cytospin Staining
2.8. Flow Cytometry Analysis of Apoptosis
2.9. Cell Cycle Analysis
2.10. Flow Cytometry Analysis
2.11. Culturing of Short-Term ALL-B Precursor Cells with the Addition of EVs
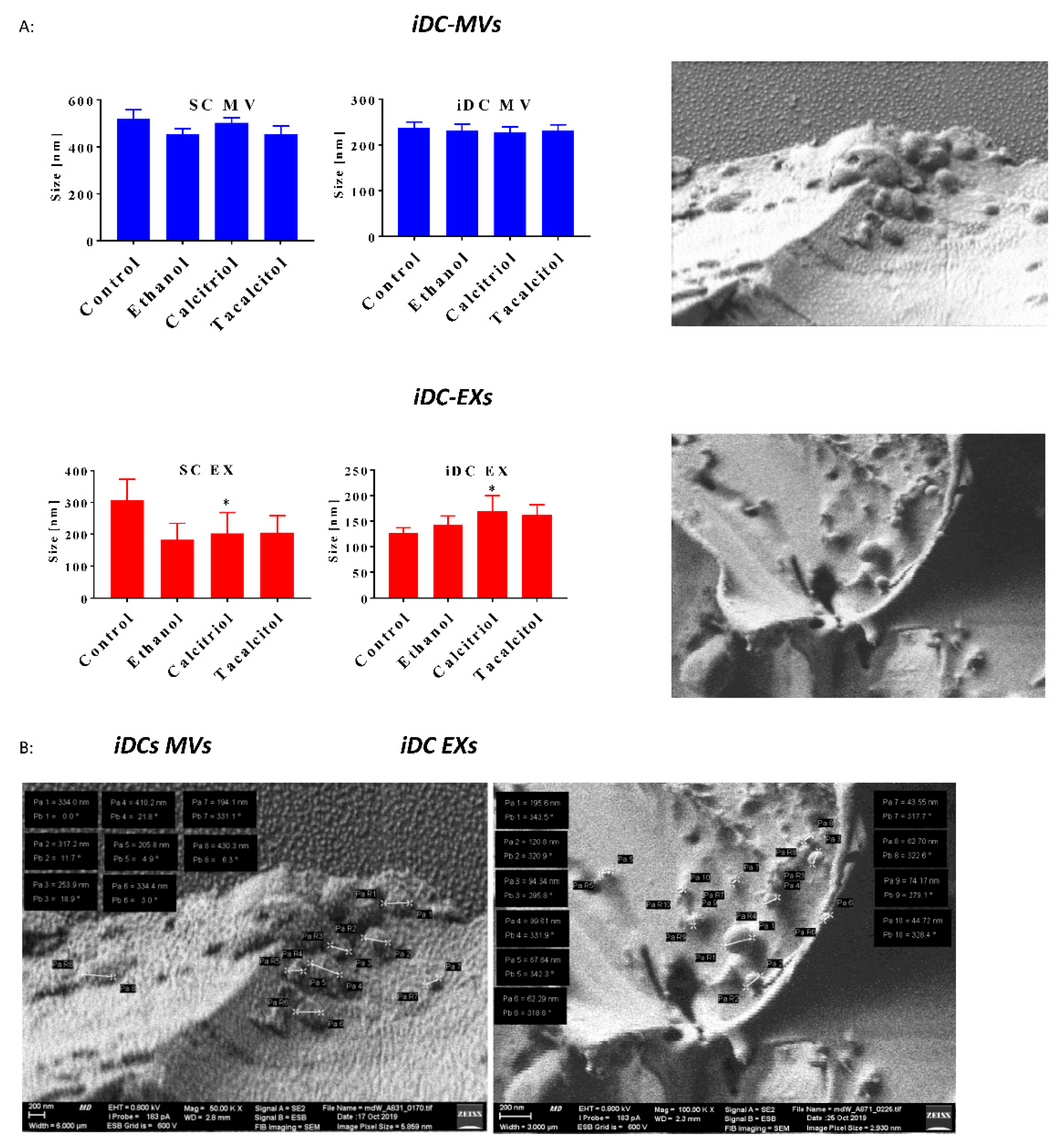
2.12. Assessment of MVs/EXs Hydrodynamic Size via DLS (Dynamic Light Scattering)
2.13. Biological Nanostructure Imaging by Cryogenic Low-Voltage Field-Emission Scanning Electron Microscopy (Cryo-LV-FESEM)
2.14. IgM and IgG Determination in Precursor B Cells
3. Results
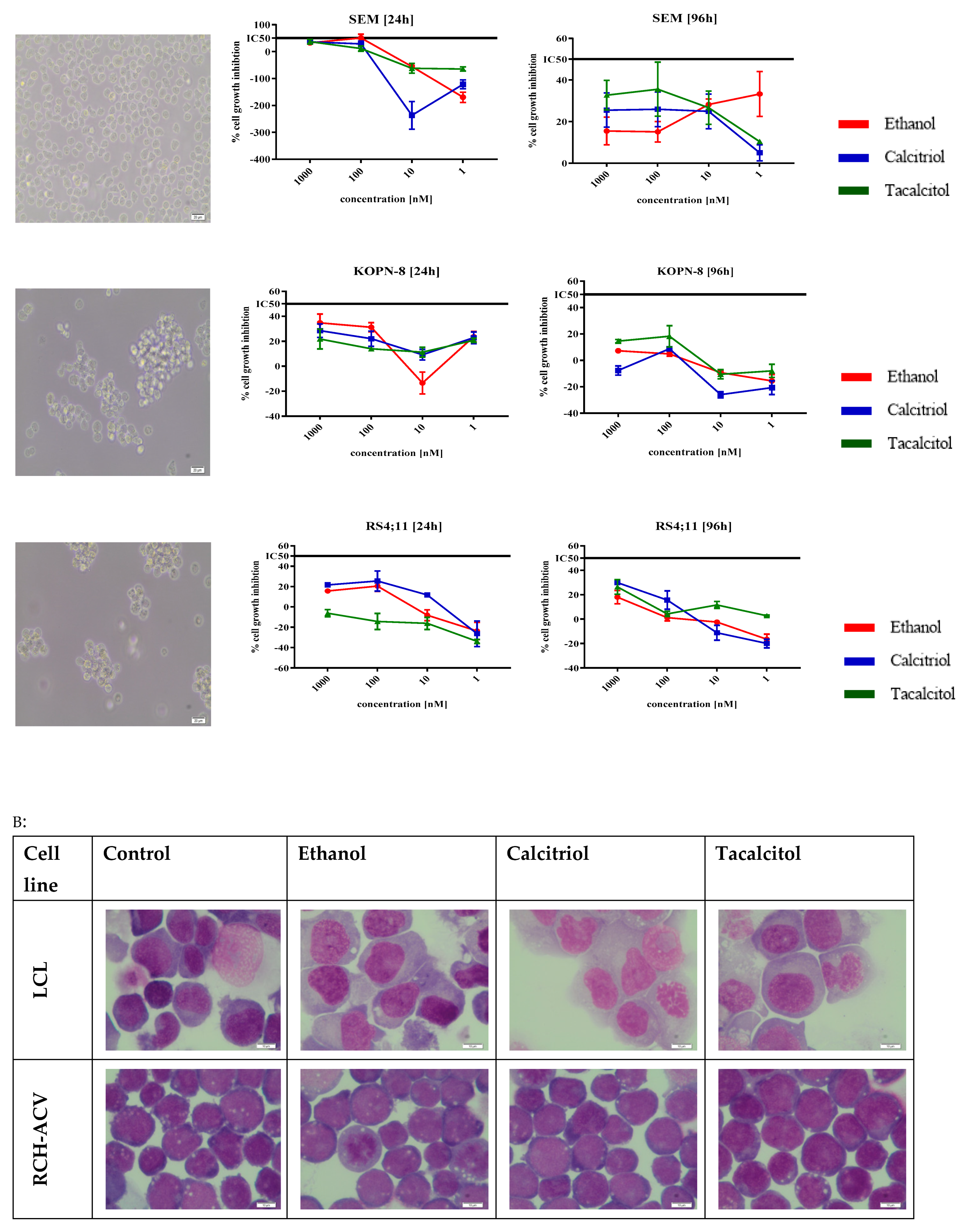
3.1. The Sensitivity of SC and iDCs on Calcitriol and Tacalcitol
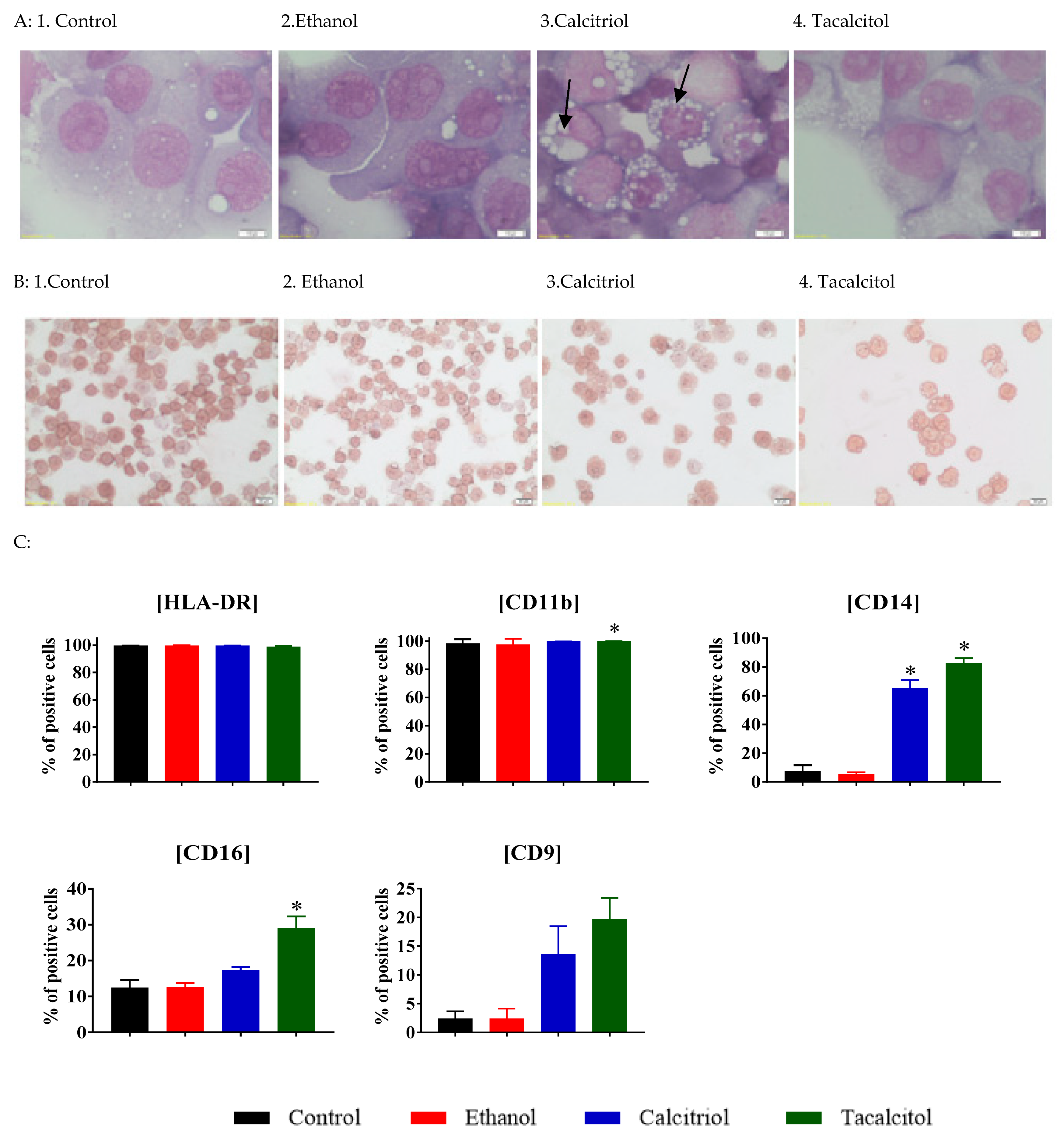
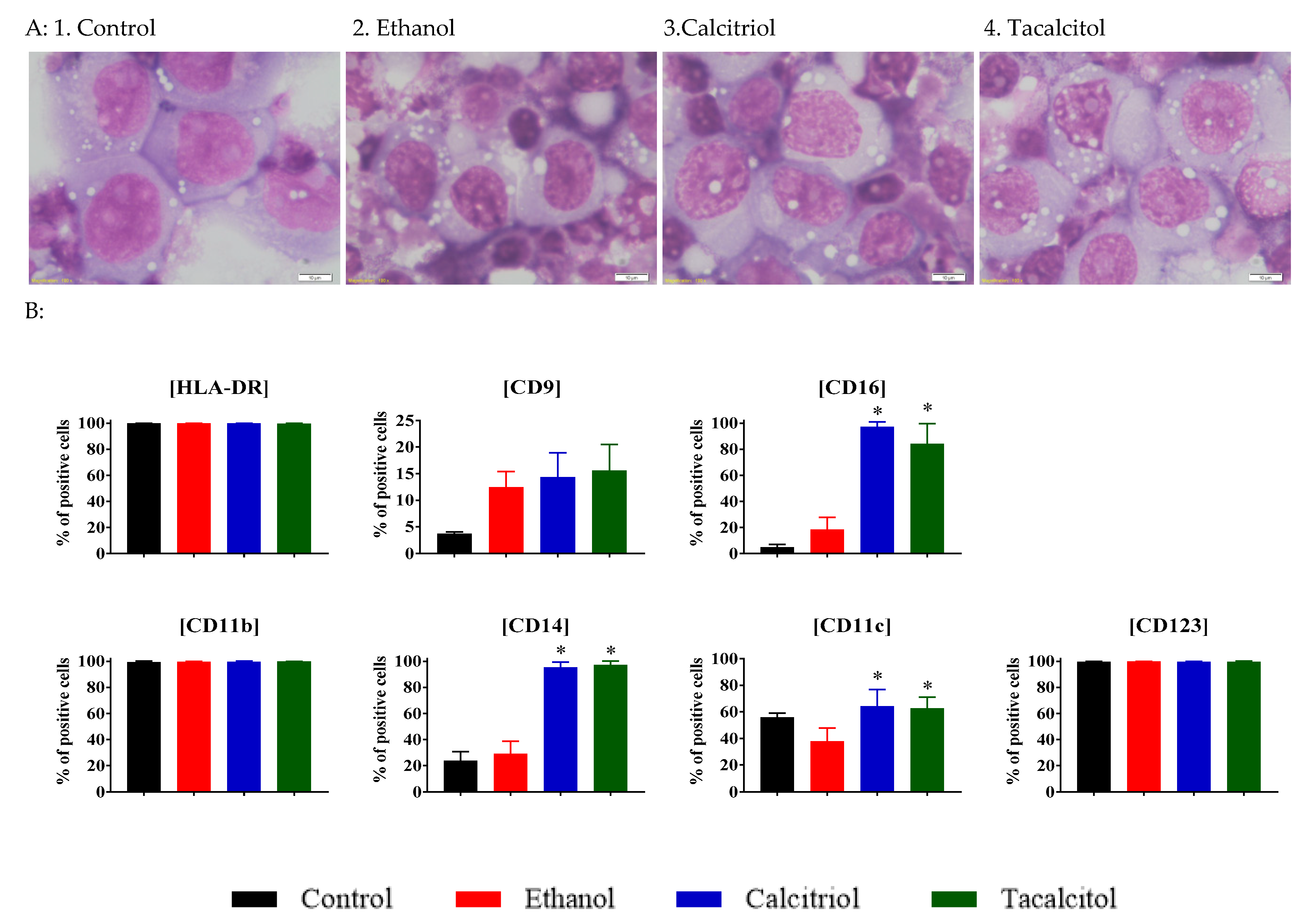
3.2. Enhanced Vacuolization in Calcitriol- and Tacalcitol-Stimulated Myeloid iDC/Mat DC Cells
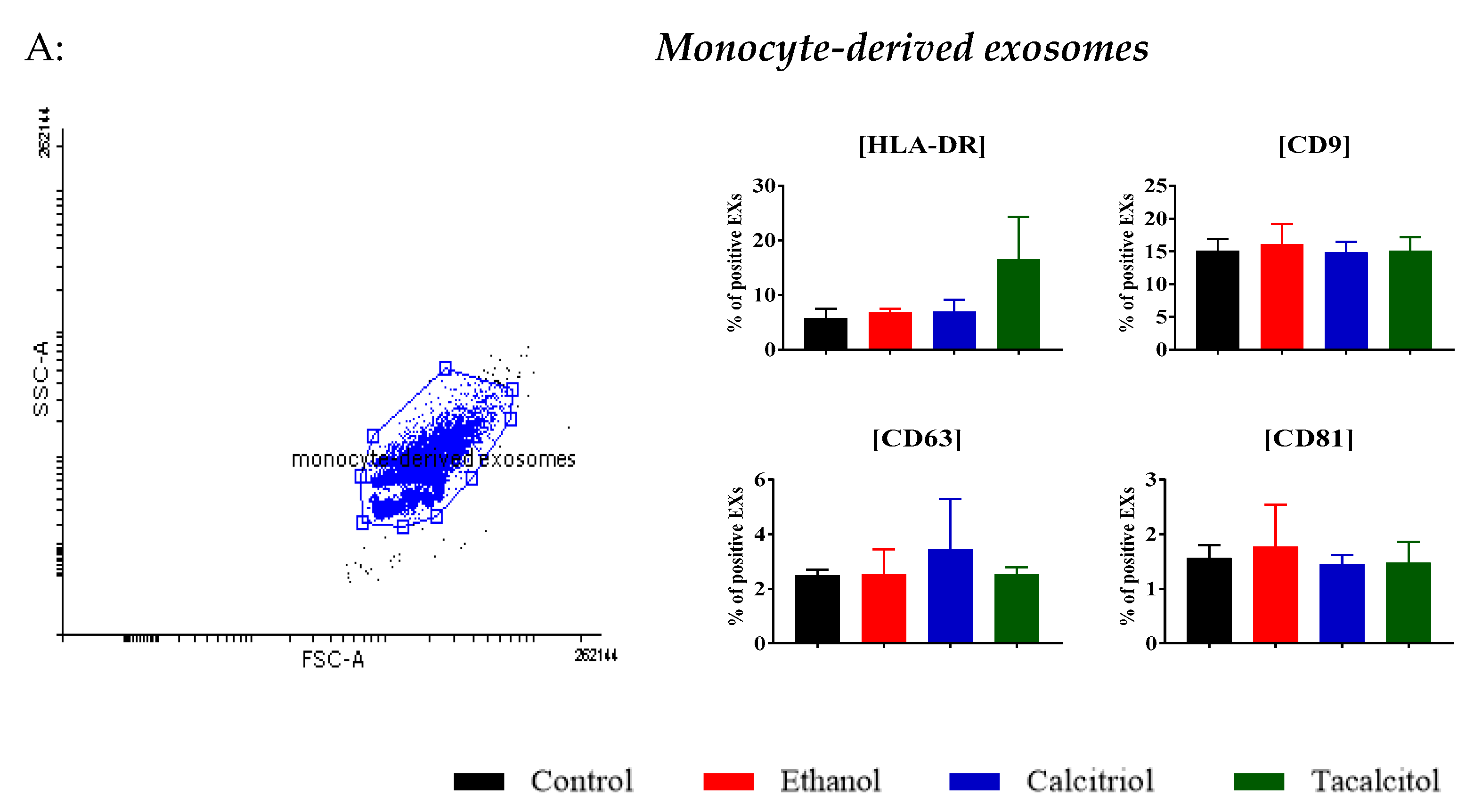
3.3. Phenotype Differentiation of EVs Derived from SC/iDCs after Calcitriol and Tacalcitol Treatment
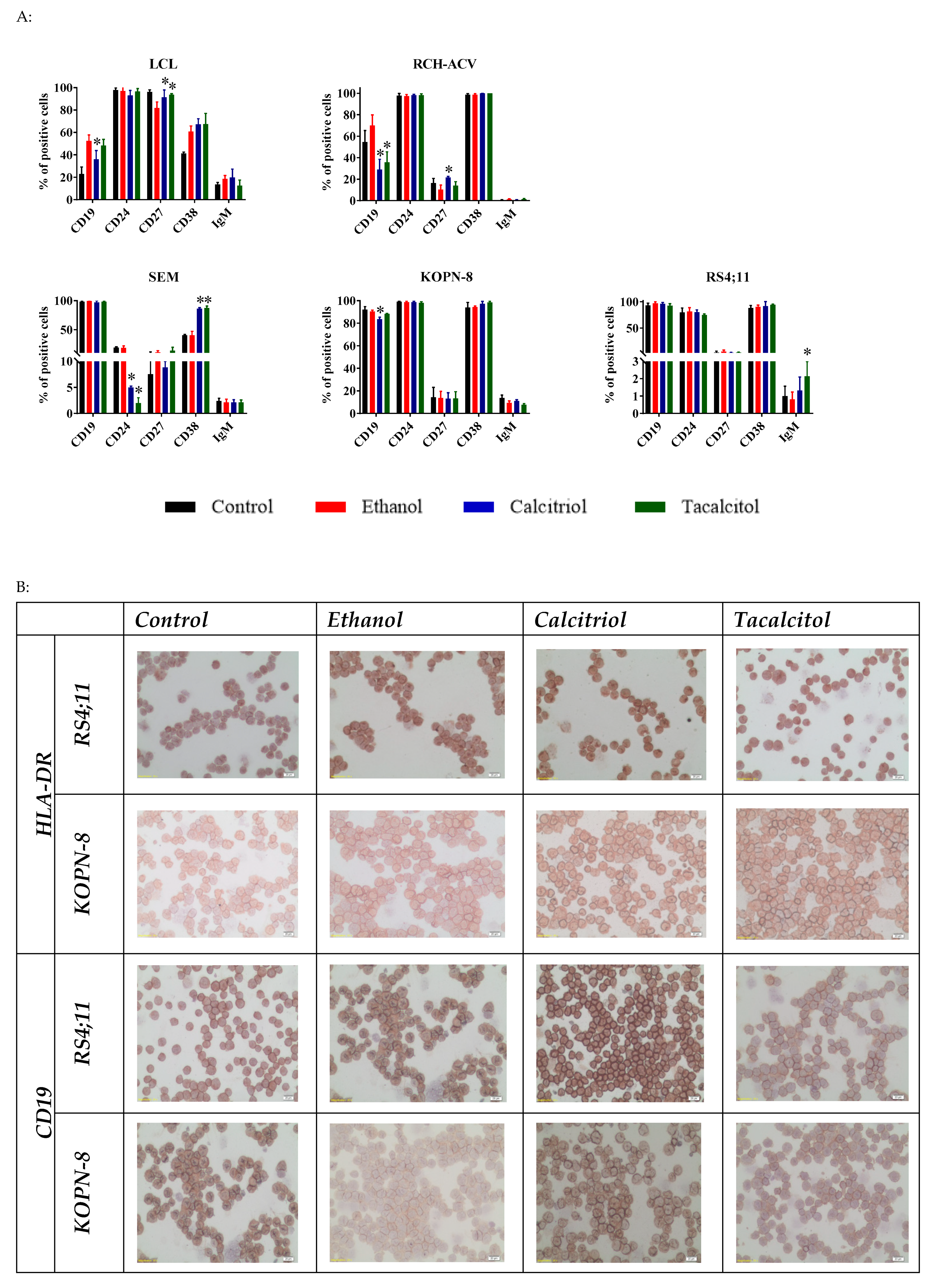
3.4. Morphology and Phenotype Changes of Acute Lymphoblastic Precursor B Cells after Calcitriol or Tacalcitol Treatment
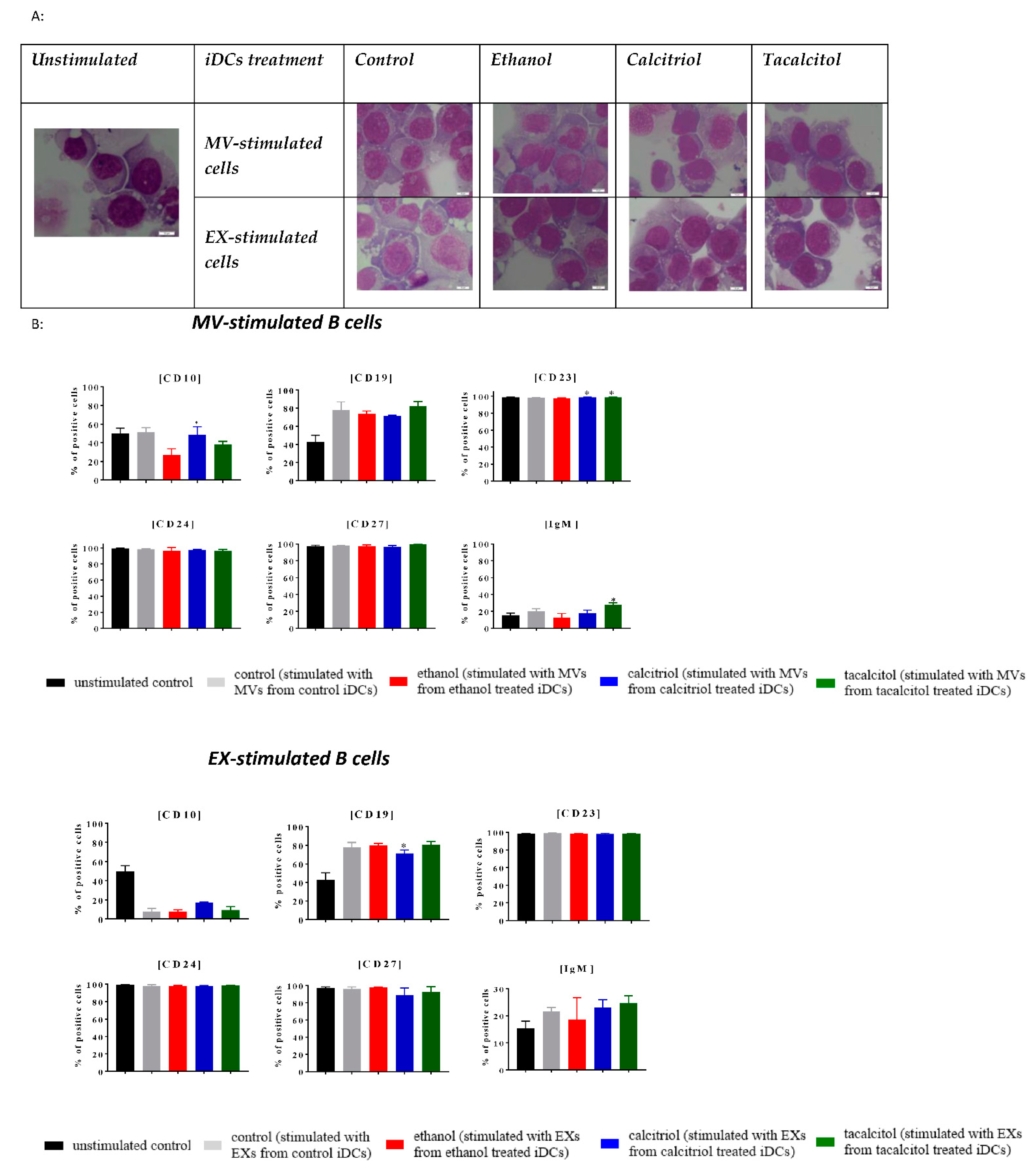
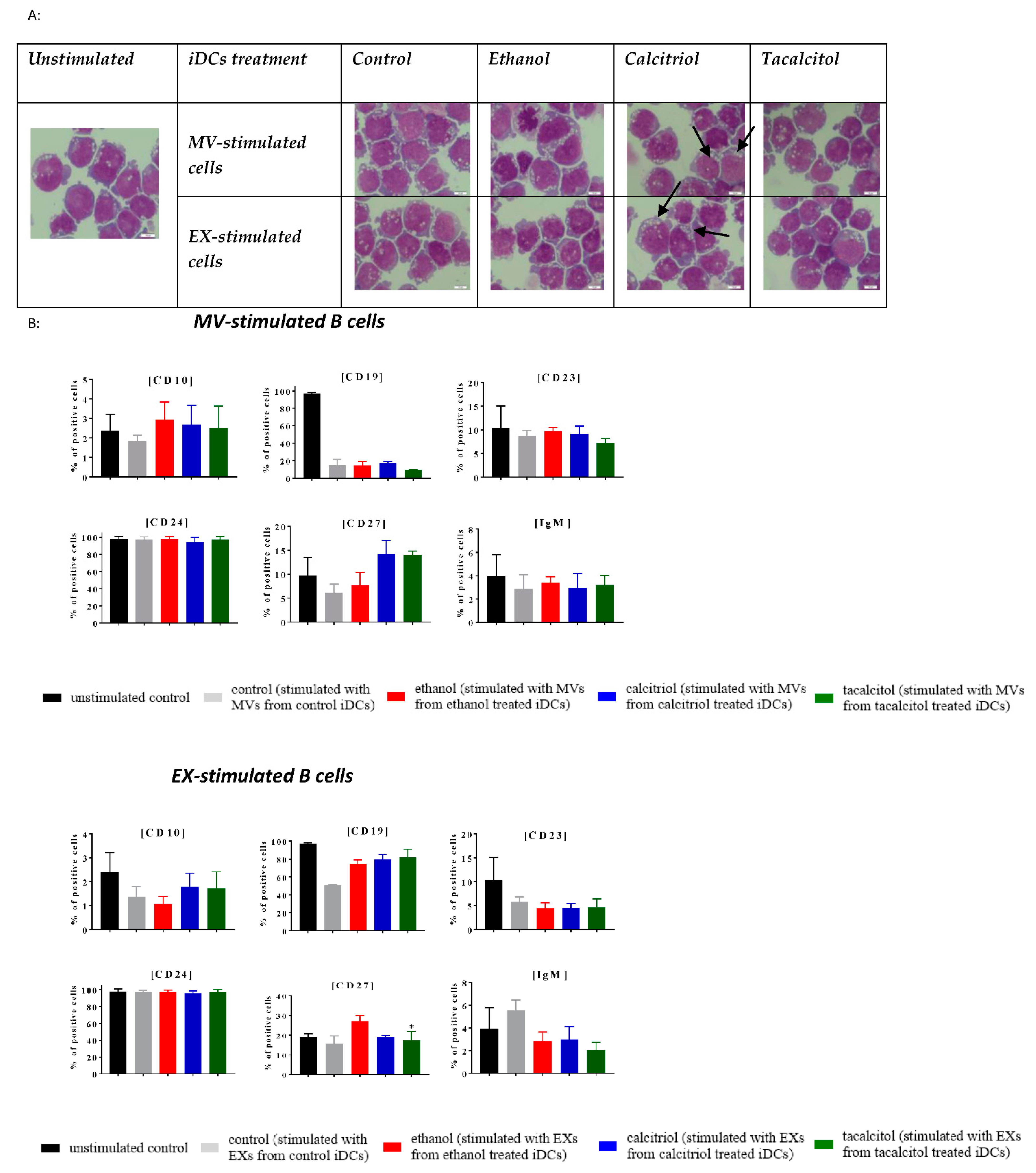
3.5. Morphology and Phenotype of Precursor B Cells Cultured with EVs Derived from Myeloid iDCs Treated with Calcitriol and Tacalcitol
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VDA | Vitamin D analog |
| ALL | Acute lymphoblastic leukemia |
| VDR | Vitamin D receptor |
| MV | Microvesicle |
| EX | Exosome |
| mDCs | Myeloid dendritic cells |
| iDCs | Immature dendritic cells |
| matDCs | Mature dendritic cells |
| EV | Extracellular vesicle |
| APCs | Antigen-presenting cells |
| pDCs | Plasmacytoid dendritic cells |
| MHC | Major histocompatibility complex |
| GM-CSF | Colony-stimulating granulocyte-macrophage factor |
| IL | Interleukin |
| ATRA | All-trans retinoic acid |
| PMA | Phorbol 12-myristate 13-acetate |
| 1,25 (OH)2D3 | Calcitriol |
| Treg | Regulatory T cell |
| MP | Microparticle |
| MVBs | Multivesicular bodies |
| ILVs | Intraluminal vesicles |
| SNARE | SNAP receptor |
| Tsg 101 | Tumor susceptibility gene 101 |
| MLL | Mixed-lineage leukemia |
| DFS | Disease-free survival |
| AF | Acute lymphoblastic leukemia 1-fused gene |
| AML | Acute myeloid leukemia |
| CYP27B1 | 25-Hydroxyvitamin D3-1a-hydroxylase |
| Th | Helper T cell |
| CDKN | Vyclin-dependent kinase inhibitor |
| ATCC | American Type Culture Collection |
| DSMZ | German Collection of Microorganisms and Cell Culture |
| EBV | Epstein–Barr virus |
| IMDM | Iscove modified Dulbecco medium |
| FBS | Fetal bovine serum |
| BSA | Bovine serum albumin |
| RPMI-1640 | Medium Roswell Park Memorial Institute 1640 |
| MEMα | Minimal essential medium |
| 1,24R (OH)2D3/ PRI-2191 | Tacalcitol |
| MTT | Colorimetric assay for assessing cell metabolic activity |
| DMSO | Dimethyl sulfoxide |
| IC50 | Half-maximal inhibitory concentration |
| MGG | May–Grunwald–Giemsa staining |
| PI | Propidium iodide |
| BSA | Bovine serum albumin |
| FACS | Fluorescence-activated cell sorter |
| DLS | Dynamic light scattering |
| Cryo-LV-FESEM | Cryogenic low-voltage field-emission scanning Electron microscopy |
| ET | Everhart–Thornley detector |
| SD | Standard deviation |
| PdI | Polydispersity index |
| CLL | Chronic lymphoblastic leukemia |
| MDSC | Myeloid-derived suppressor cell |
| DLBCL | Diffuse large B-cell lymphoma |
| PBMC | Peripheral blood mononuclear cell |
| SLE | Systemic lupus erythematosus |
| MS | Multiple sclerosis |
| IgV | Immunoglobulin variable domain |
| CALLA | Common acute lymphoblastic leukemia antigen |
| BCR | B cell receptor |
| PWM | Pokeweed mitogen |
References
- Mbongue, J.C.; Nieves, H.A.; Torrez, T.W.; Langridge, W.H.R. The Role of Dendritic Cell Maturation in the Induction of Insulin-Dependent Diabetes Mellitus. Front. Immunol. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berges, C.; Naujokat, C.; Tinapp, S.; Wieczorek, H.; Höh, A.; Sadeghi, M.; Opelz, G.; Daniel, V. A cell line model for the differentiation of human dendritic cells. Biochem. Biophys. Res. Commun. 2005, 333, 896–907. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, L.; Li, F.; Jiang, G. The plasticity and potential of leukemia cell lines to differentiate into dendritic cells. Oncol. Lett. 2012, 4, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adorini, L.; Penna, G.; Giarratana, N.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Uskokovic, M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Lutz, W.H.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem. Biophys. Res. Commun. 2000, 270, 701–708. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2016, 8, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 6. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Hefazi, M.; Litzow, M.R. Recent advances in the biology and treatment of B-cell acute lymphoblastic leukemia. Blood Lymphat. Cancer Targets Ther. 2018, 8, 47–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaretti, S.; Zini, G.; Bassan, R. Diagnosis and Subclassification of Acute Lymphoblastic Leukemia. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balgobind, B.V.; Zwaan, C.M.; Pieters, R.; Van den Heuvel-Eibrink, M.M. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia 2011, 25, 1239–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greil, J.; Gramatzki, M.; Burger, R.; Marschalek, R.; Peltner, M.; Trautmann, U.; Hansen-Hagge, T.E.; Bartram, C.R.; Fey, G.H.; Stehr, K. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br. J. Haematol. 1994, 86, 275–283. [Google Scholar] [CrossRef]
- Stong, R.C.; Kersey, J.H. In vitro culture of leukemic cells in t(4;11) acute leukemia. Blood 1985, 66, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Jack, I.; Seshadri, R.; Garson, M.; Michael, P.; Callen, D.; Zola, H.; Morley, A. RCH-ACV: A lymphoblastic leukemia cell line with chromosome translocation 1;19 and trisomy 8. Cancer Genet. Cytogenet. 1986, 19, 261–269. [Google Scholar] [CrossRef]
- Nevozhay, D. Cheburator Software for Automatically Calculating Drug Inhibitory Concentrations from In Vitro Screening Assays. PLoS ONE 2014, 9, e106186. [Google Scholar] [CrossRef] [Green Version]
- Baran, J.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Szatanek, R.; Zembala, M.; Barbasz, J.; Czupryna, A.; Szczepanik, A.; Zembala, M. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol. Immunother. CII 2010, 59, 841–850. [Google Scholar] [CrossRef]
- Schierer, S.; Ostalecki, C.; Zinser, E.; Lamprecht, R.; Plosnita, B.; Stich, L.; Dörrie, J.; Lutz, M.B.; Schuler, G.; Baur, A.S. Extracellular vesicles from mature dendritic cells (DC) differentiate monocytes into immature DC. Life Sci. Alliance 2018, 1, e201800093. [Google Scholar] [CrossRef]
- Drab, M.; Krajniak, J.; Grzelakowski, K.P. The New Methodology and Chemical Contrast Observation by Use of the Energy-Selective Back-Scattered Electron Detector. Microsc. Microanal. 2016, 22, 1369–1373. [Google Scholar] [CrossRef]
- Drab, M. Phage Aggregation–Dispersion by Ions: Striving beyond Antibacterial Therapy. Trends Biotechnol. 2018, 36, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Lagishetty, V.; Liu, N.Q.; Hewison, M. Vitamin D metabolism and innate immunity. Mol. Cell. Endocrinol. 2011, 347, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigby, W.F.; Waugh, M.; Graziano, R.F. Regulation of human monocyte HLA-DR and CD4 antigen expression, and antigen presentation by 1,25-dihydroxyvitamin D3. Blood 1990, 76, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Széles, L.; Keresztes, G.; Töröcsik, D.; Balajthy, Z.; Krenács, L.; Póliska, S.; Steinmeyer, A.; Zuegel, U.; Pruenster, M.; Rot, A.; et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009, 182, 2074–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enioutina, E.Y.; Bareyan, D.; Daynes, R.A. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J. Immunol. 2009, 182, 4296–4305. [Google Scholar] [CrossRef] [Green Version]
- Göbel, F.; Taschner, S.; Jurkin, J.; Konradi, S.; Vaculik, C.; Richter, S.; Kneidinger, D.; Mühlbacher, C.; Bieglmayer, C.; Elbe-Bürger, A.; et al. Reciprocal role of GATA-1 and vitamin D receptor in human myeloid dendritic cell differentiation. Blood 2009, 114, 3813–3821. [Google Scholar] [CrossRef] [Green Version]
- Lotze, M.; Thomson, A. Dendritic Cells. In Biology and Clinical Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Trynda, J.; Turlej, E.; Milczarek, M.; Pietraszek, A.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Antiproliferative Activity and in Vivo Toxicity of Double-Point Modified Analogs of 1,25-Dihydroxyergocalciferol. Int. J. Mol. Sci. 2015, 16, 24873–24894. [Google Scholar] [CrossRef] [Green Version]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef] [Green Version]
- Lawrie, A.S.; Albanyan, A.; Cardigan, R.A.; Mackie, I.J.; Harrison, P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009, 96, 206–212. [Google Scholar] [CrossRef]
- Alchinova, I.B.; Khaspekova, S.G.; Golubeva, N.V.; Shustova, O.N.; Antonova, O.A.; Karganov, M.Y.; Mazurov, A.V. Comparison of the size of membrane microparticles of different cellular origin by dynamic light scattering. Dokl. Biochem. Biophys. 2016, 470, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Consolini, R.; Pala, S.; Legitimo, A.; Crimaldi, G.; Ferrari, S.; Ferrari, S. Effects of vitamin D on the growth of normal and malignant B-cell progenitors. Clin. Exp. Immunol. 2001, 126, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykes, M.; MacPherson, G. Dendritic cell-B-cell interaction: Dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology 2000, 100, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Marchwicka, A.; Corcoran, A.; Berkowska, K.; Marcinkowska, E. Restored expression of vitamin D receptor and sensitivity to 1,25-dihydroxyvitamin D3 in response to disrupted fusion FOXP2-FGFR1 gene in acute myeloid leukemia cells. Cell Biosci. 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Uchida, H.; Hasegawa, Y.; Takahashi, H.; Makishima, M. 1α-dihydroxyvitamin D3 and retinoic acid increase nuclear vitamin D receptor expression in monocyte THP-1 cells. Anticancer. Res. 2016, 12, 6297–6301. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, X.; Studziński, G.P. 1,25-dihydroxyvitamin D3 induces monocytic differentiation of human myeloid leukemia cells by regulating C/EBPβ expression through MEF2C. J. Steroid Biochem. Mol. Biol. 2015, 148, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Xu, J.; Gu, Y.; Gu, X.; Li, W.; Wang, Y. Vitamin D suppresses oxidative stress-induced microparticle release by human umbilical vein endothelial cells. Biol. Reprod. 2017, 96, 199–210. [Google Scholar] [CrossRef]
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J., Jr.; McHugh, K.P.; Holliday, L.S. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J. Dent. Res. 2016, 95, 673–679. [Google Scholar] [CrossRef]
- Bruns, H.; Böttcher, M.; Qorraj, M.; Fabri, M.; Jitschin, S.; Dindorf, J.; Busch, L.; Jitschin, R.; Mackensen, A.; Mougiakakos, D. CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D. Leukemia 2017, 31, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Kozielewicz, P.; Grafton, G.; Kutner, A.; Curnow, S.J.; Gordon, J.; Barnes, N.M. Novel vitamin D analogues; cytotoxic and anti-proliferative activity against a diffuse large B-cell lymphoma cell line and B-cells from healthy donors. J. Steroid Biochem. Mol. Biol. 2016, 164, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linker-Israeli, M.; Elstner, E.; Klinenberg, J.R.; Wallace, D.J.; Koeffler, H.P. Vitamin D(3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin. Immunol. 2001, 99, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, R.H.; Racila, E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk. Lymphoma 1995, 18, 385–397. [Google Scholar] [CrossRef]
- Pawlik, A.; Anisiewicz, A.; Filip-Psurska, B.; Nowak, M.; Turlej, E.; Trynda, J.; Banach, J.; Gretkierewicz, P.; Wietrzyk, J. Calcitriol and Its Analogs Establish the Immunosuppressive Microenvironment That Drives Metastasis in 4T1 Mouse Mammary Gland Cancer. Int. J. Mol. Sci. 2018, 19, 2116. [Google Scholar] [CrossRef] [Green Version]
- Rolf, L.; Muris, A.-H.; Hupperts, R.; Damoiseaux, J. Vitamin D effects on B cell function in autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef]
- Haas, J.; Schwarz, A.; Korporal-Kuhnke, M.; Faller, S.; Jarius, S.; Wildemann, B. Hypovitaminosis D upscales B-cell immunoreactivity in multiple sclerosis. J. Neuroimmunol. 2016, 294, 18–26. [Google Scholar] [CrossRef]
- Tangye, S.G.; Good, K.L. Human IgM + CD27 + B cells: Memory B cells or ‘memory’ B cells? J. Immunol. 2007, 179, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Gerasimčik, N.; Camponeschi, A.; Tan, Y.; Wu, Q.; Brynjolfsson, S.; Zheng, J.; Abrahamsson, J.; Nordlund, J.; Lönnerholm, G.; et al. CD27 expression and its association with clinical outcome in children and adults with pro-B acute lymphoblastic leukemia. Blood Cancer J. 2017, 7, e575. [Google Scholar] [CrossRef] [Green Version]
- Uckun, F.M.; Song, C.W. Lack of CD24 antigen expression in B-lineage acute lymphoblastic leukemia is associated with intrinsic radiation resistance of primary clonogenic blasts. Blood 1993, 81, 1323–1332. [Google Scholar] [CrossRef] [Green Version]
- Funaro, A.; Morra, M.; Calosso, L.; Zini, M.G.; Ausiello, C.M.; Malavasi, F. Role of the human CD38 molecule in B cell activation and proliferation. Tissue Antigens 1997, 49, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Béné, M.C.; Faure, G.C. CD10 in acute leukemias. GEIL (Groupe d’Etude Immunologique des Leucémies). Haematologica 1997, 82, 205–210. [Google Scholar] [PubMed]
- Chung, J.B.; Sater, R.A.; Fields, M.L.; Erikson, J.; Monroe, J.G. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int. Immunol. 2002, 14, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabin, E.; Cong, Y.Z.; Wortis, H.H. Loss of CD23 is a consequence of B-cell activation. Implications for the analysis of B-cell lineages. Ann. N. Y. Acad. Sci. 1992, 651, 130–142. [Google Scholar] [CrossRef]
- Hartmann, B.; Heine, G.; Babina, M.; Steinmeyer, A.; Zügel, U.; Radbruch, A.; Worm, M. Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy 2011, 66, 540–548. [Google Scholar] [CrossRef]
- DiRaimondo, F.; Albitar, M.; Huh, Y.; O’Brien, S.; Montillo, M.; Tedeschi, A.; Kantarjian, H.; Lerner, S.; Giustolisi, R.; Keating, M. The clinical and diagnostic relevance of CD23 expression in the chronic lymphoproliferative disease. Cancer 2002, 94, 1721–1730. [Google Scholar] [CrossRef] [Green Version]
- Shirakawa, A.-K.; Nagakubo, D.; Hieshima, K.; Nakayama, T.; Jin, Z.; Yoshie, O. 1,25-Dihydroxyvitamin D3 Induces CCR10 Expression in Terminally Differentiating Human B Cells. J. Immunol. 2008, 180, 2786–2795. [Google Scholar] [CrossRef] [Green Version]
- Rolf, L.; Muris, A.; Hupperts, R.; Damoiseaux, J. Illuminating vitamin D effects on B cells—The multiple sclerosis perspective. Immunology 2016, 147, 275–284. [Google Scholar] [CrossRef]
- Britten, O.; Ragusa, D.; Tosi, S.; Kamel, Y.M. MLL-Rearranged Acute Leukemia with t(4;11)(q21;q23)—Current Treatment Options. Is There a Role for CAR-T Cell Therapy? Cells 2019, 8, 1341. [Google Scholar] [CrossRef] [Green Version]
- Antony, R.; Sheng, X.; Ehsanipour, E.A.; Ng, E.; Pramanik, R.; Klemm, L.; Ichihara, B.; Mittelman, S.D. Vitamin D protects acute lymphoblastic leukemia cells from dexamethasone. Leuk. Res. 2012, 36, 591–593. [Google Scholar] [CrossRef] [Green Version]
- Fukasawa, H.; Kato, A.; Fujigaki, Y.; Yonemura, K.; Furuya, R.; Hishida, A. Hypercalcemia in a patient with B-cell acute lymphoblastic leukemia: A role of proinflammatory cytokine. Am. J. Med. Sci. 2001, 322, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.R.; Neira, L.C.; Fischer, S.G.; Torres, M.C.T.; Milinarsky, A.T.; Giadrosich, V.R.; Arriagada, M.M.; Arinoviche, R.S.; Casanova, D.M. Effect of 1,25(OH)2-vitamin D on bone mass in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2008, 30, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, D.; Lin, S.; Li, P. CD34 and CD38 are prognostic biomarkers for acute B lymphoblastic leukemia. Biomark. Res. 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]






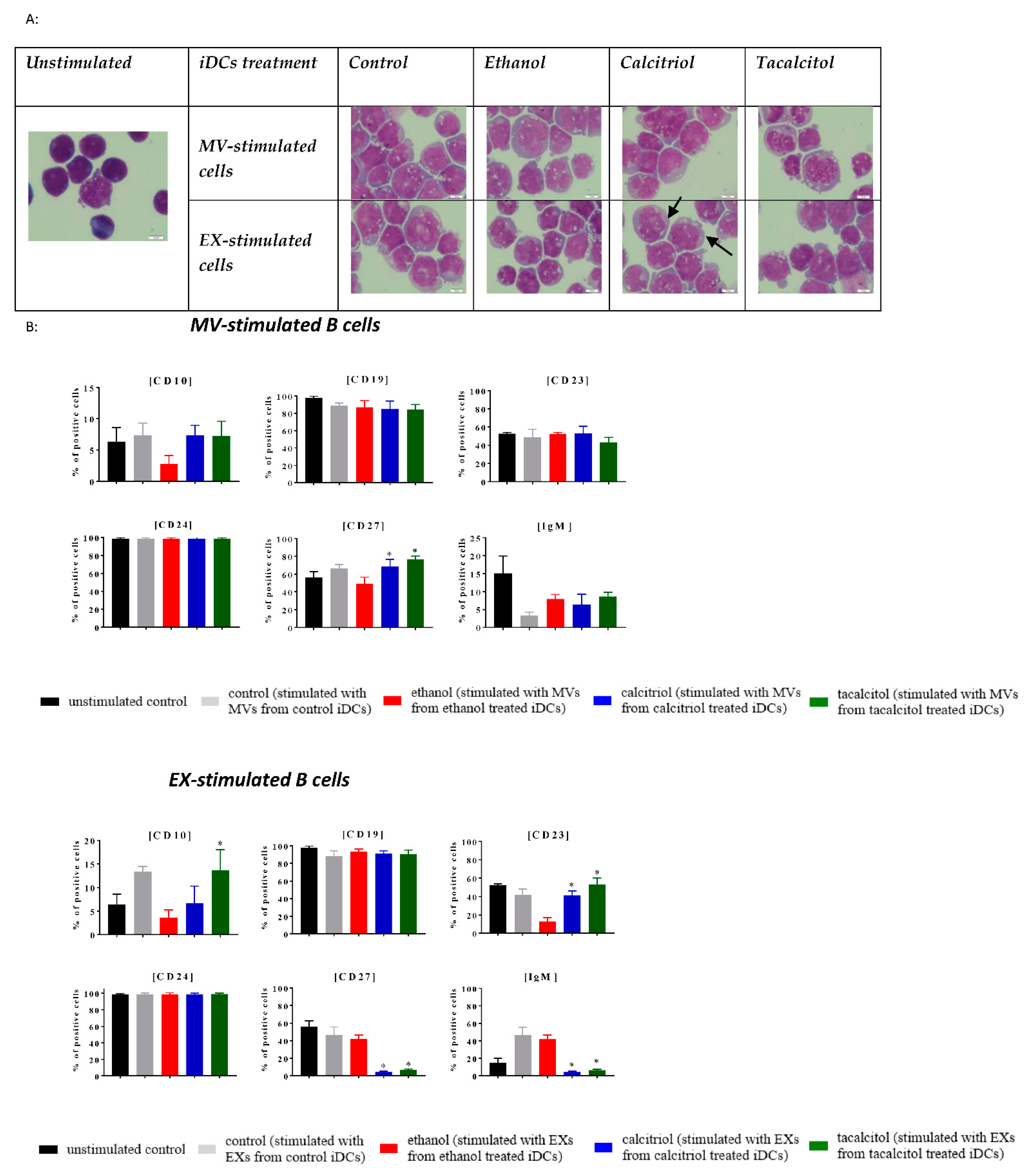
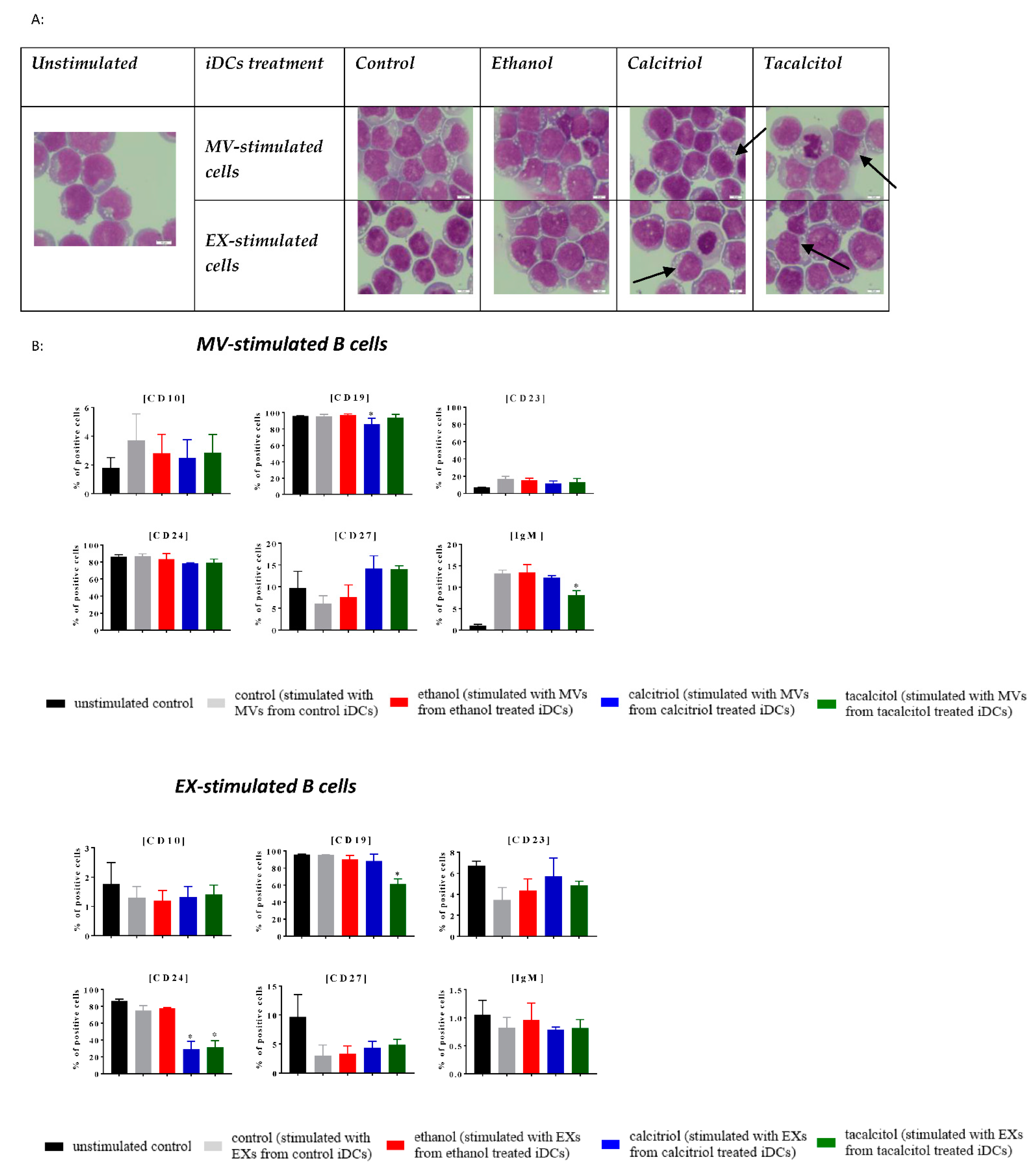
| Cells/Microvesicles/Exosomes | Antibody (Clone) Fluorescent Probe | Microvesicles/Exosomes | Antibody (Clone) Fluorescent Probe |
|---|---|---|---|
| Monocyte | CD9 (M-L13) PE-CF594 | Exosomes (monocyte) | CD9 (M-L13) PE-CF594 |
| CD11b PE | CD81 (JS-81) APC | ||
| CD14 (M5E2) APC | CD63(H5C6) PE | ||
| CD16 (3G8) BV421 | HLA-DR (G46-6) BV421 | ||
| HLA-DR (G46-6) BV421 | Exosomes (dendritic cell) | CD9 (M-L13) PE-CF594 | |
| Dendritic cell (immature/mature) | CD9(M-L13) PE-CF594 | CD81 (JS-81) APC | |
| CD11c (B-ly6) APC | CD63 (H5C6) PE | ||
| CD11b PE | HLA-DR (G46-6) BV421 | ||
| CD81 (JS-81) APC | CD11b PE | ||
| CD123 (7G3) PE | CD40 (5C3) PE | ||
| CD14 (M5E2) APC | CD86 (FUN-1)APC | ||
| CD16 (3G8) BV421 | Annexin V APC | ||
| HLA-DR (G46-6) BV421 | Microvesicles (monocyte) | CD11b PE | |
| Precursor B cell (before/after stimulation by microvesicles/exosomes) (monocyte) | CD10 (HI10a) BV421 | CD14(M5E2) APC | |
| CD19 (HIB19) PE | HLA-DR (G46-6)BV421 | ||
| CD20 (2H7) APC | Microvesicles (dendritic cell) | CD11c (B-ly6) APC | |
| CD23 (M-L233) BB700 | CD123(7G3) PE | ||
| CD24 (ML5) BV421 | CD11b PE | ||
| CD27 (M-T271) BV421 | CD14 (M5E2) APC | ||
| CD34 APC | HLA-DR(G46-6) BV421 | ||
| CD38 (HIT2) BB515 | |||
| HLA-DR(G46-6) BV421 | |||
| IgM (G20-127) APC | |||
| IgD (IA6-2) PE | |||
| LCL | RCH-ACV | SEM | KOPN-8 | RS4;11 | ||
|---|---|---|---|---|---|---|
| Translocation | - | t(11,19) | t(4;11) | t(4;11) | t(4;11) | |
| Calcitriol/Tacalcitol cell stimulation | ||||||
| Direct | CD19↓ CD27↑↑ | CD19↓↓ CD27↑ | CD24↓↓ CD38↑↑ | CD19↓ | IgM↑ | |
| Indirect | MVs | CD10↑ CD23↑↑ * IgM↑ | - | - | CD27↑↑ | CD19↓ IgM↓ |
| EXs | CD19↓ | CD27↓ | CD24↑ | CD10↑ CD23↑↑ CD27↓↓ IgM↓↓ | CD19↓ CD24↓↓ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turlej, E.; Goszczyński, T.M.; Drab, M.; Orzechowska, B.; Maciejewska, M.; Banach, J.; Wietrzyk, J. The Impact of Exosomes/Microvesicles Derived from Myeloid Dendritic Cells Cultured in the Presence of Calcitriol and Tacalcitol on Acute B-Cell Precursor Cell Lines with MLL Fusion Gene. J. Clin. Med. 2022, 11, 2224. https://doi.org/10.3390/jcm11082224
Turlej E, Goszczyński TM, Drab M, Orzechowska B, Maciejewska M, Banach J, Wietrzyk J. The Impact of Exosomes/Microvesicles Derived from Myeloid Dendritic Cells Cultured in the Presence of Calcitriol and Tacalcitol on Acute B-Cell Precursor Cell Lines with MLL Fusion Gene. Journal of Clinical Medicine. 2022; 11(8):2224. https://doi.org/10.3390/jcm11082224
Chicago/Turabian StyleTurlej, Eliza, Tomasz Marek Goszczyński, Marek Drab, Beata Orzechowska, Magdalena Maciejewska, Joanna Banach, and Joanna Wietrzyk. 2022. "The Impact of Exosomes/Microvesicles Derived from Myeloid Dendritic Cells Cultured in the Presence of Calcitriol and Tacalcitol on Acute B-Cell Precursor Cell Lines with MLL Fusion Gene" Journal of Clinical Medicine 11, no. 8: 2224. https://doi.org/10.3390/jcm11082224






