Genetic Characteristics According to Subgroup of Acute Myeloid Leukemia with Myelodysplasia-Related Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Molecular Analysis
2.3. Statistical Analysis and Response Assessment
3. Results
3.1. Patient Characteristics
3.2. Genetic Characteristics and Their Association with AML-MRC Subgroups
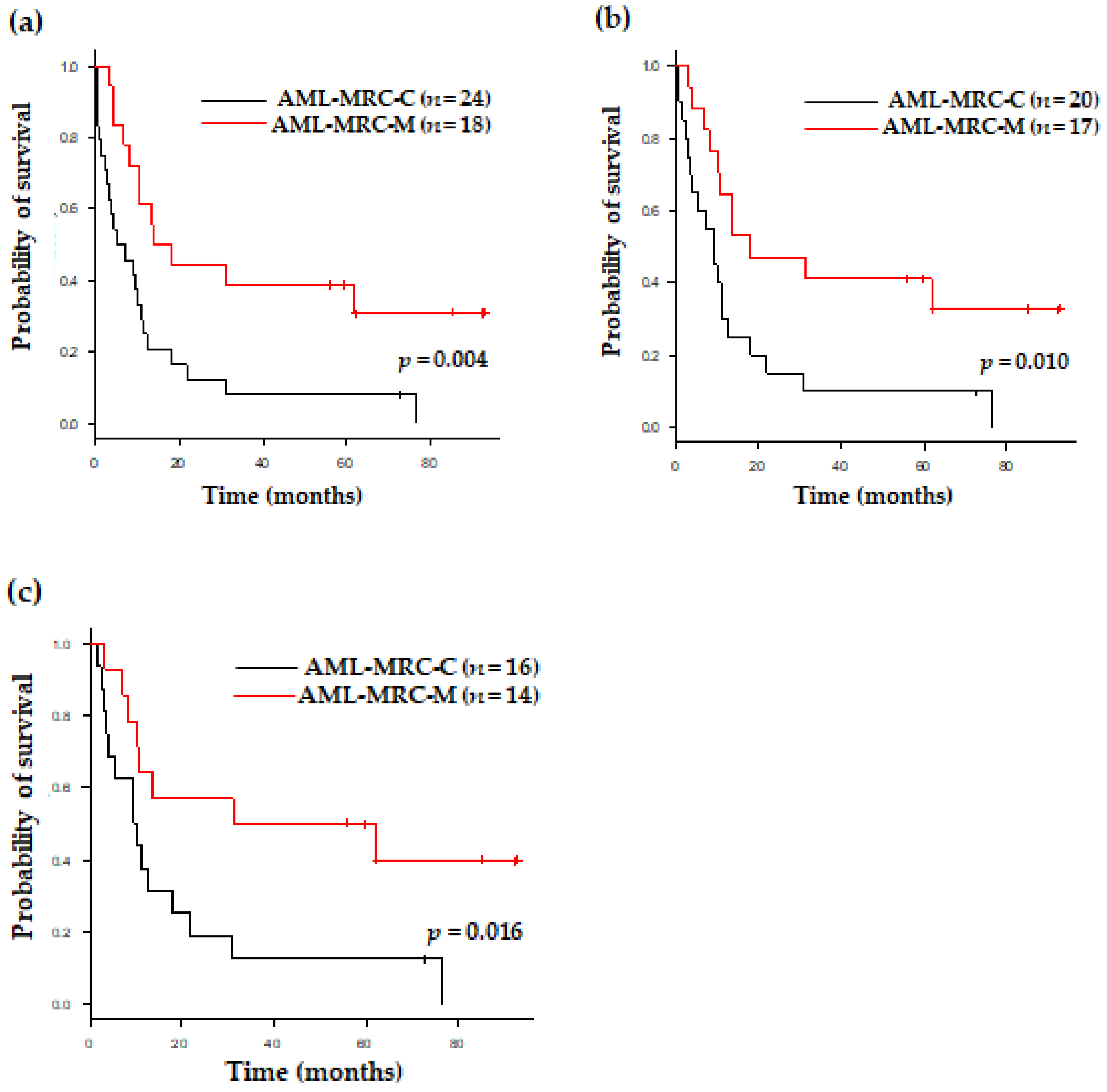
3.3. Clinical Outcomes of Patients with AML-MRC Based on Disease Subgroup and Therapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Wandt, H.; Schäkel, U.; Kroschinsky, F.; Prange-Krex, G.; Mohr, B.; Thiede, C.; Pascheberg, U.; Soucek, S.; Schaich, M.; Ehninger, G. MLD according to the WHO classification in AML has no correlation with age and no independent prognostic relevance as analyzed in 1766 patients. Blood 2008, 111, 1855–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miesner, M.; Haferlach, C.; Bacher, U.; Weiss, T.; Macijewski, K.; Kohlmann, A.; Klein, H.-U.; Dugas, M.; Kern, W.; Schnittger, S.; et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: A comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood 2010, 116, 2742–2751. [Google Scholar] [PubMed]
- Weinberg, O.K.; Pozdnyakova, O.; Campigotto, F.; DeAngelo, D.J.; Stone, R.M.; Neuberg, D.; Hasserjian, R.P. Reproducibility and prognostic significance of morphologic dysplasia in de novo acute myeloid leukemia. Mod. Pathol. 2015, 28, 965–976. [Google Scholar] [CrossRef] [PubMed]
- McGowan-Jordan, J.; Hastings, R.J.; Moore, S. ISCN 2020. An International System for Human Cytogenomic Nomenclature (2020); Karger: Basel, Switzerland, 2020; p. 170. [Google Scholar]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kim, Y.; Kang, D.; Kim, H.S.; Lee, J.M.; Kim, M.; Cho, B.S. Prognostic value of measurable residual disease monitoring by next-generation sequencing before and after allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Blood Cancer J. 2021, 11, 109. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, G.D.; Park, J.; Yoon, J.-H.; Kim, H.-J.; Min, W.-S.; Kim, M. Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 2015, 5, e336. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Yahng, S.-A.; Kwon, A.; Park, J.; Jeon, Y.-W.; Yoon, J.-H.; Shin, S.-H.; Lee, S.-E.; Cho, B.-S.; Eom, K.-S.; et al. Mutation in TET2 or TP53 predicts poor survival in patients with myelodysplastic syndrome receiving hypomethylating treatment or stem cell transplantation. Bone Marrow Transpl. 2015, 50, 1132–1134. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Cho, B.-S.; Kim, H.-J.; Han, E.; Jang, W.; Han, K.; Lee, J.-W.; Chung, N.-G.; Cho, B.; Kim, M.; et al. Reclassification of Acute Myeloid Leukemia According to the 2016 WHO Classification. Ann. Lab. Med. 2019, 39, 311–316. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; WHO: Geneva, Switzerland, 2017; p. 586.
- Hollink, I.H.; van den Heuvel-Eibrink, M.M.; Arentsen-Peters, S.T.; Pratcorona, M.; Abbas, S.; Kuipers, J.E.; van Galen, J.F.; Beverloo, H.B.; Sonneveld, E.; Kaspers, G.-J.J.L.; et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood 2011, 118, 3645–3656. [Google Scholar] [CrossRef] [Green Version]
- Thol, F.; Kölking, B.; Hollink, I.H.I.; Damm, F.; Van Den Heuvel-eibrink, M.M.; Michel Zwaan, C.; Bug, G.; Ottmann, O.; Wagner, K.; Morgan, M.; et al. Analysis of NUP98/NSD1 translocations in adult AML and MDS patients. Leukemia 2013, 27, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Flach, J.; Shumilov, E.; Joncourt, R.; Porret, N.; Tchinda, J.; Legros, M.; Scarpelli, I.; Hewer, E.; Novak, U.; Schoumans, J.; et al. Detection of rare reciprocal RUNX1 rearrangements by next-generation sequencing in acute myeloid leukemia. Genes Chromosomes Cancer 2020, 59, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, C.; Dicker, F.; Herholz, H.; Schnittger, S.; Kern, W. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia 2008, 22, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, R.S.; Ma, L.; Merker, J.D.; Gotlib, J.R.; Schrijver, I.; Zehnder, J.L.; A Arber, D. Next-generation sequencing of acute myeloid leukemia identifies the significance of TP53, U2AF1, ASXL1, and TET2 mutations. Mod. Pathol. 2015, 28, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.; Li, S.; Adams, P.D.; Deshpande, A.J. The role of TP53 in acute myeloid leukemia: Challenges and opportunities. Genes Chromosomes Cancer 2019, 58, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.M.; Richardson, D.R.; Galeotti, J.; Esparza, S.; Zhu, A.; Fedoriw, Y.; Weck, K.E.; Foster, M.C.; Coombs, C.C.; Zeidner, J.F.; et al. Acute Myeloid Leukemia with Co-mutated ASXL1 and SRSF2 Exhibits Monocytic Differentiation and has a Mutational Profile Overlapping with Chronic Myelomonocytic Leukemia. Hemasphere 2019, 3, e292. [Google Scholar] [CrossRef]
- Prats-Martín, C.; Burillo-Sanz, S.; Morales-Camacho, R.M.; Pérez-López, O.; Suito, M.; Vargas, M.T.; Caballero-Velázquez, T.; Carrillo-Cruz, E.; González, J.; Bernal, R.; et al. ASXL1 mutation as a surrogate marker in acute myeloid leukemia with myelodysplasia-related changes and normal karyotype. Cancer Med. 2020, 9, 3637–3646. [Google Scholar] [CrossRef] [Green Version]
- Devillier, R.; Gelsi-Boyer, V.; Brecqueville, M.; Carbuccia, N.; Murati, A.; Vey, N.; Birnbaum, D.; Mozziconacci, M.-J. Acute myeloid leukemia with myelodysplasia-related changes are characterized by a specific molecular pattern with high frequency of ASXL1 mutations. Am. J. Hematol. 2012, 87, 659–662. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, R.; Yang, Y.; Zhao, Y.; Wakeham, A.; Li, W.Y.; Tseng, A.; Leca, J.; Berger, T.; Saunders, M.; et al. IDH1 mutation contributes to myeloid dysplasia in mice by disturbing heme biosynthesis and erythropoiesis. Blood 2021, 137, 945–958. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Stein, E.M.; Dinardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; DiNardo, C.D. Acute myeloid leukemia with IDH1 and IDH2 mutations: 2021 treatment algorithm. Blood Cancer J. 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Bacher, U.; Schnittger, S.; Macijewski, K.; Grossmann, V.; Kohlmann, A.; Alpermann, T.; Kowarsch, A.; Nadarajah, N.; Kern, W.; Haferlach, C.; et al. Multilineage dysplasia does not influence prognosis in CEBPA-mutated AML, supporting the WHO proposal to classify these patients as a unique entity. Blood 2012, 119, 4719–4722. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T.; Stengel, A.; Eckstein, S.; Perglerová, K.; Alpermann, T.; Kern, W.; Meggendorfer, M. The new provisional WHO entity ‘RUNX1 mutated AML’ shows specific genetics but no prognostic influence of dysplasia. Leukemia 2016, 30, 2109–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasik, S.; Eckardt, J.-N.; Kramer, M.; Röllig, C.; Krämer, A.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brümmendorf, T.H.; Naumann, R.; et al. Impact of PTPN11 mutations on clinical outcome analyzed in 1529 patients with acute myeloid leukemia. Blood Adv. 2021, 5, 3279–3289. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ren, Y.; Gunawan, S.; Teng, P.; Chen, Z.; Lawrence, H.R.; Cai, J.; Lawrence, N.J.; Wu, J. Selective inhibition of leukemia-associated SHP2(E69K) mutant by the allosteric SHP2 inhibitor SHP099. Leukemia 2018, 32, 1246–1249. [Google Scholar] [CrossRef]
- Seymour, J.F.; Döhner, H.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. Azacitidine improves clinical outcomes in older patients with acute myeloid leukaemia with myelodysplasia-related changes compared with conventional care regimens. BMC Cancer 2017, 17, 852. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Koenig, K.L.; Sahasrabudhe, K.D.; Sigmund, A.M.; Bhatnagar, B. AML with Myelodysplasia-Related Changes: Development, Challenges, and Treatment Advances. Genes 2020, 11, 845. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.; Hong, S.E.; Kang, D.; Choi, H.; Lee, J.M.; Yoon, J.H.; Cho, B.S.; Lee, S.; Kim, H.J.; et al. Integrative Analysis of Gene Expression Data by RNA Sequencing for Differential Diagnosis of Acute Leukemia: Potential Application of Machine Learning. Front. Oncol. 2021, 11, 717616. [Google Scholar] [CrossRef] [PubMed]
- Devillier, R.; Mansat-De Mas, V.; Gelsi-Boyer, V.; Demur, C.; Murati, A.; Corre, J.; Prebet, T.; Bertoli, S.; Brecqueville, M.; Arnoulet, C.; et al. Role of ASXL1 and TP53 mutations in the molecular classification and prognosis of acute myeloid leukemias with myelodysplasia-related changes. Oncotarget 2015, 6, 8388–8396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, O.K.; Gibson, C.J.; Blonquist, T.M.; Neuberg, D.; Pozdnyakova, O.; Kuo, F.; Ebert, B.L.; Hasserjian, R.P. Association of mutations with morphological dysplasia in de novo acute myeloid leukemia without 2016 WHO Classification-defined cytogenetic abnormalities. Haematologica 2018, 103, 626–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalban-Bravo, G.; Kanagal-Shamanna, R.; Class, C.A.; Sasaki, K.; Ravandi, F.; Cortes, J.E.; Daver, N.; Takahashi, K.; Short, N.J.; DiNardo, C.D.; et al. Outcomes of acute myeloid leukemia with myelodysplasia related changes depend on diagnostic criteria and therapy. Am. J. Hematol. 2020, 95, 612–622. [Google Scholar] [CrossRef]
- Jiang, G.; Capo-Chichi, J.M.; Liu, A.; Atenafu, E.G.; Guo, R.; Tierens, A.; Minden, M.D.; Chang, H. Acute myeloid leukemia with myelodysplasia-related changes diagnosed with multilineage dysplasia alone demonstrates a superior clinical outcome. Hum. Pathol. 2020, 104, 117–126. [Google Scholar] [CrossRef]
- Badar, T.; Szabo, A.; Sallman, D.; Komrojki, R.; Lancet, J.; Padron, E.; Song, J.; Hussaini, M.O. Interrogation of molecular profiles can help in differentiating between MDS and AML with MDS-related changes. Leuk. Lymphoma 2020, 61, 1418–1427. [Google Scholar] [CrossRef]
- Yu, J.; Du, Y.; Jalil, A.; Ahmed, Z.; Mori, S.; Patel, R.; Varela, J.C.; Chang, C.C. Mutational profiling of myeloid neoplasms associated genes may aid the diagnosis of acute myeloid leukemia with myelodysplasia-related changes. Leuk. Res. 2021, 110, 106701. [Google Scholar] [CrossRef]
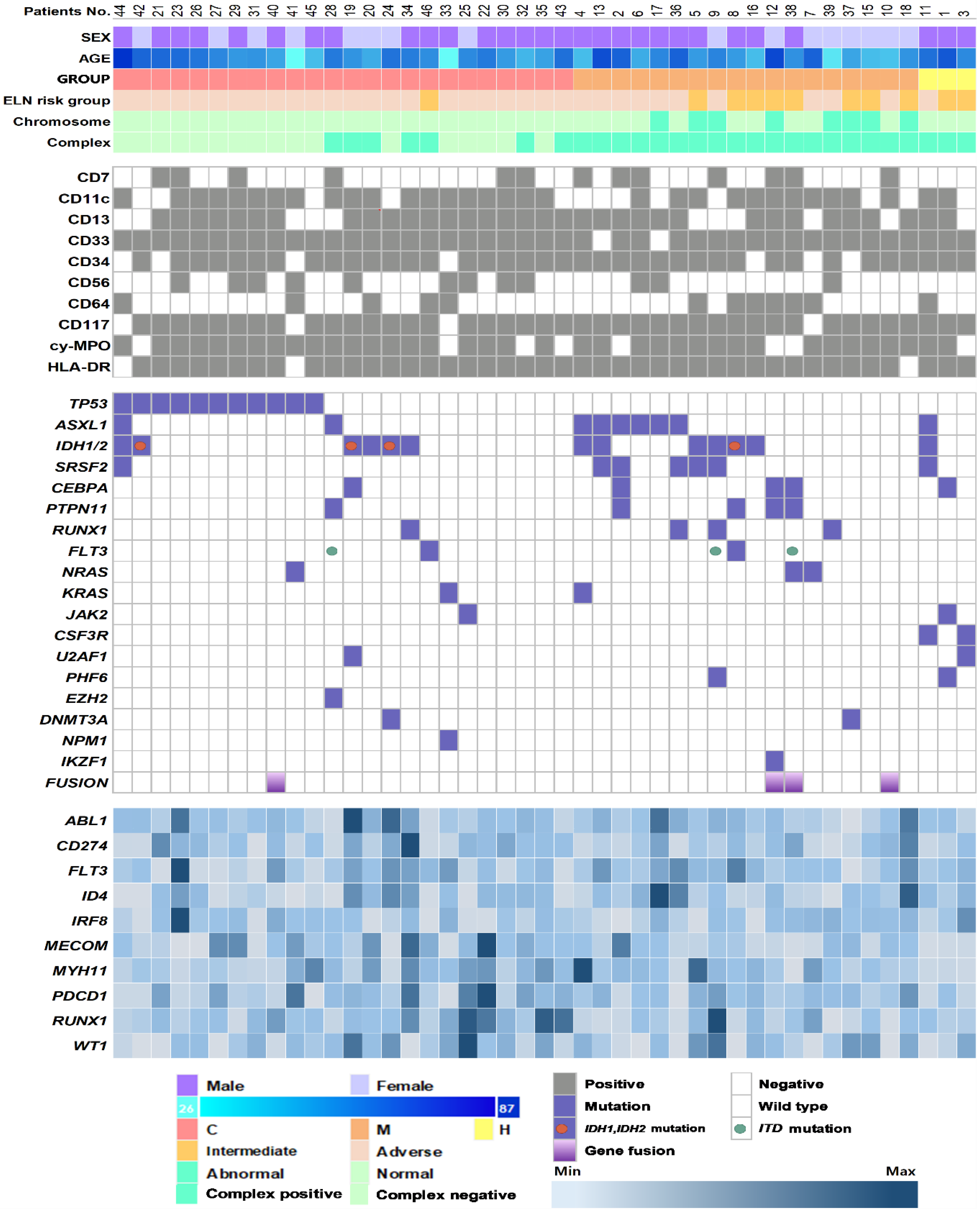
| Variables | Overall | AML-MRC-H | AML-MRC-C | AML-MRC-M | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis, years | 0.324 | ||||
| Median (range) | 60 (26–87) | 69 (61–76) | 60.5 (26–87) | 57 (34–81) | |
| Sex, n (%) | 0.312 | ||||
| Male | 29 (64.4) | 3(100) | 16 (66.7) | 10 (55.6) | |
| Female | 16 (35.6) | 0 | 8 (33.3) | 8 (44.4) | |
| WBC count at diagnosis | 0.780 | ||||
| Median (range) | 3.19 (0.78–363.5) | 12.64 (0.9–42.85) | 3.67 (0.78–169.6) | 2.91 (0.84–363.5) | |
| WBC group at diagnosis, n (%) | 0.629 | ||||
| <50 × 10⁹/L | 41 (91.1) | 3 (100) | 21 (87.5) | 17 (94.4) | |
| ≥50 × 10⁹/L | 4 (8.9) | 0 | 3 (12.5) | 1(5.6) | |
| 2017 ELN risk group, n (%) | 0.002 | ||||
| Favorable | 0 | 0 | 0 | 0 | |
| Intermediate | 11 (24.4) | 2 (66.7) | 1 (4.2) | 8 (55.6) | |
| Adverse | 34 (75.6) | 1 (33.3) | 23 (95.8) | 10 (44.4) | |
| Treatment, n (%) | 0.469 | ||||
| Low-intensity treatment | 8 (20.5%) | 3 (17.6%) | 4 (20.0%) | 1 (50.0%) | |
| Intensive chemotherapy | 31 (79.5%) | 14 (82.4%) | 16 (80.0%) | 1 (50.0%) | |
| Treatment detail, n (%) | 1.000 | ||||
| Hypomethylating agent (HMA) | 5 (12.8%) | 3 (17.6%) | 1 (5.0%) | 1 (50.0%) | |
| Low-dose cytarabine | 3 (7.7%) | 0 (0.0%) | 3 (15.0%) | 0 (0.0%) | |
| Intensive chemo only | 12 (30.8%) | 4 (23.5%) | 8 (40.0%) | 0 (0.0%) | |
| Intensive chemo + transplantation | 19 (48.7%) | 10 (58.8%) | 8 (40.0%) | 1 (50.0%) |
| Variables | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.049 | 1.016–1.084 | 0.004 |
| WBC at diagnosis | 1.000 | 1.000–1.000 | 0.273 |
| BM blast | 1.007 | 0.993–1.020 | 0.318 |
| AML-MRC subgroup | 0.018 | ||
| Mutation | |||
| TP53 | 4.580 | 2.156–9.729 | <0.001 |
| ASXL1 | 0.844 | 0.371–1.921 | 0.686 |
| IDH2 | 0.687 | 0.286–1.649 | 0.100 |
| SRSF2 | 1.025 | 0.428–2.458 | 0.955 |
| CEBPA | 1.281 | 0.494–3.323 | 0.611 |
| PTPN11 | 1.250 | 0.482–3.243 | 0.647 |
| IDH1 | 2.139 | 0.734–6.235 | 0.164 |
| RUNX1 | 1.160 | 0.408–3.295 | 0.781 |
| Characteristics | p-Value | Hazard Ratio (95% CI) |
|---|---|---|
| Age (continuous variable) | 0.003 | 1.061 (1.021–1.103) |
| WBC at diagnosis (continuous variable) | 0.451 | 1.000 (1.000–1.000) |
| BM blasts (continuous variable) | 0.655 | 1.003 (0.989–1.017) |
| AML-MRC-C vs. AML-MRC-M | 0.004 | 3.055 (1.425–6.547) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Jung, J.; Park, S.; Cho, B.-S.; Kim, H.-J.; Kim, Y.; Lee, J.-M.; Kim, H.S.; Ahn, A.; Kim, M.; et al. Genetic Characteristics According to Subgroup of Acute Myeloid Leukemia with Myelodysplasia-Related Changes. J. Clin. Med. 2022, 11, 2378. https://doi.org/10.3390/jcm11092378
Kang D, Jung J, Park S, Cho B-S, Kim H-J, Kim Y, Lee J-M, Kim HS, Ahn A, Kim M, et al. Genetic Characteristics According to Subgroup of Acute Myeloid Leukemia with Myelodysplasia-Related Changes. Journal of Clinical Medicine. 2022; 11(9):2378. https://doi.org/10.3390/jcm11092378
Chicago/Turabian StyleKang, Dain, Jin Jung, Silvia Park, Byung-Sik Cho, Hee-Je Kim, Yeojae Kim, Jong-Mi Lee, Hoon Seok Kim, Ari Ahn, Myungshin Kim, and et al. 2022. "Genetic Characteristics According to Subgroup of Acute Myeloid Leukemia with Myelodysplasia-Related Changes" Journal of Clinical Medicine 11, no. 9: 2378. https://doi.org/10.3390/jcm11092378






